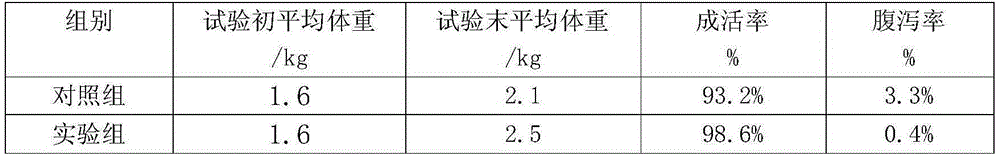
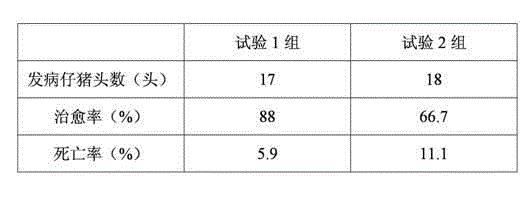
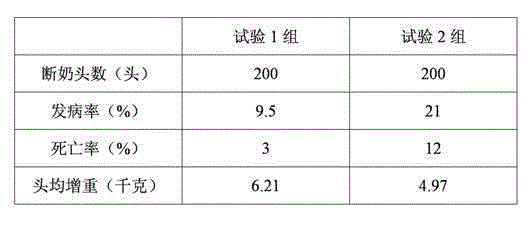
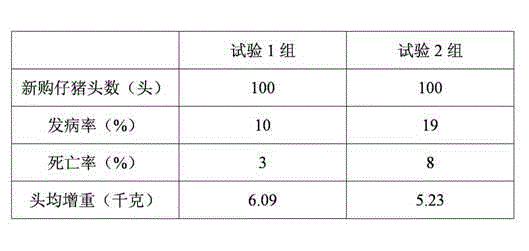
Patents
Literature
56 results about "Diarrheal diseases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diarrheal diseases are a collection of diseases caused by multiple viral, bacterial, and parasitic organisms that share the common symptom of diarrhea, defined as the passage of three or more loose or liquid stools per day.
Heartleaf houttuynia herb livestock and poultry feed additive and preparation method thereof
InactiveCN101926411AImprove conversion ratePreventive protection rateAnimal feeding stuffBiotechnologyFeed conversion ratio
The invention relates to a heartleaf houttuynia herb livestock and poultry feed additive, which comprises the following components in part by weight: 25 to 40 parts of heartleaf houttuynia herb, 20 to 40 parts of pine needle, 10 to 20 parts of indigowoad root, 10 to 20 parts of astragalus, 5 to 10 parts of honeysuckle flower and 10 to 20 parts of dandelion. The heartleaf houttuynia herb livestock and poultry feed additive has the advantages of: (1) special effect on common diarrheal disease of livestock and poultry, prevention protection rate of over 99 percent and mortality of zero; (2) average daily gain of 0.74 kilogram in the whole course feeding process of live pigs, which is improved by 7.2 percent compared with that of a blank control group of 0.69 kilogram, and improvement of feed conversion rate of over 9.7 percent; and (3) average daily gain per broiler of 43.7 grams, which is improved by 5.3 percent compared with that of the blank control group of 41.5 grams, and improvement of feed conversion rate of 5.9 percent.
Owner:LISHUI ACAD OF FORESTRY
Preparation method and application of traditional Chinese medicinal immunoglobulin compound preparation
InactiveCN102302778AWide variety of sourcesNo pollution in the processAntibacterial agentsPowder deliveryDiarrheal diseasesVeterinary Drugs
The invention discloses a production method of an immunoglobulin compound preparation for especially preventing and treating porcine diarrhea, and relates to the field of veterinary drugs and feed additives. The production method comprises the following steps: adding part of extracts or powder and the like to specific immunoglobulin or a substance which is not extracted and contains the specific immunoglobulin according to a certain proportion; and then evenly mixing to obtain the immunoglobulin compound preparation. By compositing the advantages of the immunoglobulin extract or powder, a novel antibiotic substitute is formed, thereby achieving the purposes of treating both symptoms and causes, accelerating the rehabilitation speed of a diarrheal animal, preventing and treating porcine diarrhea and meanwhile lowering cost; and the immunoglobulin compound preparation is a safe and hormone-free green product with special good effect on porcine diarrhea. The immunoglobulin compound preparation is taken as a feed additive or mixed with water and drunk. The immunoglobulin compound preparation is especially applicable to prevention and treatment of porcine diarrhea and also suitable for diarrheal diseases of animals such as various poultry, livestock and the like.
Owner:方希修
Bacterial strain capable of preventing and treating cattle and sheep diarrheal diseases
ActiveCN109161509ASimple production processImprove immunityBacteriaMicroorganism based processesMicroorganismDiarrheal diseases
The invention relates to a bacterial strain capable of preventing and treating cattle and sheep diarrheal diseases. The bacterial strain capable of preventing and treating the cattle and sheep diarrheal diseases is bacillus subtilis and preserved in China General Microbiological Culture Collection Center; and the preservation number is CGMCC NO: 15545. The bacillus subtilis can be applied to plant-eating animal feeding, can also be applied to preparation of plant-eating animal feed additives and can further be applied to preparation of plant-eating animal feed. The bacterial strain provided bythe invention has relatively strong adhesion capacity and antibacterial capacity, has very good acid-resistant capacity, gastrointestinal tract resistant capacity and bile salt tolerance, and can effectively prevent and treat the cattle and sheep diarrheal diseases.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
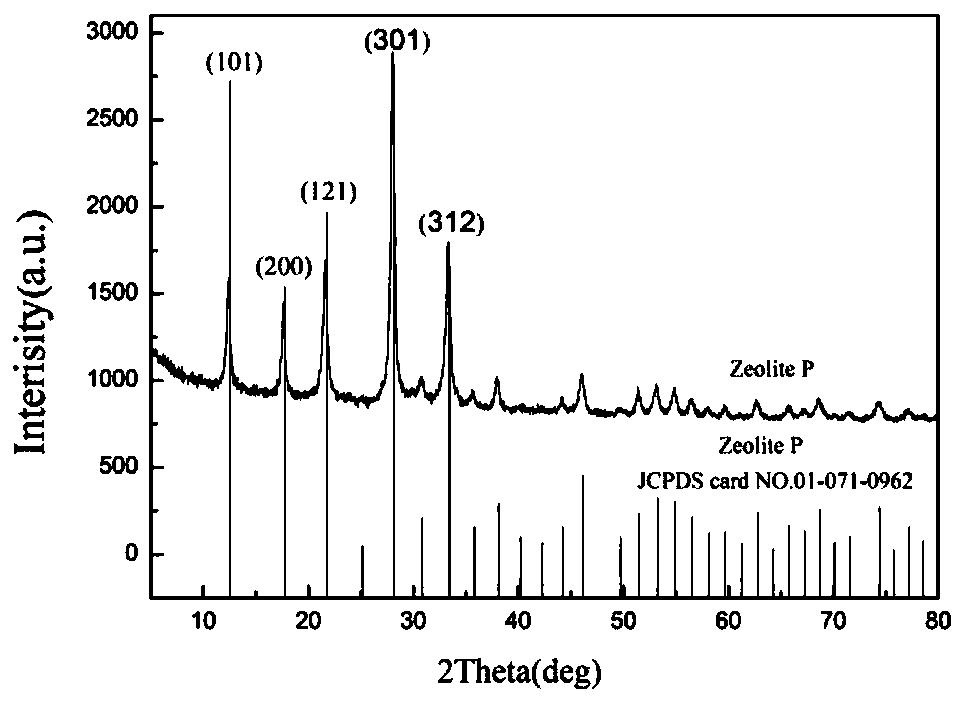
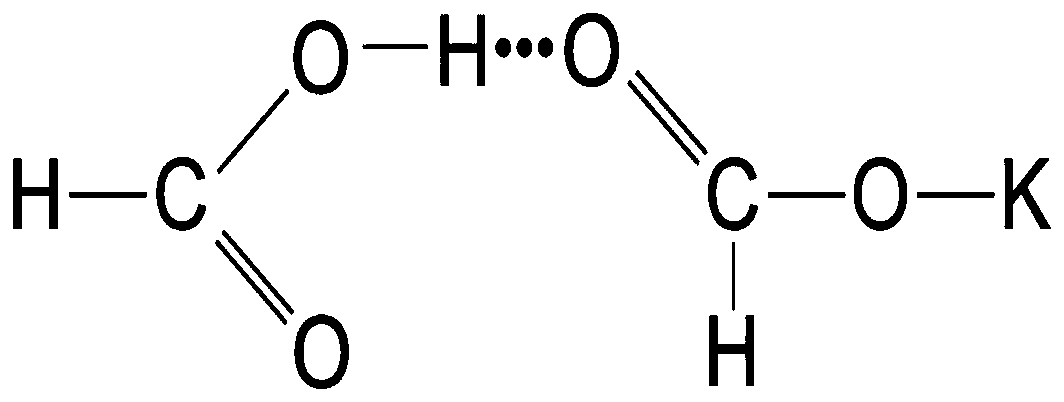
Preparation method of P-type molecular sieve-chitosan-sodium alga acid-potassium diformate slow-release antibacterial agent
ActiveCN109731103AGood prospects for development and utilizationAntibacterial agentsPharmaceutical non-active ingredientsEscherichia coliDiarrheal diseases
The invention discloses a P-type molecular sieve-chitosan-sodium alga acid-potassium diformate compound and a method for forming a livestock antibacterial agent with a slow-release effect. The defectsare overcome that heavy metal in a silver zeolite antibacterial agent easily deposits in an organism and cannot be applied to fodder, and potassium diformate as an acidifying agent cannot exert effects in the intestinal tract. With a P-type molecular sieve being a carrier, chitosan and sodium alga acid are sued a bridge. Potassium diformate as the acidifying agent is adsorbed, and the livestock slow-release antibacterial agent is prepared. Acidity detection and in-vitro antibacterial testing show that the slow-release antibacterial agent has slow-release performance, has an excellent antibacterial effect on escherichia coli, and can prevent and treat a diarrheal disease caused by escherichia coli.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Construction method of IPEC-J2 cell with APN gene knockout
InactiveCN108220338AKnockout worksAids in functional studiesHydrolasesStable introduction of DNAAgricultural scienceDiarrheal diseases
The invention discloses a construction method of an IPEC-J2 cell with APN gene knockout and belongs to the technical field of gene engineering. According to the construction method, a CRISPR / Cas9 technology is adopted to knock out an APN gene sequence in an IPEC-J2 cell line genome, and an established intestinal epithelial cell with APN gene knockout can provide more direct and effective study model to deeply reveal a diarrhea pathogen pathogenic mechanism and to cultivation and application of a diarrheal disease resistance transgenic pig.
Owner:YANGZHOU UNIV
Method of using IL-11 for inflammation associated with acute pancreatitis
Provided by the present invention are methods of treating a variety of disorders including AIDS, arthritis (rheumatoid arthritis, osteoarthritis, spondyloarthropathies), antibiotic induced diarrheal diseases (Closbidium difficile), multiple sclerosis, osteoporosis, gingivitis, peptic ulcer disease, esophagitis, diabetes, retinitis, uveitis, reperfusion injury after myocardial infarction (MI) or cerebral vascular accident (CVA), aphthous ulcers (oral), atherosclerosis (plaque rupture), prevention of tumor metastases, asthma, preeclampsia, acute pancreatitis, psoriasis, infertility and allergic disorders such as rhinitis, conjunctivitis, and urticaria.
Owner:GENETICS INST INC
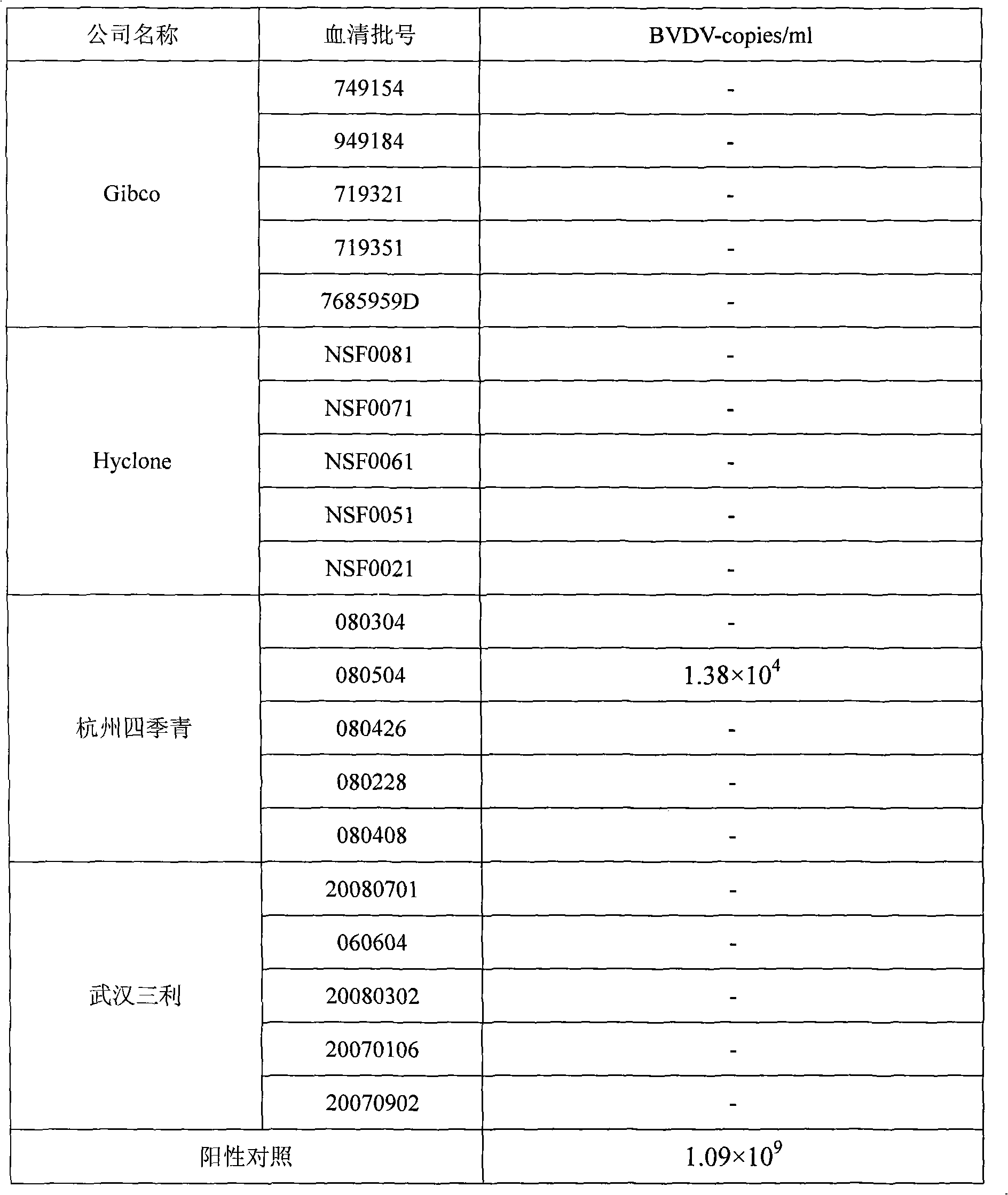
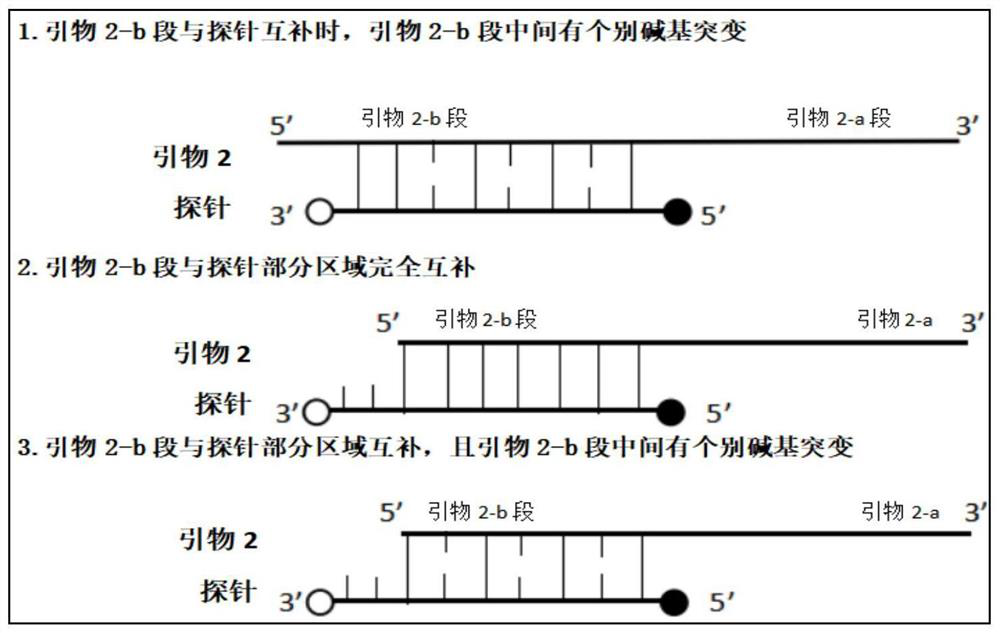
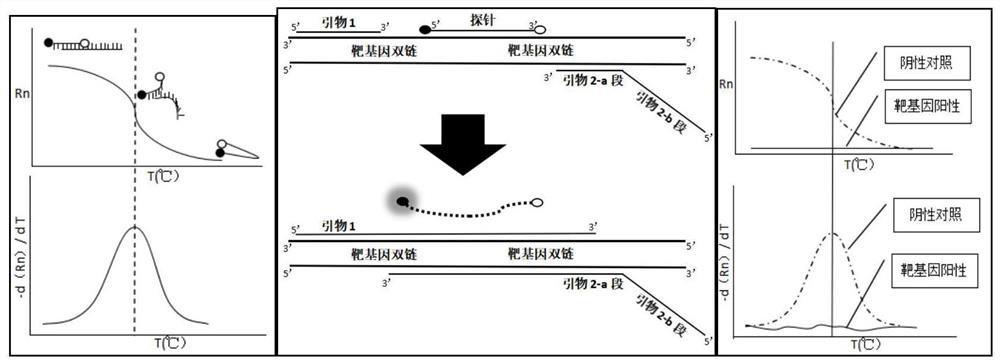
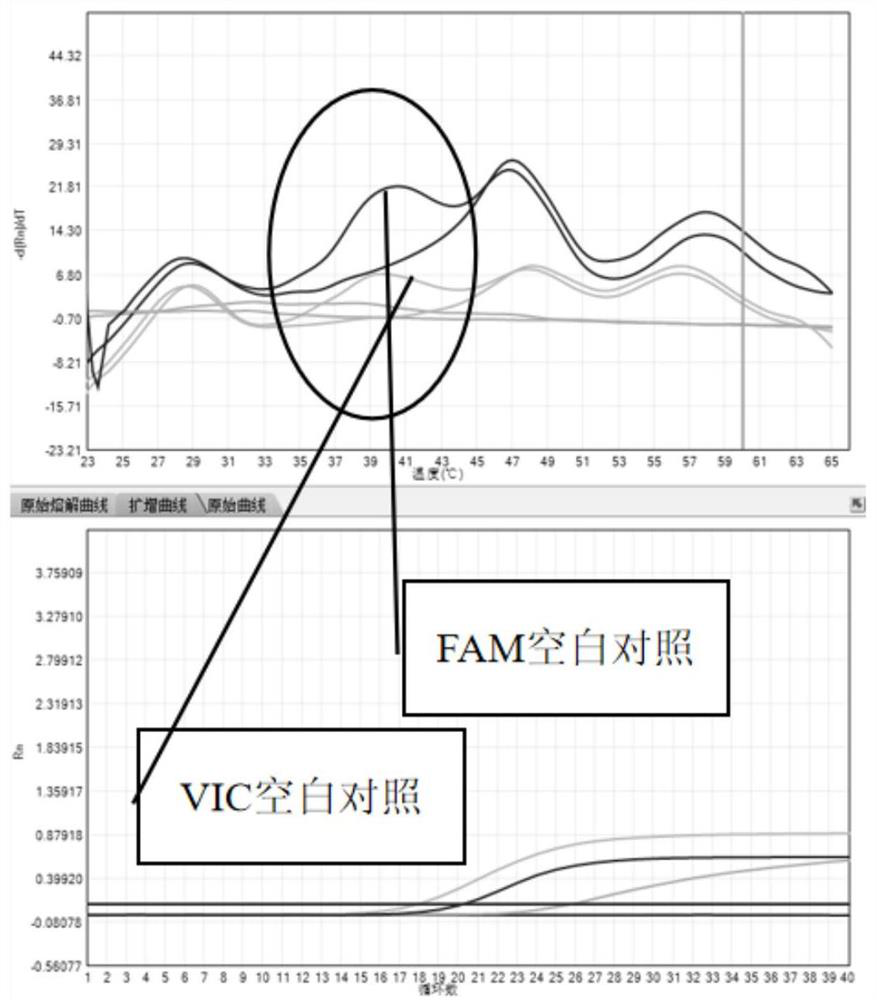
Fluorescence quantitative PCR kit for detecting calf diarrhea virus and application
ActiveCN101560572AAccurate determination of starting copy numberIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseFluorescence
The invention discloses a fluorescence quantitative PCR kit for detecting calf diarrhea virus and application. The kit comprises a) RNA extract, b) reverse transcriptase reaction liquid, c) reverse transcriptase, d) RNA enzyme inhibitor, e) primers and TaqMan probe, f) standard positive DNA template, and g) fluorescence quantitative PCR reaction liquid. The kit is characterized in that in a positive primer, a negative primer and a fluorescence probe sequence, a 5' end of the probe marks a fluorescence emitting group FAM, a 3' end of the probe marks a fluorescence quenching group TAMRA, the standard positive DNA template converts colon bacillus DH5a by a pGEM-T carrier inserted into a 185bp fragment of a calf diarrhea virus 5'-UTR area, plasmids are extracted after multiplication, and A260 is measured by an ultraviolet spectrophotometer to definite quantity and is diluted by 10 times of gradient. Preparation for the kit comprises the following steps: A, treatment of a specimen and a standard product; and B, RT-PCR amplification by a two-step method and real-time fluorescence detection, and application of the fluorescence quantitative PCR kit in medicaments for quantitatively detecting calf diarrhea virus. The quantitative result is more accurate, reliable and stable, and the repeatability is good.
Owner:WUHAN SANLI BIO TECH
Breeding method of new disease-resistance large white pig strain
InactiveCN106973854AEnhance immune responseReduce morbidityAnimal feeding stuffBiological testingInterleukin 6Lean meat
The invention discloses a breeding method of a new disease-resistance large white pig strain. The method includes the following steps that 1, a feed adding method is adopted to conduct an Escherichia coli infection experiment on weaned piglets of a core group in large white pigs, and an Escherichia coli resistant breeding basic group in the large white pigs is established; 2, concentration determination is conducted on cytokines of interleukin 1beta, interleukin 4, interleukin 6, interleukin 8, interleukin 10, transforming growth factor beta, tumor necrosis factor alpha and interferon gamma of an Escherichia coli resistant population in the basic group; reproductive performances are detected and selected, growth speeds, lean meat ratios and carcass quality characters, and a first generation is established; 3, a group subculture selective breeding method and a molecular-marker-assisted selection method are adopted. In this way, by the adoption of the breeding method of the new disease-resistance large white pig strain, after four to five generations, a vested breeding target is achieved gradually, a new-strain core group is established, and the morbidity of piglet diarrheal diseases is expected to be reduced.
Owner:TAICANG JINZHU AGRI DEV
Pharmaceutical composition for preventing and treating animal diarrheal diseases and preparation method of pharmaceutical composition
ActiveCN104523822AImprove immunityPromote growthAntibacterial agentsDigestive systemDiseaseORIGANUM OIL
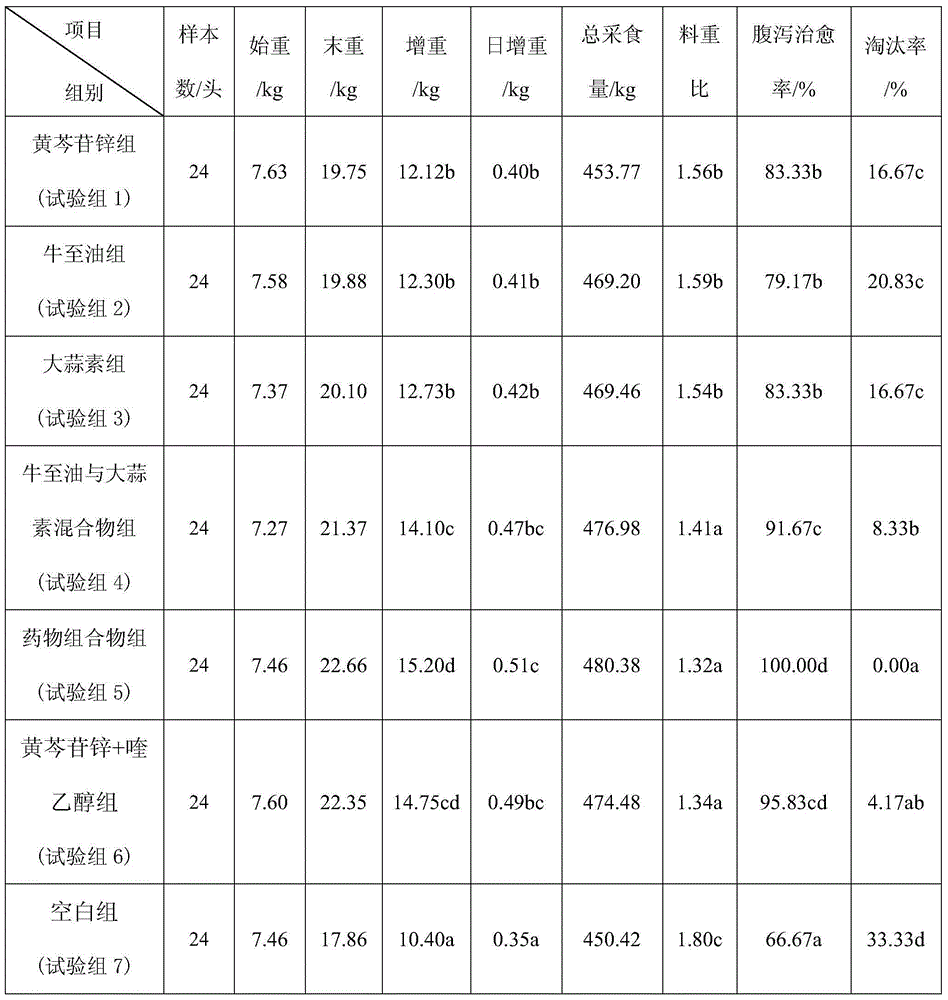
The invention provides a pharmaceutical composition for preventing and treating animal diarrheal diseases and a preparation method of the pharmaceutical composition. The pharmaceutical composition is prepared by mixing the following components in parts by weight: 2.5-30 parts of baicalin-Zn, 2.5-30 parts of origanum oil and 5-30 parts of allicin. The pharmaceutical composition has favorable preventing and treating effects on animal diarrheal diseases, has the effects of trapping and improving the intestinal florae of animals, and can be used for effectively improving the immunities of the animals, promoting the treatment of the diseases and promoting the growth of the animals if being added for a long term.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Chinese herbal medicine feed additive to improve immunity of rex rabbit and prevent diarrheal disease and feed containing the same
InactiveCN105233094AEnhance physical fitnessImprove the body's immunityDigestive systemInanimate material medical ingredientsDiseaseBetel
A Chinese herbal medicine feed additive to improve immunity of rex rabbit and prevent diarrheal diseases is composed of, by weight, 30-50g of Polygonum multiflorum, 15-30g of dandelion, 15-45g of Radix Codonopsis, 15-45g of Poria cocos, 5-15g of Eucommia, 5-15g of Phellodendron, 5-15g of Scutellaria baicalensis, 5-15g of Chinese angelica, 5-15g of Chinese herbaceous peony, 5-15g of radices saussureae, 15-45g of scorch-fried medicated leaven, 15-45g of scorch-fried malt, 15-45g of scorch-fried sanxian, 5-8g of aloe, 6-10g of isatis root, 30-90g of fossil fragments, 10-30g of lily, 30-80g of licorice, 10-20g of hawthorn and 10-20g of betel nut. According to the principle of compatibility of traditional Chinese medicine, and combined with the physiological characteristics of rex rabbit, the invention applies a medicament composed of Chinese herbal medicine as an additive to rex rabbit feed, so as to enhance constitution of rex rabbit, improve immunity, prevent and treat diarrhea, strengthen calcium and iron absorption, reduce the occurrence of malnutrition and disease.
Owner:陈红媛 +2
Traditional Chinese medicine composition for preventing piglet dysentery and preparation method thereof
InactiveCN102743657ACompatibility is reasonableGood treatment effectDigestive systemPlant ingredientsBiotechnologyEscherichia coli
The invention discloses a traditional Chinese medicine composition for preventing piglet dysentery and a preparation method thereof. The traditional Chinese medicine composition, by weight, comprises 5-20 parts of bulk pharmaceutical chemicals hawthorn, 5-20 parts of malt, 5-25 parts of pericarpium citri reticulatae, 5-25 parts of medicated leaven, 10-30 parts of radix bupleuri, 10-30 parts of root of Chinese pulsatilla and 10-30 parts of root of common peony. Firstly needed bulk pharmaceutical chemicals are processed to be clean, then drying and smashing are carried out, various bulk pharmaceutical chemical powder are weighted to be sufficiently and evenly mixed, finally split charging is carried out. The traditional Chinese medicine composition can effectively prevent and treat various irritability diarrheas, trophism diarrheas and yellow and white dysentery caused by escherichia coli, has obvious effects on viral diarrheas, can protect and repair intestinal mucosa and recovers digestion capability of piglets.
Owner:河南中亚神鹏医药科技有限公司
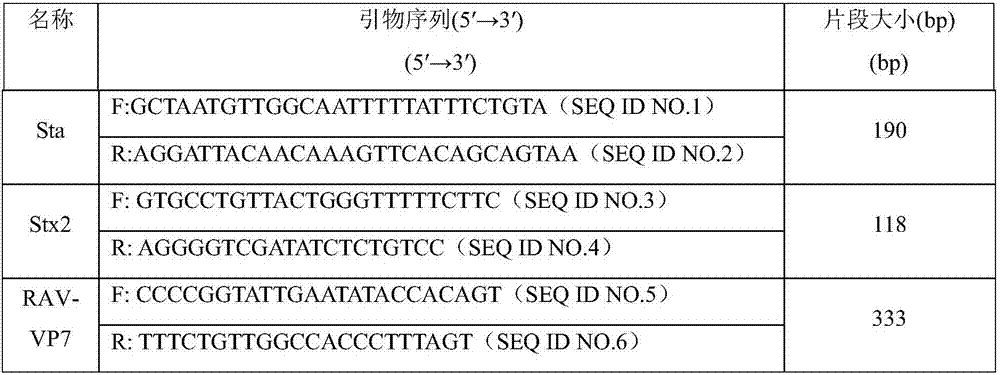
Multiplex RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer probe group for real-time fluorescent quantitative detection of four porcine diarrhea viruses, kit and detection method
PendingCN113462820AThe test result is accurateReal-time quantitative detectionMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexPig farms
The invention discloses a multiplex RT-PCR (reverse transcription-polymerase chain reaction) primer probe group for real-time fluorescent quantitative detection of four porcine epidemic diarrhea viruses. The multiplex RT-PCR primer probe group comprises an upstream primer, a downstream primer and a specific fluorescent probe of a porcine epidemic diarrhea virus M gene, an upstream primer, a downstream primer and a specific fluorescent probe of a swine transmissible gastroenteritis virus S gene; an upstream primer, a downstream primer and a specific fluorescent probe of a porcine rotavirus VP6 gene; and an upstream primer, a downstream primer and a specific fluorescent probe of a porcine D-type coronavirus M gene. The kit assembled on the basis of the primer probe group has the advantages of high sensitivity, high specificity, low pollution and real-time detection, and provides a reliable technology and product for early warning, early diagnosis and prevention and control monitoring of clinical diarrhea virus in a first-line pig farm.
Owner:HEBEI UNIV OF ENG
Neutralizing antigenic epitope fusion protein of three porcine diarrhea-causing viruses and construction method and application of neutralizing antigenic epitope fusion protein
ActiveCN111217916AImproving immunogenicitySsRNA viruses positive-senseAntibody mimetics/scaffoldsFusion Protein ExpressionIntraperitoneal route
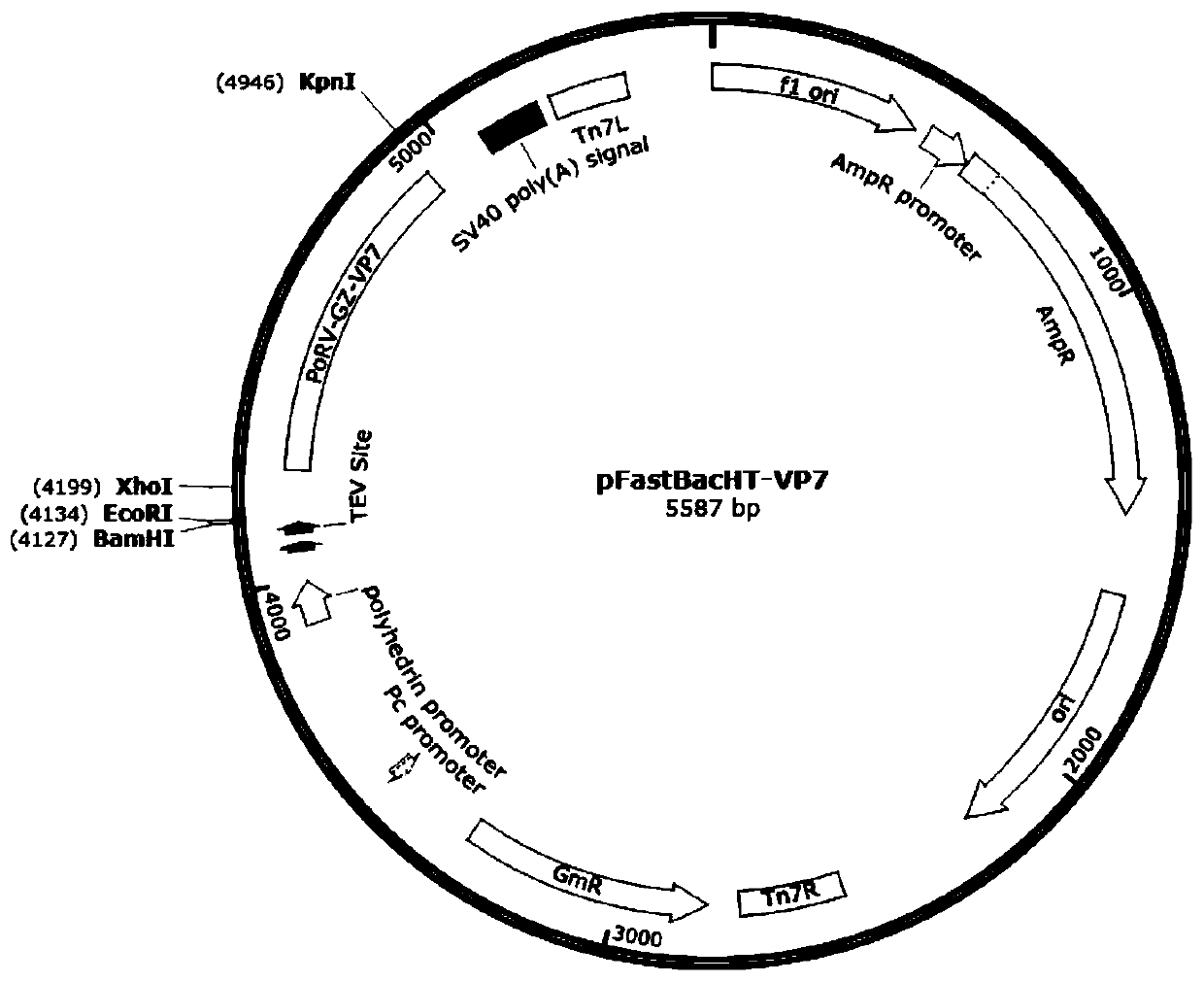
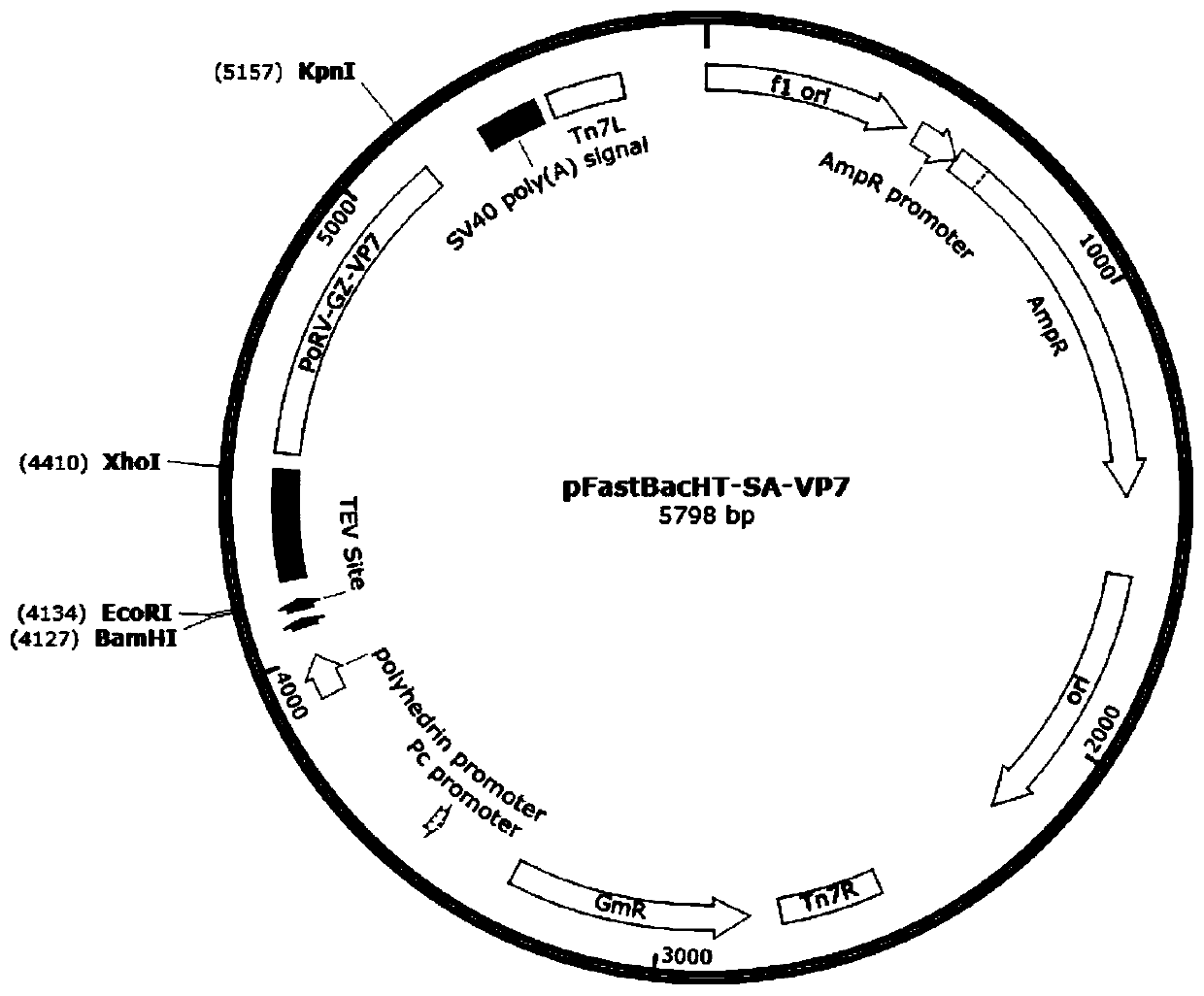
The invention relates to a neutralizing antigenic epitope fusion protein reconstructed body of three porcine diarrhea-causing viruses, a preparation method and application of neutralizing antigenic epitope fusion protein. An amino acid sequence of the neutralizing antigenic epitope fusion protein of the three porcine diarrhea-causing viruses PEDV, TGEV, and PoRV is shown as SEQ ID NO.2. A gene sequence encoding the neutralizing antigenic epitope fusion protein is shown as SEQ ID NO.1. Main neutralizing antigenic epitope genes of PEDV, TGEV, and PoRV are selected correspondingly and spliced, and then tandem genes are subjected to fusion protein expression by a recombinant baculovirus eukaryotic expression system. Recombinant viruses expressing the tandem gene fusion proteins are used for immunizing mice through an intraperitoneal injection method, a body can be induced to generate an immune response, and the immunogenicity is high. A new method is provided for the prevention of PEDV, TGEV, and PoRV infection, and a foundation is further laid for the development of a novel vaccine for porcine diarrhea viruses.
Owner:YANGZHOU UNIV
Breeding method of new Berkshire pig disease-resistant variety
InactiveCN107494422AImprove reproductive performanceSimple structureAnimal husbandryDiseaseLean meat
The invention relates to a breeding method of a new Berkshire pig disease-resistant variety. The breeding method comprises the steps that firstly, weaned piglets of a Berkshire pig core group are subjected to Escherichia coli infection experiments by adopting a feed adding method, according to a phenotype identification result, a Berkshire pig Escherichia coli disease-resistant breeding base group is established; secondly, the base group is subjected to molecule breeding with general resistance to diseases by using a proprietary technology obtained in the early stage, meanwhile characters such as breeding performance, growing speed, lean meat percentage and carcass quality are detected and selected, and a first generation is built; thirdly, a group continuous progeny breeding and molecular marker assisted selection method is adopted, after four to five generations, an established breeding aim is achieved step by step, and a new variety core group is established. The new Berkshire pig disease-resistant variety has the advantages of resistance to weaned piglet diarrheal diseases, high stress resistance, high environmental adaptability and the like, and can further provide guidance for disease-resistant breeding and new variety selection and breeding of other pig varieties.
Owner:YANGZHOU UNIV
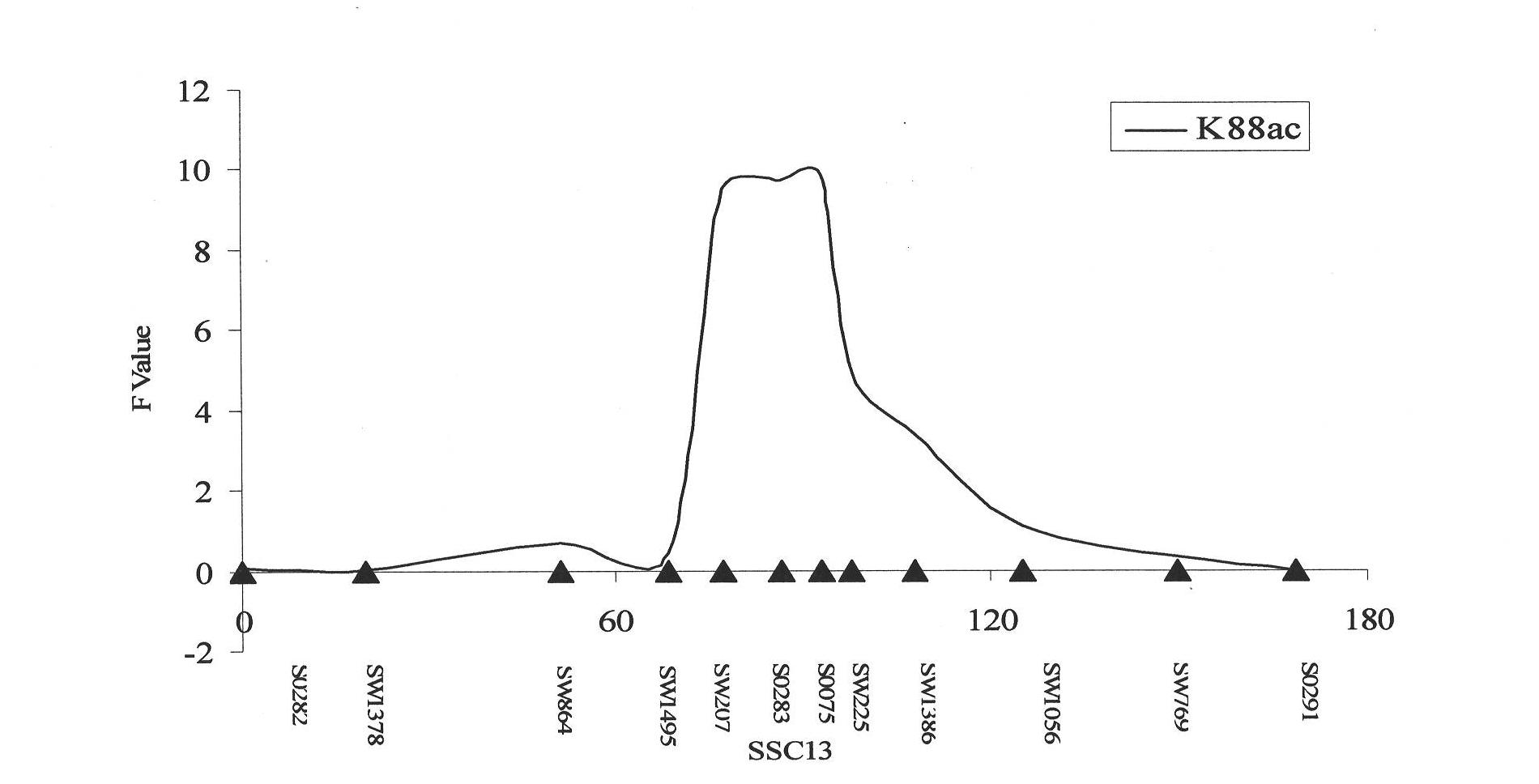
Key marker sites and applications of muc13 gene affecting susceptibility/resistance to f4ac diarrhea in piglets
ActiveCN102286480AReduce mortalityImprove resistance to diarrheal diseaseMicrobiological testing/measurementDNA/RNA fragmentationDiarrheal diseasesBOAR
The invention discloses a susceptible / resistant MUC13 (mucoprotein) gene key sign locus affecting piglet F4ac diarrhea and an application thereof in pig genetic improvement. A large scale of white Duroc*Erhualian Boars resource groups and 15 Chinese and foreign pig species distant groups are subjected to complete-genome scanning linkage position, target zone high-density SNP (Single Nucleotide Polymorphism) multi-point linkage precise positioning, breakpoint recombination analysis and distant group associativity analysis based on linkage unbalance to finally lock a coding ETEC (Enterotoxigenic escherichia coil) F4ac receptor target gene-MUC13 gene and separate and colon the MUC13 gene. 15 key mutantsites are indentified in the MUC13 gene. The 15 mutantsites and ETEC F4ac in vitro adhesive phenotype are basically mutually separated in 144 pure breed Duroc, Landrace and big white piglet from core groups of five provinces. The accuracy for the strongest relevant sign to judge susceptible / resistant individual is 97% (P=1.59*10-21). The invention provides new method for breeding for disease resistance for boar ETEC F4ac diarrheal disease.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Anti-campylobacter jejuni antibodies and uses therefor
Campylobacter jejuni is a leading cause of bacterial food-borne diseases in humans, ranging from acute diarrheal disease to neurological disorders. An isolated or purified antibody or fragment thereof specific to C. jejuni is described. The antibody or fragment thereof binds to a flagellar protein and reduces motility of C. jejuni. The antibody or fragment thereof is derived from a heavy chain IgG variable domain fragment (VHH) of a camelid animal immunized with C. jejuni flagellar protein. A multivalent form, as well as a phage format, of the antibody or fragment thereof is described. Methods of reducing presence of C. jejuni in an animal or an animal environment, methods and formulations for treating C. jejuni infection, and method of detecting C. jejuni are also described.
Owner:NAT RES COUNCIL OF CANADA
Method for preventing and controlling porcine epidemic diarrhea
The present invention discloses a method for preventing and controlling porcine epidemic diarrhea. A return feeding method is adopted, a natural infection method is simulated, feces or intestinal tract and intestinal contents of porcine epidemic diarrhea virus (PEDV) infected animals are crushed, ground and diluted, then antibiotics are added, non-infected animals are infected through mouths, the total group can be infected in the same period, mucosal immunoreaction is stimulated to be generated, IgA antibodies are generated, so that the anti-PEDV effect is achieved, and finally the pestilence can be controlled. The return feeding method is tried out through a small group, the specificity sIgA antibody level is detected by using an indirect ELASA method, the return feeding dosage is determined according to antibody level change conditions, then large group return feeding is carried out, and return feeding failure caused by the fact that the return feeding dosage is not exact in the past is largely reduced. According to the method, the porcine epidemic diarrhea can be effectively prevented and controlled, the spread of diseases can be rapidly and effectively controlled especially when the epidemic diarrhea diseases outbreak, and greater economic losses and disease outbreak again can be prevented; and the return feeding process is simple and easy to operate, and cost is low.
Owner:SHANGHAI XINNONG FEED
Drug for treating enteric disease of beasts and birds
InactiveCN103169825APromote absorptionQuick resultsDigestive systemPlant ingredientsDiseaseDiarrheal diseases
The invention provides a traditional Chinese medicine preparation for treating enteric disease of beasts and birds and a preparation method of the traditional Chinese medicine preparation. The traditional Chinese medicine preparation comprises the following components by weight percent: 20-80% of spider perfume and 20-80% of grassleaf sweelflag rhizome. The preparation method comprises the following extraction technology: weighing medicinal materials according to a prescription, smashing into coarse powder, thoroughly soaking the medicinal materials by water, extracting volatile oil completely by means of steam distillation, filtering out liquid medicine, boiling dregs of a decoction by water for once, merging two filter liquor, concentrating under a reduced pressure to be proper in quantity, carrying out alcohol precipitation, leaching liquid supernatant, and recovering ethanol, i.e. the purified liquid medicine, and adding the volatile oil into the drug by a proper method when a finished product is prepared. The drug can be made into oral liquid, granules, tablets and capsules. The oral preparation of the drug is quick to absorb, and good to take effect, the granule of the drug is easy to store and take, and the tablet and the capsule of the drug are exact in dose, convenient to use, and unique in curative effect to the diarrheal diseases of the beasts and birds. Compared with an existing common drug, the traditional Chinese medicine preparation has the advantages of being high in safety, good in curative effect, free from vestigital and the like, thereby being a product for well treating the enteric disease of beasts and birds.
Owner:SHENYANG VICA ANIMAL HUSBANDRY TECH +1
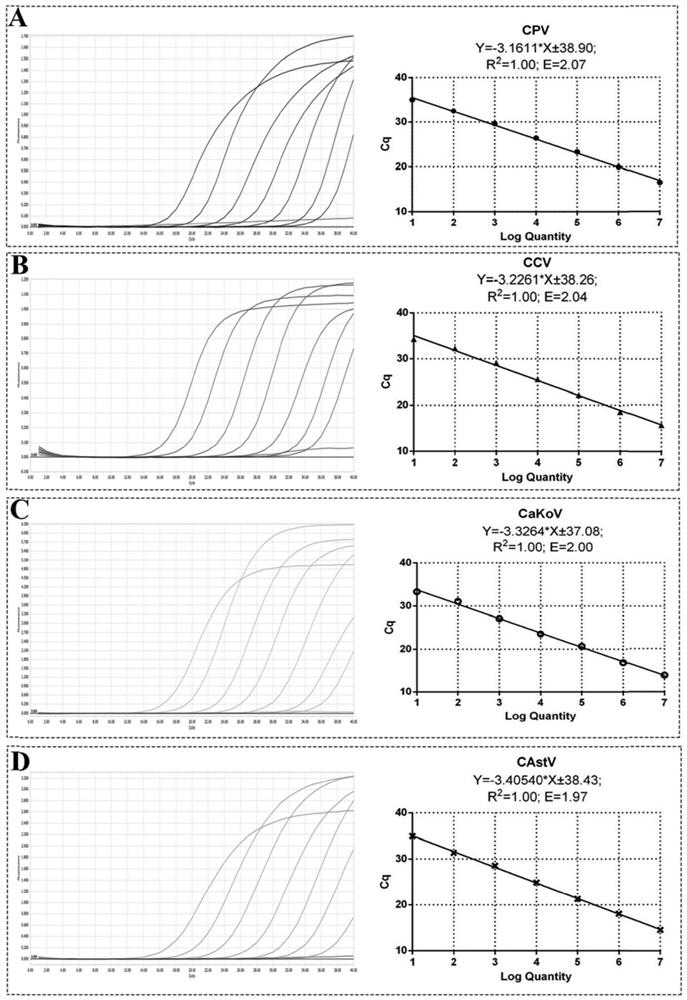
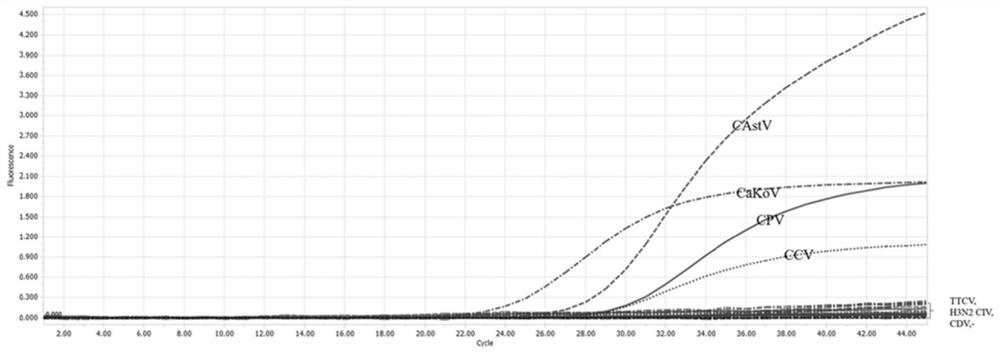
Primer pair for detecting canine diarrhea viruses through one-step method, TaqMan probe, method and application
ActiveCN111763766ANo mutual interferenceSimilar amplification efficiencyMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexDiarrheal diseases
The invention discloses a primer pair for detecting canine diarrhea viruses through a one-step method, a TaqMan probe, a method and application. Exploratory research on multiplex fluorescent PCR is carried out in multiple aspects of the specificity of a primer probe, a key factor in a reaction system, quality control construction and the like; a detection fragment is amplified by nucleic acid or reverse transcription products of a sample to be detected, and connected to a pMD18-T vector; plasmids are successfully constructed and used as a template; a multiplex real-time fluorescent quantitative PCR method of canine parvovirus, canine coronavirus, canine astrovirus and canine kobuviruses is established; the patent allows that at most four viruses causing canine diarrhea can be simultaneously detected in one reaction tube; the detection sensitivity is up to 1*10<2> copies; the detection sensitivity and the specificity of four canine diarrhea viruses can be obviously improved; furthermore, the detection period is effectively shortened; and thus, the patent has important meanings.
Owner:NANJING AGRICULTURAL UNIVERSITY
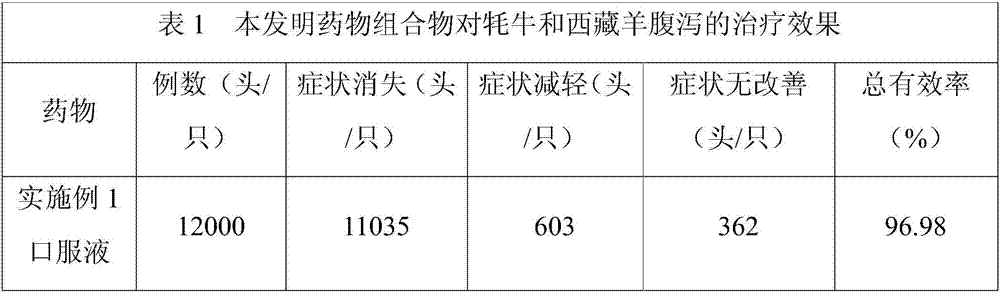
Chinese and Tibetan medicine composition for preventing and controlling livestock diarrhea
ActiveCN107233454AWill not affect milking salesSignificant effect in diarrheal diseaseAntibacterial agentsDigestive systemBetelDiarrheal diseases
The invention discloses a pharmaceutical composition for preventing and controlling livestock diarrhea. The pharmaceutical composition is an agent prepared from the following raw medicinal materials in parts by weight: 1-4 parts of Chinese herbaceous peony, 1-5 parts of scutellaria baicalensis, 1-5 parts of coptis chinensis, 1-5 parts of angelica sinensis, 1-5 parts of rheum officinale, 1-5 parts of elecampane, 1-5 parts of betel nut, 1-5 parts of lonicerae flos, 1-5 parts of purslane, 1-5 parts of rhododendron micranthum leaf, 1-5 parts of polygonum viviparum, 1-4 parts of bergenia purpurascens and 1-4 parts of liquorice. Clinical veterinary use results prove that the medicine disclosed by the invention has an obvious effect used for treating diarrheal diseases of livestock such as yak, Tibetan sheep and the like. Moreover, compared with the current Western medicine used for treating livestock diarrhea, the medicine disclosed by the invention has the advantages that milking and milk vending of animal owners are not influenced during the milking period of cattle and sheep, the advantages are obvious, and a novel medication choice is provided for clinical treatment of livestock diarrhea diseases.
Owner:阿坝藏族羌族自治州畜牧科学技术研究所
Triple fluorescent quantitative PCR kit for simultaneously detecting bovine rotavirus, bovine coronavirus and bovine viral diarrhea virus and application method of triple fluorescent quantitative PCR kit
InactiveCN112760421AStrong specificityImprove reaction efficiencyMicrobiological testing/measurementAgainst vector-borne diseasesBovine rotavirusBovine Viral Diarrhea Viruses
The invention provides a triple fluorescent quantitative PCR kit for simultaneously detecting a bovine rotavirus, a bovine coronavirus and a bovine viral diarrhea virus and an application, and belongs to the technical field of virus detection. The triple fluorescent quantitative PCR kit comprises three groups of specific primers and three corresponding probes aiming at the bovine rotavirus, the bovine coronavirus and the bovine viral diarrhea virus. The primer and the probe for detecting the three viruses have the advantages of strong specificity and high reaction efficiency. The triple real-time fluorescent quantitative PCR kit has the characteristics of high amplification efficiency, strong specificity, stable detection effects and good repeatability, and is suitable for dairy cow diarrhea pathogen detection, regular monitoring, dairy cow diarrhea epidemiological investigation and the like. The kit disclosed by the invention can be used for simultaneously detecting three dairy cow diarrhea pathogens in a real-time fluorescent quantitative PCR reaction system, so that the detection time is saved, the detection cost is reduced, a plurality of samples can be simultaneously detected, and the detection efficiency is high.
Owner:北京三元集团畜牧兽医总站
Triple PCR detection method for simultaneously detecting three feline diarrhea viruses and application of triple PCR detection method
PendingCN113528708AImprove detection efficiencyStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesFeline astrovirusDiarrheal diseases
The invention discloses a triple PCR detection method for simultaneously detecting three feline diarrhea viruses and application of the triple PCR detection method. Specific primers for detecting cat astrovirus, cat parvovirus and cat intestinal coronavirus are provided, and the triple PCR detection method for the cat astrovirus, the cat parvovirus and the cat intestinal coronavirus is established. The specific primers and the detection method are high in specificity, high in sensitivity and good in repeatability and stability, and a rapid, sensitive and specific clinical detection method is provided for differential diagnosis of cat diarrheal diseases.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Mongolian veterinary drug for resisting escherichia coli diarrhea and preparation method thereof
ActiveCN113559181AHigh activityIncrease growth rateAntibacterial agentsDigestive systemEscherichia coliInfant animal
The invention discloses a Mongolian veterinary drug for resisting Escherichia coli diarrhea and a preparation method thereof, the Mongolian veterinary drug comprises kusnezoff monkshood leaf, myrobalan, oxytropis myriophylla, rubia cordifolia, black myrobalan, rhizoma nardostachyos and rheum officinale according to a mass ratio of 2: 1: 1: 1: 1: 2: 2. According to the invention, the anti-inflammatory, immune and intestinal barrier effects of diarrhea model animals and target animals are adjusted through improving the structure of animal intestinal flora., the antibacterial, anti-inflammatory and intestinal barrier functions of the animals are improved, the morbidity of escherichia coli diarrhea is reduced, the survival rate of young animals is further increased, and economic benefits are improved.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Materials and methods for prevention and treatment of diarrhea and inflammation in the gastrointestinal tract
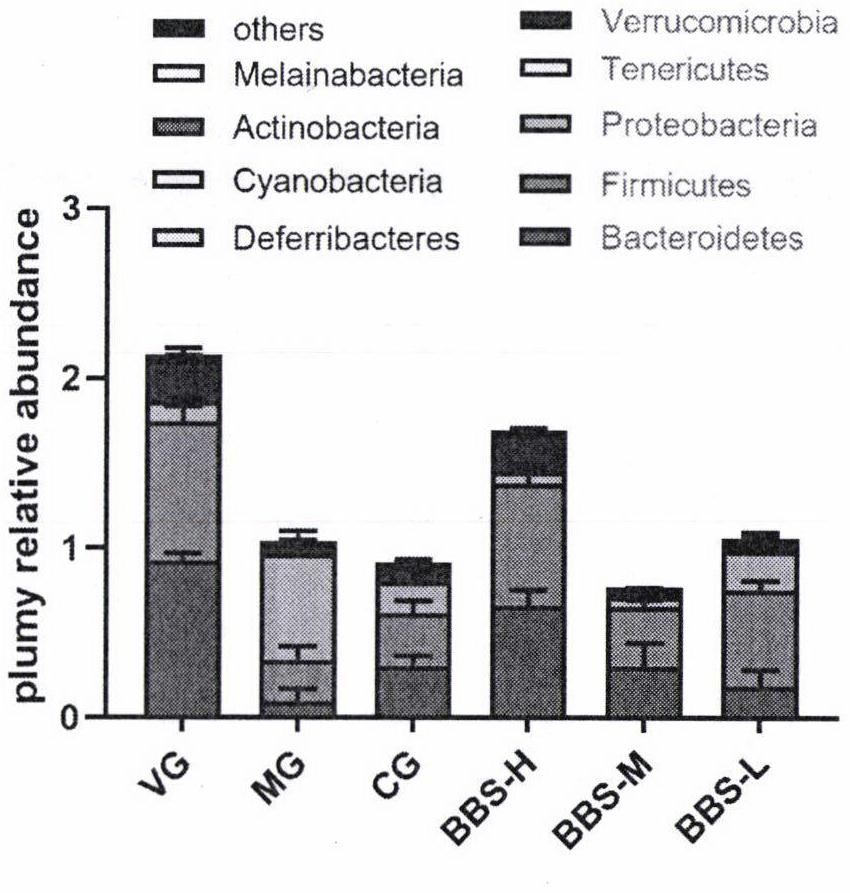
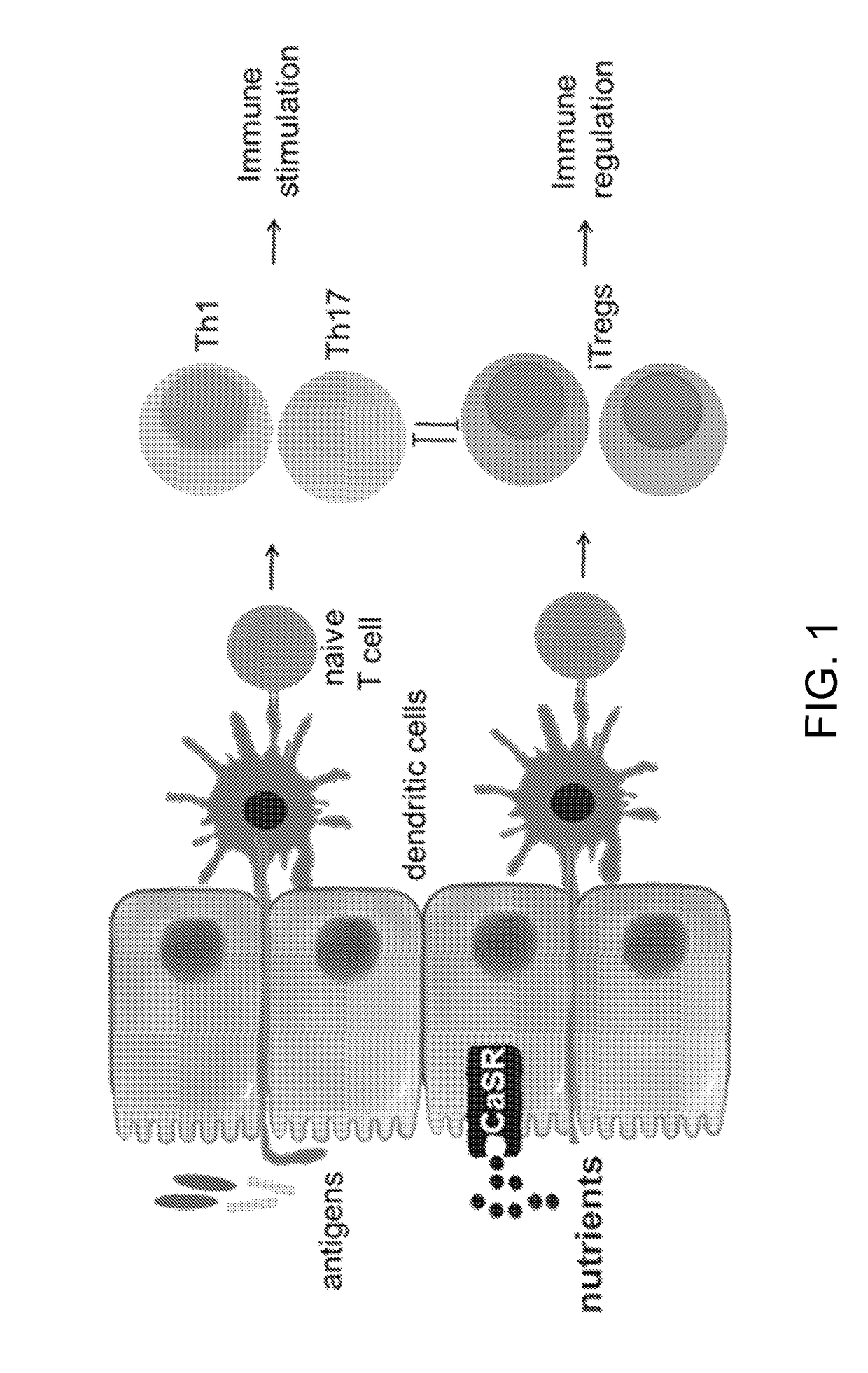
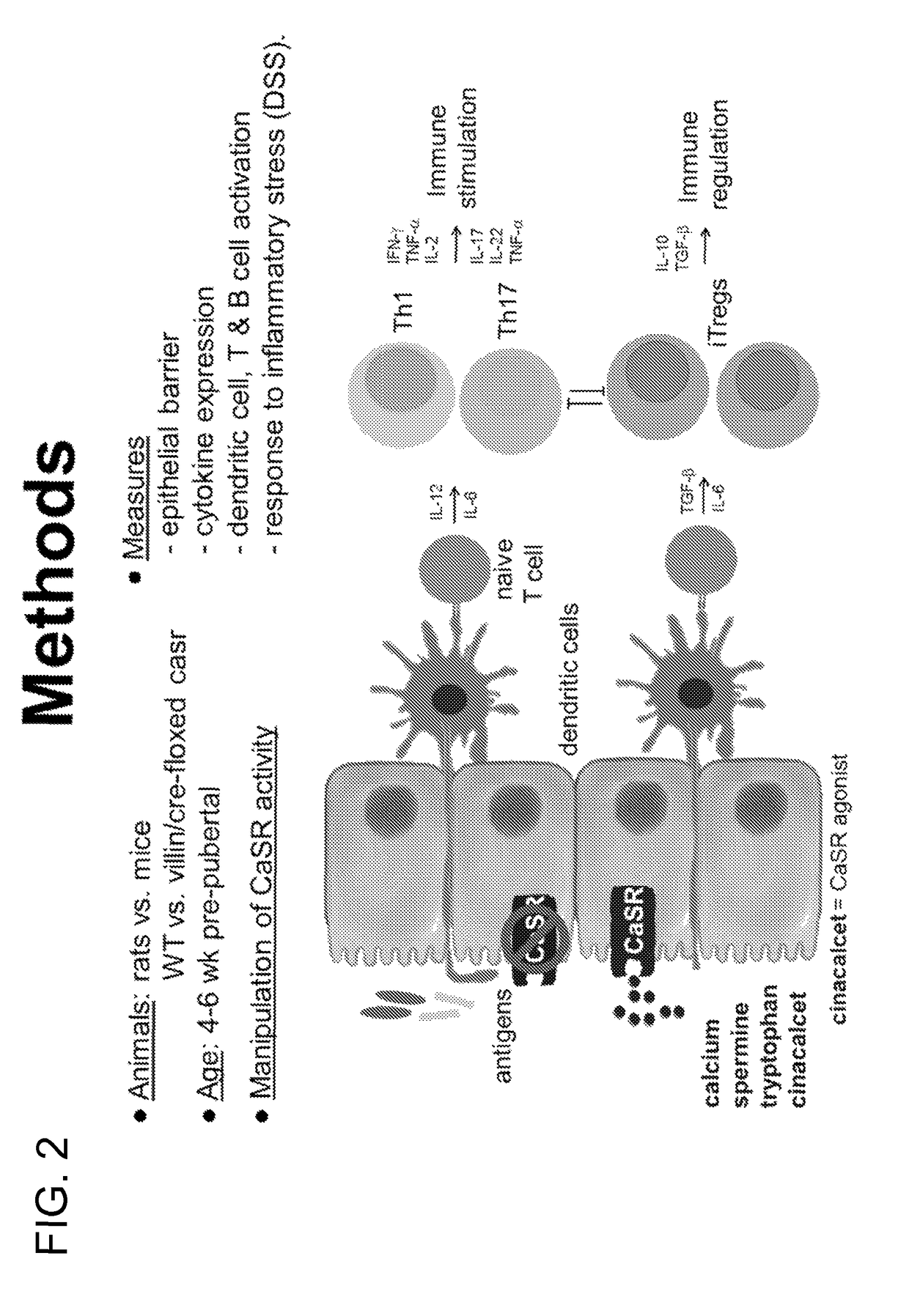
The present invention pertains to the use of the calcium-sensing receptor (CaSR)-activating nutrients (designated as “CaSR-based nutrients”) for the prevention and / or treatment of diarrheal diseases and inflammation in the gastrointestinal tract. In one embodiment, the current invention is formulated for oral administration. The anti-diarrheal composition of the present invention is useful for treating diarrheal and gastrointestinal inflammatory conditions in infants and young children.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for simultaneously detecting multiple diarrhea viruses by utilizing melting curve
PendingCN112575118AImprove throughputApplicable screeningMicrobiological testing/measurementDNA/RNA fragmentationDiarrheal diseasesMelting curve analysis
The invention discloses a method for simultaneously detecting multiple diarrhea viruses by utilizing a melting curve. The method comprises the following steps: designing eight groups of specific primer probes according to gene conservative fragments of viruses; designing a pair of reference gene primer probes EF, ER and EP according to the human ribonucleotidase P gene; combining all the primer probes and materials required by PCR into a reaction solution; adding sample nucleic acid into the reaction solution, and amplifying on a fluorescent PCR instrument by using a PCR amplification technology in combination with melting curve analysis; and judging whether the sample is positive or not according to the fluorescence amplification signals of different channels, and typing the positive nucleic acid in the corresponding fluorescence channels by adopting a melting curve analysis result.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
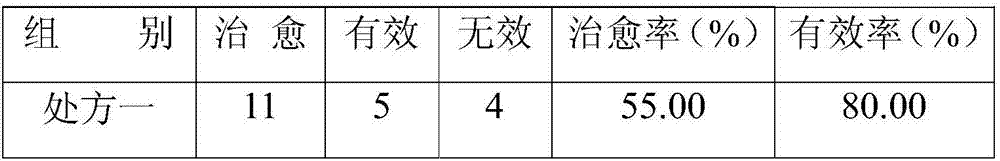
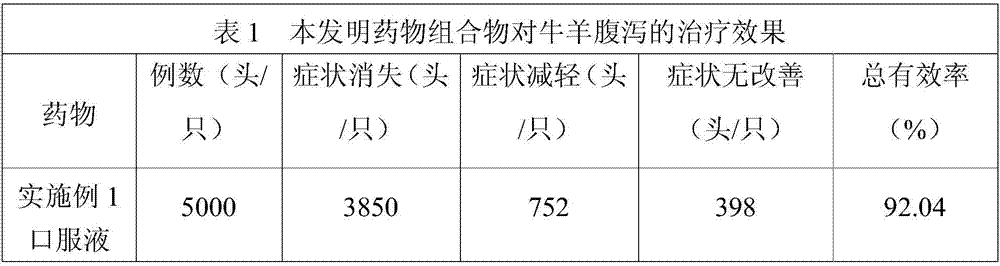
Medicinal composition for preventing livestock diarrhea
ActiveCN107441267ASignificant effect in diarrheal diseaseRelieve symptomsDigestive systemPlant ingredientsTreatment effectDiarrheal diseases
The invention discloses a medicinal composition for preventing livestock diarrhea. The medicinal composition is a preparation which is prepared from the raw medicinal materials in parts by weight: 4 to 8 parts of lacquer tree fruit, 1 to 5 parts of pomegranate bark, 2 to 6 parts of cortex cinnamomi, 1 to 3 parts of pterocephalus hookeri heck, 3 to 7 parts of holarrhena pubescens, 2 to 7 parts of navicular aconite, 3 to 7 parts of plantain herb, 3 to 7 parts of triglochin martimum, 2 to 6 parts of myricaria prostrate, 1 to 6 parts of hypecoum leptocarpum, and 2 to 4 parts of medicated leaven. The medicament disclosed by the invention has a remarkable effect of treating diarrheal diseases of the livestock, such as cattle and sheep; symptoms are obviously lightened after the medicament is taken for three days continuously, and the diarrhea can be cured after five to six days; and the total effective rate reaches 92 percent and above. Moreover, the medicament disclosed by the invention has an obvious treatment effect on the livestock diarrhea caused by the factors, such as bacteria, viruses and dyspepsia, is wide in application range, and has a wide application prospect.
Owner:阿坝藏族羌族自治州畜牧科学技术研究所
Application of iodine citrate in the preparation of medicines for the treatment of diseases of the digestive system of livestock and poultry
The invention discloses the application of iodine citrate in the preparation of medicine for treating diseases of the digestive system of livestock and poultry. Clinical experiments have proved that iodine citrate has a good therapeutic effect on poultry candidiasis and livestock and poultry epidemic diarrhea.
Owner:FOSHAN STANDARD BIO TECH
Antibody detection kit of porcine epidemic diarrhea virus IgG
InactiveCN105116144AConvenient for on-site testingEasy to operateMaterial analysisDiarrheal diseasesSpecific igg
The invention belongs to the field of biotechnology, and concretely relates to an antibody detection kit of porcine epidemic diarrhea virus IgG. The kit prepared by the invention has a simple operation flow for clinic sample detection, the detection is completed during 3.5 hours, only a common inverted microscope is needed for determination of result, special apparatuses are not needed, specific dyeing is visible to naked eye, non-specific dyeing is easy to be distinguished, so the kit prepared by the invention is convenient for laboratory and clinic on-site detection. A secondary antibody used in the kit prepared by the invention is an enzyme-labeling SPA which can be combined with the mammal IgG with good specificity; the kit provided by the invention can be used for detecting pigs which are infected by the porcine epidemic diarrhea virus or specific IgG antibody in the vaccine inoculated pig, simultaneously is used for evaluating level of the porcine epidemic diarrhea virus antibody IgG during the porcine epidemic diarrhea virus immunization research using mice or rabbit as experiment animal.
Owner:NANYANG NORMAL UNIV
Norovirus and sapovirus antigens
Immunogenic compositions that elicit immune responses against Norovirus and Sapovirus antigens are described. In particular, the invention relates to polynucleotides encoding one or more capsid proteins or other immunogenic viral polypeptides from one or more strains of Norovirus and / or Sapovirus, coexpression of such immunogenic viral polypeptides with adjuvants, and methods of using the polynucleotides in applications including immunization and production of immunogenic viral polypeptides and viral-like particles (VLPs). Methods for producing Norovirus- or Sapovirus-derived multiple epitope fusion antigens or polyproteins and immunogenic compositions comprising one or more immunogenic polypeptides, polynucleotides, VLPs, and / or adjuvants are also described. The immunogenic compositions of the invention may also contain antigens other than Norovirus or Sapovirus antigens, including antigens that can be used in immunization against pathogens that cause diarrheal diseases, such as antigens derived from rotavirus.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Norovirus and sapovirus antigens
Immunogenic compositions that elicit immune responses against Norovirus and Sapovirus antigens are described. In particular, the invention relates to polynucleotides encoding one or more capsid proteins or other immunogenic viral polypeptides from one or more strains of Norovirus and / or Sapovirus, coexpression of such immunogenic viral polypeptides with adjuvants, and methods of using the polynucleotides in applications including immunization and production of immunogenic viral polypeptides and viral-like particles (VLPs). Methods for producing Norovirus- or Sapovirus-derived multiple epitope fusion antigens or polyproteins and immunogenic compositions comprising one or more immunogenic polypeptides, polynucleotides, VLPs, and / or adjuvants are also described. The immunogenic compositions of the invention may also contain antigens other than Norovirus or Sapovirus antigens, including antigens that can be used in immunization against pathogens that cause diarrheal diseases, such as antigens derived from rotavirus.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com