Novel prime-boost combinations of attenuated mycobacterium
A technology for mycobacteria and mycobacterial antigens, which can be applied in the directions of drug combination, bacteria, antibody medical components, etc., can solve the problems of not providing cost-effectiveness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
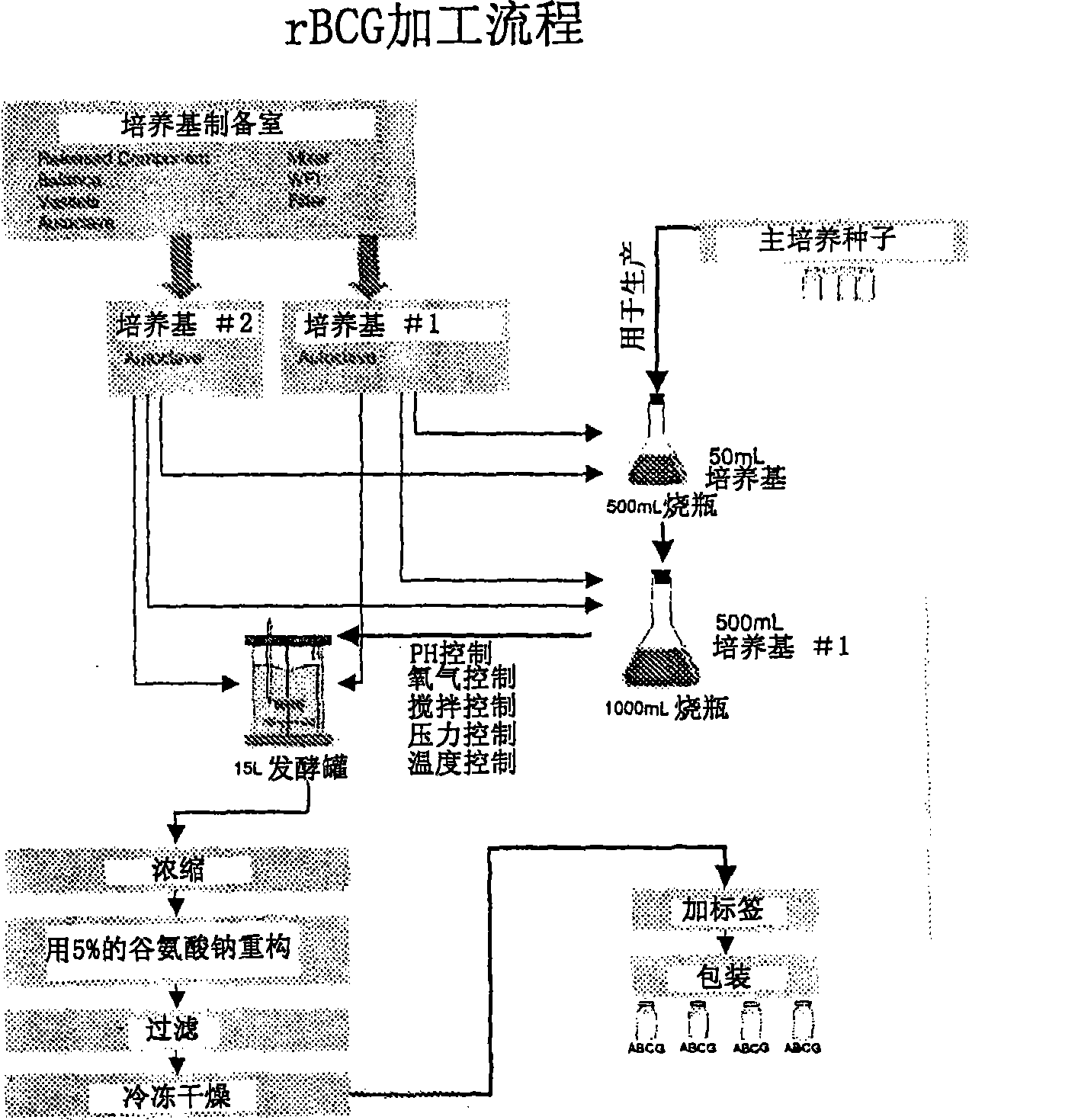
Image
Examples
Embodiment 1
[0146] Example 1. Construction of recombinant BCG strains that overexpress TB antigens and escape endosomes
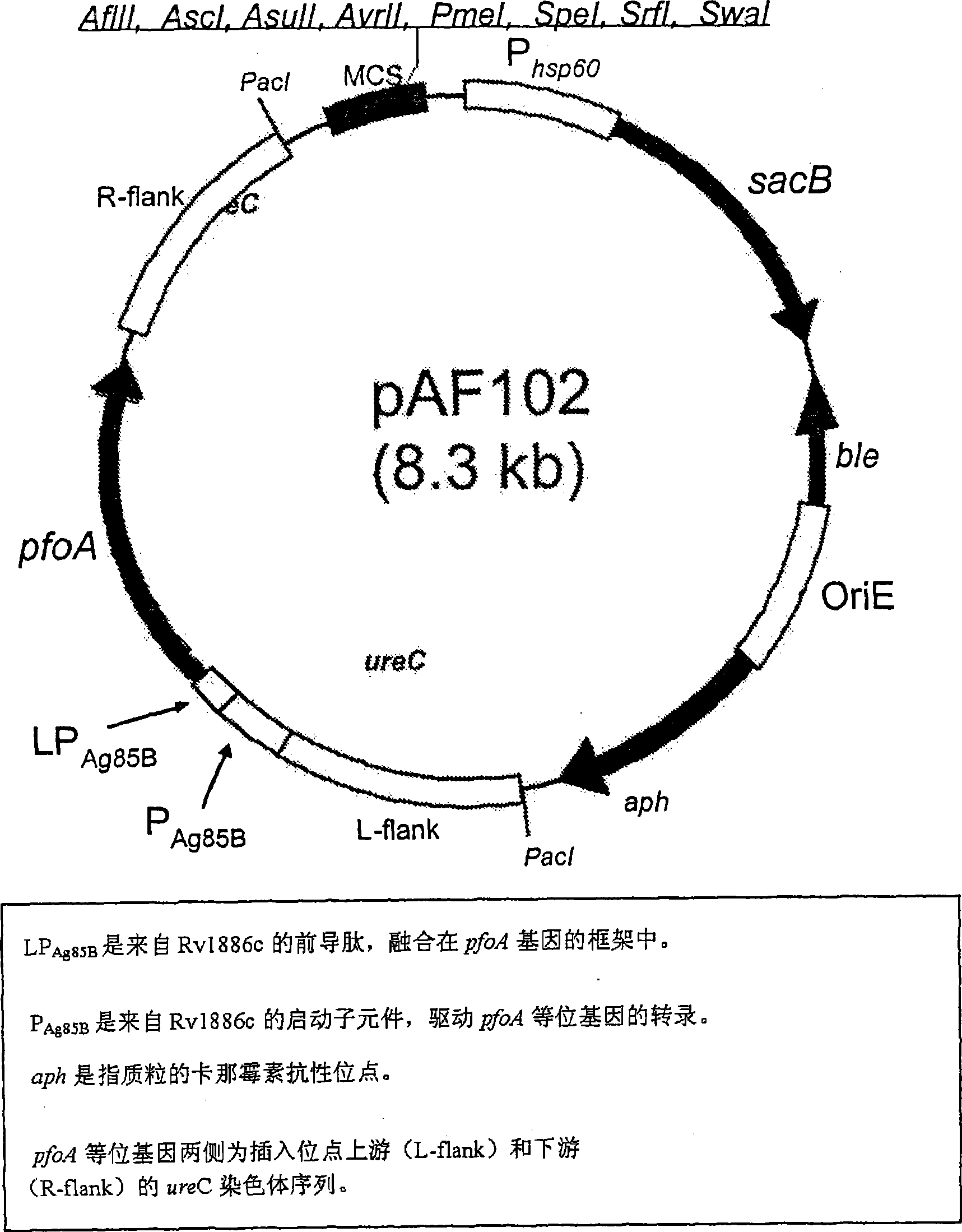
[0147] To generate strains that escape endosomes, we developed BCG1331 derivatives expressing perfringolysin O(Pfo), a cytolysin normally secreted by Clostridium perfringens, encoded by the pfoA gene (GenBank accession number CPE0163 ). PfoA mediates escape from phagosomes when expressed in Clostridium and in Bacillus subtilis (52). However, unlike Llo, PfoA is active at both pH 5.0 and pH 7.0 (52). To limit the cytotoxicity of Pfo, a mutant form of this protein with a G137Q substitution (PfoAG137Q) was used, since this strain has a short half-life in the host cell cytosol and is also capable of mediating endosomes over a wide pH range escape of (52).
[0148] In order to study the usefulness of PfoAG137Q, we constructed a recombinant BCG that secretes this protein and named it AFV102 (ie BCG1331 ureC::pfoAG137Q). The strain was constructed by allelic exchange with t...
Embodiment 2
[0163] Example 2. Optimization of Oral Recombinant BCG Vaccine Formulation and Dosage
[0164] Before testing a new two-component TB vaccine, studies were conducted to determine the optimal oral formulation and dosage. Groups containing 16 BALB / c mice were inoculated via gastric cannulation as shown in Table 5. 72 hours after inoculation, 3 mice per group were sacrificed and the number of viable AFV102 bacteria in intestine, Peyer's patch, lung and spleen were counted by the direct plate count method described above. This experiment shows that oral formulations containing Cera Vacx including gastric neutralizing components are more effective than formulations without them in their ability to deliver living organisms to mucosal tissues.
[0165] Six weeks after inoculation, 5 animals in each group were sacrificed and the immune responses to Rv3804c, Rv1886 and Rv0288 were measured by flow cytometric analysis. Briefly, mice were sacrificed by cervical dislocation and spleens w...
Embodiment 3
[0172] Example 3. Optimization of Vaccination Strategies
[0173] The purpose of this experiment was to optimize the prime-boost strategy of the candidate attenuated mycobacterial vaccine strain AFRO-1 (Example 1) in SPF male Hartley guinea pigs (250-300 grams). Therefore, 10 animals per group were vaccinated as shown in Figure 6 in order to evaluate prime-booster intervals of 10, 14 and 17 weeks.
[0174] Table 6. Guinea Pig Strategy Study Design
[0175] Group Trigger I
(Day 1) Initiate II
(week 3) Trigger III
(week 7) strengthen
(week 17) 1 salt water (id) - - 3 AFRO-1(id) - - AFRO-1(po) 4 - AFRO-1(id) - AFRO-1(po) 5 - - AFRO-1(id) AFRO-1(po) 6 - - - AFRO-1(po)
[0176] Initiate with 0.1ml of 10% glycerol containing 10 6 Dosing of cfu was performed by intradermal administration. Control mice received only 0.1 ml of 10% glycerol intradermally. Fourteen weeks after priming, guinea pigs were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com