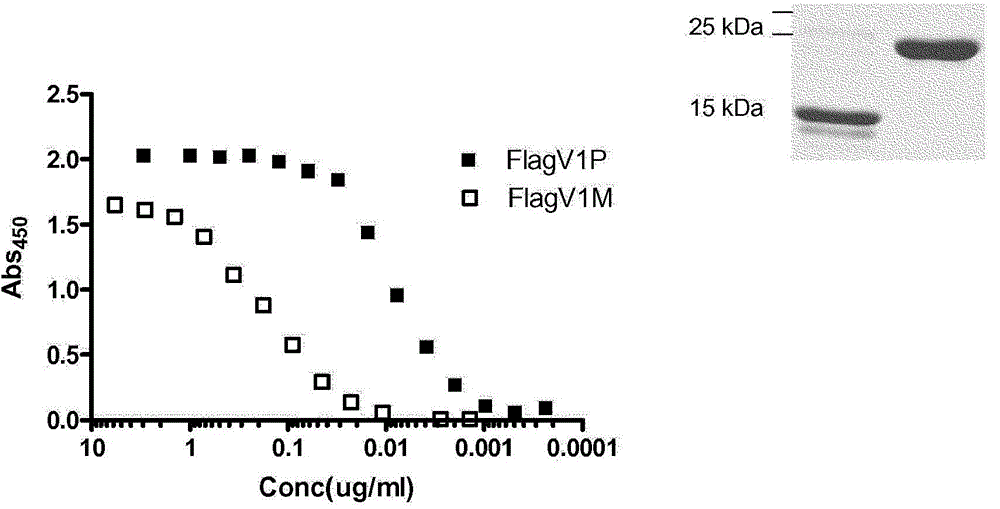
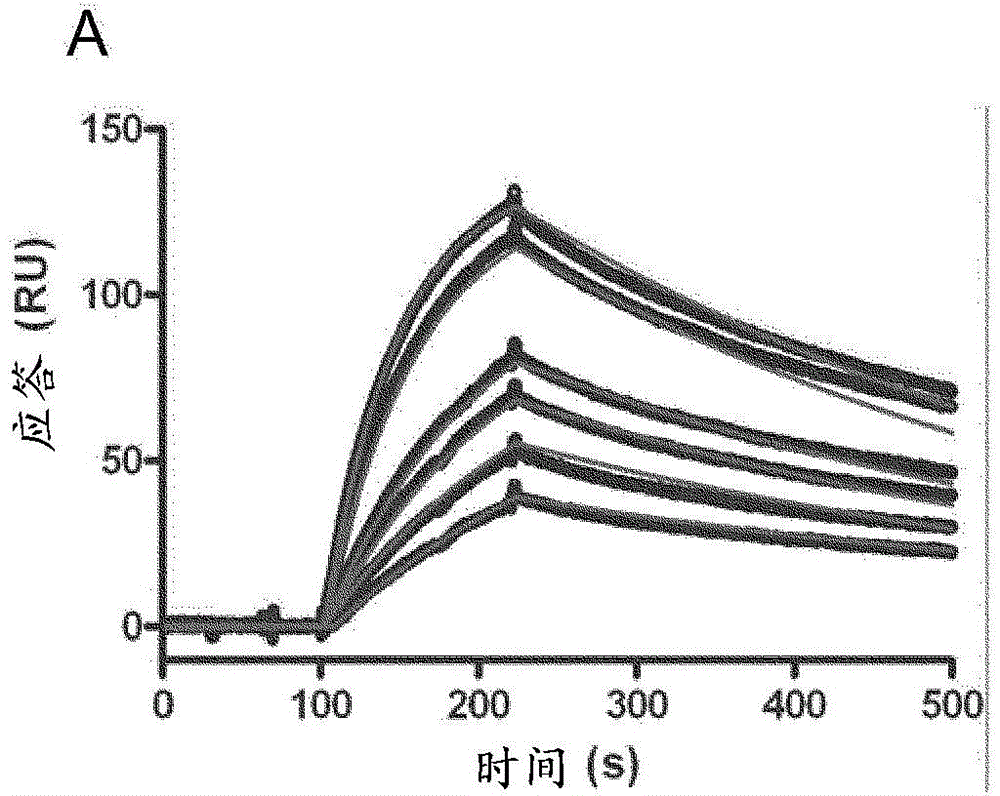
Anti-campylobacter jejuni antibodies and uses therefor
A technology of Campylobacter jejuni and antibodies, which can be applied in the direction of antibodies, antibacterial drugs, antibacterial immunoglobulins, etc., can solve the problems of unrealistic cost and feasibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] [Example 1: Preparation of Antigen]
[0155] Flagella were prepared for use as antigens in subsequent examples.
[0156] C. jejuni (strain 81-176) flagella were isolated as previously described (Power et al., 2003). Briefly, to prepare flagella, Campylobacter jejuni (C. jejuni) was cultured overnight, and cells were scraped into Muller-Hinton broth and incubated overnight. Cells were then harvested by centrifugation and resuspended in 100 mL of Tris-buffered saline. Flagella were sheared from the cells using a Waring mixer on ice. Cell debris was pelleted by centrifugation, and the supernatant was transferred to an ultracentrifuge tube. Flagella were pelleted by centrifugation at 45,000 rpm for 1 hour. Further purification was performed by resuspension in 2% SDS and centrifugation of samples. Resuspend the pellet in 200-500 μL of dH 2 O.
Embodiment 2
[0157] [Example 2: Alpaca immunity and serum response]
[0158] To isolate the V targeting the flagella of Campylobacter jejuni (C. jejuni) H H, alpacas were immunized with the flagellar antigen obtained in Example 1.
[0159] Male alpacas (Lama glama) were immunized subcutaneously with C. jejuni flagella (Example 1). A total of 7 injections were performed and, for each injection, 100 μg of antigen in a total volume of 0.5 ml was mixed with an equivalent volume of complete (day 1) or incomplete (day 21,35,49,63) Freund's Agent (Sigma) mixed. The last 2 injections (days 76 and 90) were performed with 100 μg of antigen without adjuvant. Pre-immune blood (15-20 ml) was collected before the first injection and on days 21, 49, 76 and 90. Specific immune responses were analyzed by ELISA using total preimmune and immune sera. Alpaca serum from day 90 was fractionated according to Hamers-Casterman et al. (1993). Protein G and A columns (GE HEALTHCARE) were used for serum fractio...
Embodiment 3
[0161] [Example 3: Flagella-binding V H Library construction and selection for H]
[0162] Construction of hyperimmune alpaca V based on RNA isolated from serum collected in Example 2 H H library.
[0163] Phage display libraries were constructed as previously described (Arbabi Ghahroudi et al., 2009). Briefly, approximately 1 × 10 7 Lymphocytes, total RNA was isolated using the QIAamp RNA blood mini kit (Qiagen, Mississauga, Ontario, Canada). 1-strand cDNA was synthesized with oligo(dT) primers using 5 μg of total RNA as template according to the manufacturer's recommendations (GE HEALTHCARE) (Arbabi Ghahroudi et al., 2009). By using oligonucleotide MJ1-3 (sense) and two kinds of CH2 domain antisense primers CH2 and CH2b3 (primer sequences see Arbabi Ghahroudi et al., 2009) to amplify the variable and part of the constant region DNA, and the heavy chain fragment (550-650 bp in length) was gel-purified by using the QIA Quick Gel Extraction Kit (Qiagene). The variable reg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com