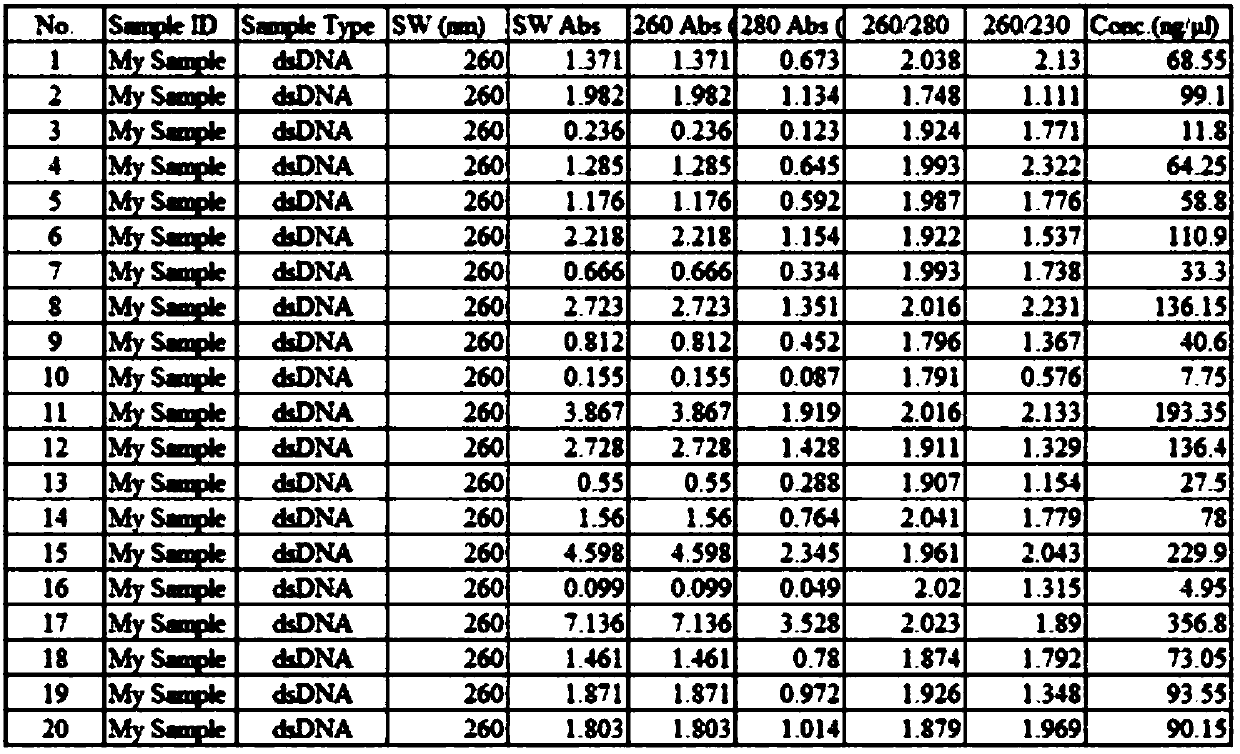
Patents
Literature
117 results about "Diarrheal disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method and application of traditional Chinese medicinal immunoglobulin compound preparation
InactiveCN102302778AWide variety of sourcesNo pollution in the processAntibacterial agentsPowder deliveryDiarrheal diseasesVeterinary Drugs
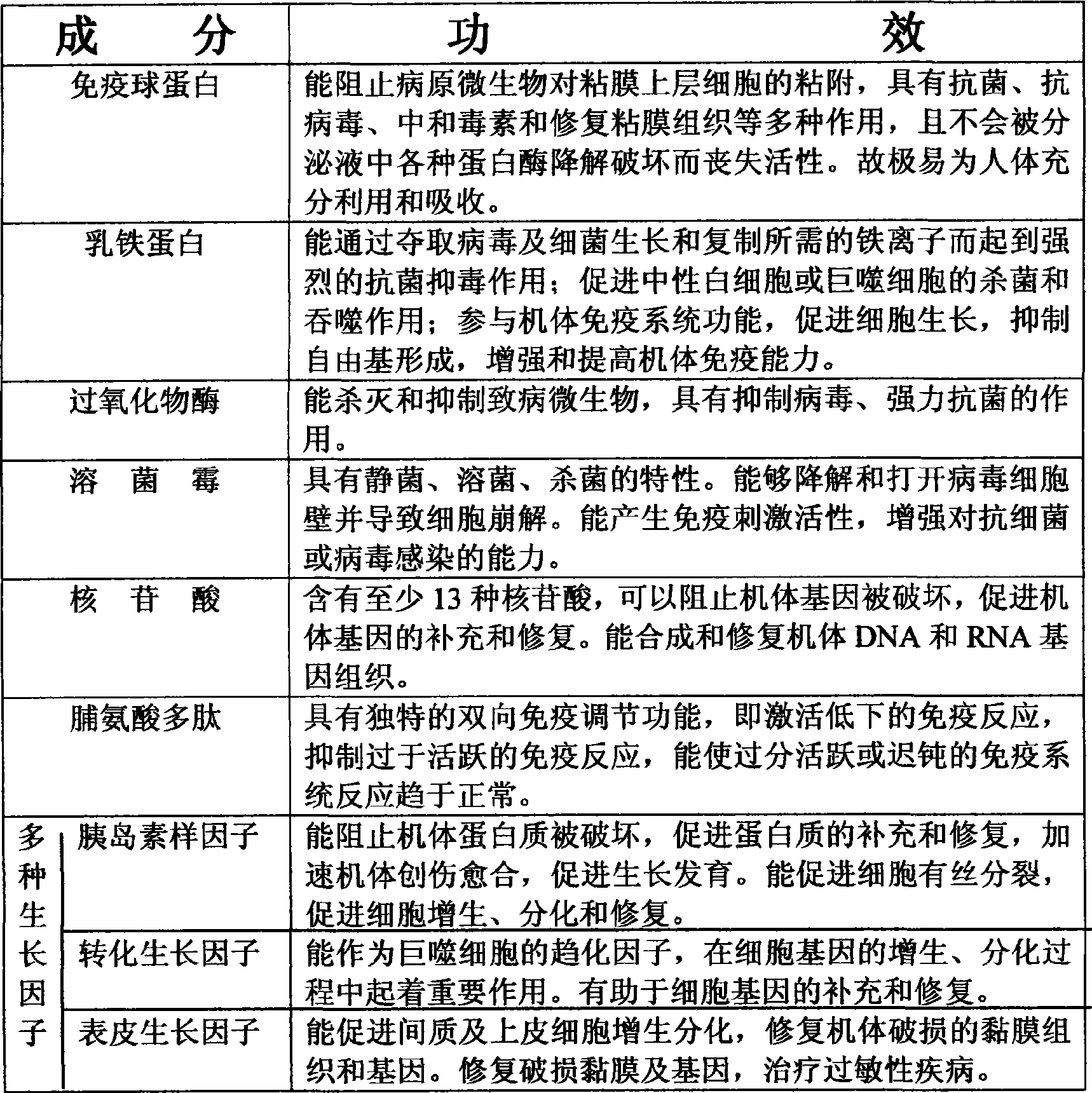
The invention discloses a production method of an immunoglobulin compound preparation for especially preventing and treating porcine diarrhea, and relates to the field of veterinary drugs and feed additives. The production method comprises the following steps: adding part of extracts or powder and the like to specific immunoglobulin or a substance which is not extracted and contains the specific immunoglobulin according to a certain proportion; and then evenly mixing to obtain the immunoglobulin compound preparation. By compositing the advantages of the immunoglobulin extract or powder, a novel antibiotic substitute is formed, thereby achieving the purposes of treating both symptoms and causes, accelerating the rehabilitation speed of a diarrheal animal, preventing and treating porcine diarrhea and meanwhile lowering cost; and the immunoglobulin compound preparation is a safe and hormone-free green product with special good effect on porcine diarrhea. The immunoglobulin compound preparation is taken as a feed additive or mixed with water and drunk. The immunoglobulin compound preparation is especially applicable to prevention and treatment of porcine diarrhea and also suitable for diarrheal diseases of animals such as various poultry, livestock and the like.
Owner:方希修
Anti-diarrhea yelk antibody feed additive for swine and injection formulation, and preparation method
InactiveCN1484972ASimple preparation processLow costPharmaceutical delivery mechanismAnimal feeding stuffRotavirusAnti-diarrhea
A feed additive containing yolk antibodies against diarrhea in pigs. The antibodies are preparing from bacilluscoli, epidemic diarrhea or rotavirus, or immunizing non-immunitive hens laying eggs withcombined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens. A yolk antibody injecting agent, which is prepared from bacilluscoli, epidemic diarrheaor rotavirus, or immunizing non-immunitive hens laying eggs with combined vaccine of bacilluscoli and / or epidemic diarrhea and / or rotavirus, then collecting yolks of the hens.
Owner:HEBEI KEXING PHARMA
Attenuated bacteria useful in vaccines
InactiveUS20050054075A1Improve protectionReliable and rapid isolationAntibacterial agentsBiocideBacterial strainImmunogenicity
The invention provides strains of bacteria, especially enterotoxigenic E. coli, attenuated by mutations in the genes encoding enterotoxins (LT, ST, EAST1) and optionally further attenuated by deletion of additional chromosomal genes. In addition the invention provides strains of attenuated bacteria expressing immunogenic but non-toxic variants of one or more of these enterotoxins. These bacteria are useful as a vaccine against diarrhoeal disease.
Owner:ACAMBIS RES LTD
Bacterial strain capable of preventing and treating cattle and sheep diarrheal diseases
ActiveCN109161509ASimple production processImprove immunityBacteriaMicroorganism based processesMicroorganismDiarrheal diseases
The invention relates to a bacterial strain capable of preventing and treating cattle and sheep diarrheal diseases. The bacterial strain capable of preventing and treating the cattle and sheep diarrheal diseases is bacillus subtilis and preserved in China General Microbiological Culture Collection Center; and the preservation number is CGMCC NO: 15545. The bacillus subtilis can be applied to plant-eating animal feeding, can also be applied to preparation of plant-eating animal feed additives and can further be applied to preparation of plant-eating animal feed. The bacterial strain provided bythe invention has relatively strong adhesion capacity and antibacterial capacity, has very good acid-resistant capacity, gastrointestinal tract resistant capacity and bile salt tolerance, and can effectively prevent and treat the cattle and sheep diarrheal diseases.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
External use plaster for treating child heat diarrhea and preparing method
InactiveCN1899455APromote secretionRegulates gastrointestinal motilityAnthropod material medical ingredientsAerosol deliveryVerbenaDiuresis
The present invention discloses a kind of externally applied plaster for treating infant heat diarrhea and its preparation process. The externally applied plaster is developed based on the physiological features of infant and pathological features and pathogenesis of infant heat diarrhea, and is prepared with 12 kinds of Chinese medicinal materials, including eucalyptus leaf, membrnaceous marshmarigold, European verbena, etc. and through decoction, concentrating, crushing, and other steps. The externally applied plaster has the functions of clearing away heat, promoting diuresis, regulating qi and middle Jiao, invigorating spleen and arresting diarrhea. It is used for treating infant heat diarrhea.
Owner:INNER MONGOLIA DAMO PHARMA
Bacillus subtilis and application thereof
ActiveCN111187730AImprove toleranceGrowth inhibitionBacteriaDigestive systemBiotechnologyInfant animal
The invention discloses a Bacillus subtilis BSC16a and application thereof. The Bacillus subtilis is preserved in China General Microbiological Culture Collection Center on September 6, 2019, and thepreservation number is CGMCC NO: 18473. The strain has a good in-vitro probiotic property. The strain disclosed by the invention plays a good probiotic role in the aspects of improving the growth performance and body immunity of growing and fattening pigs and reducing enteropathogenic bacteria. In addition, the Bacillus subtilis BSC16a disclosed by the invention is used as a probiotic preparationfor feeding diarrhea young animals. The strain has a good prevention and treatment effect on diarrhea animals. The strain can also be used as a feed additive for preventing and treating diarrhea of piglets, lambs and calves.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Diarrhea treating chinese medicine composition
ActiveCN100358549CInhibition of spontaneous movementNo obvious toxic reactionDigestive systemAgainst vector-borne diseasesInfective diarrhoeaToxic material
The diarrhea treating Chinese medicine composition is prepared with Chinese goldthread, skullcap root, white peony root, patchouly, guava leaf and tuckahoe as material. It has the functions of clearing away heat and toxic material, moisturizing and stopping diarrhea, and can kill diarrhea causing pathogenic bacteria and viruses to cure acute infectious diarrhea in high efficiency, quickly and safely.
Owner:惠州市九惠药业有限公司
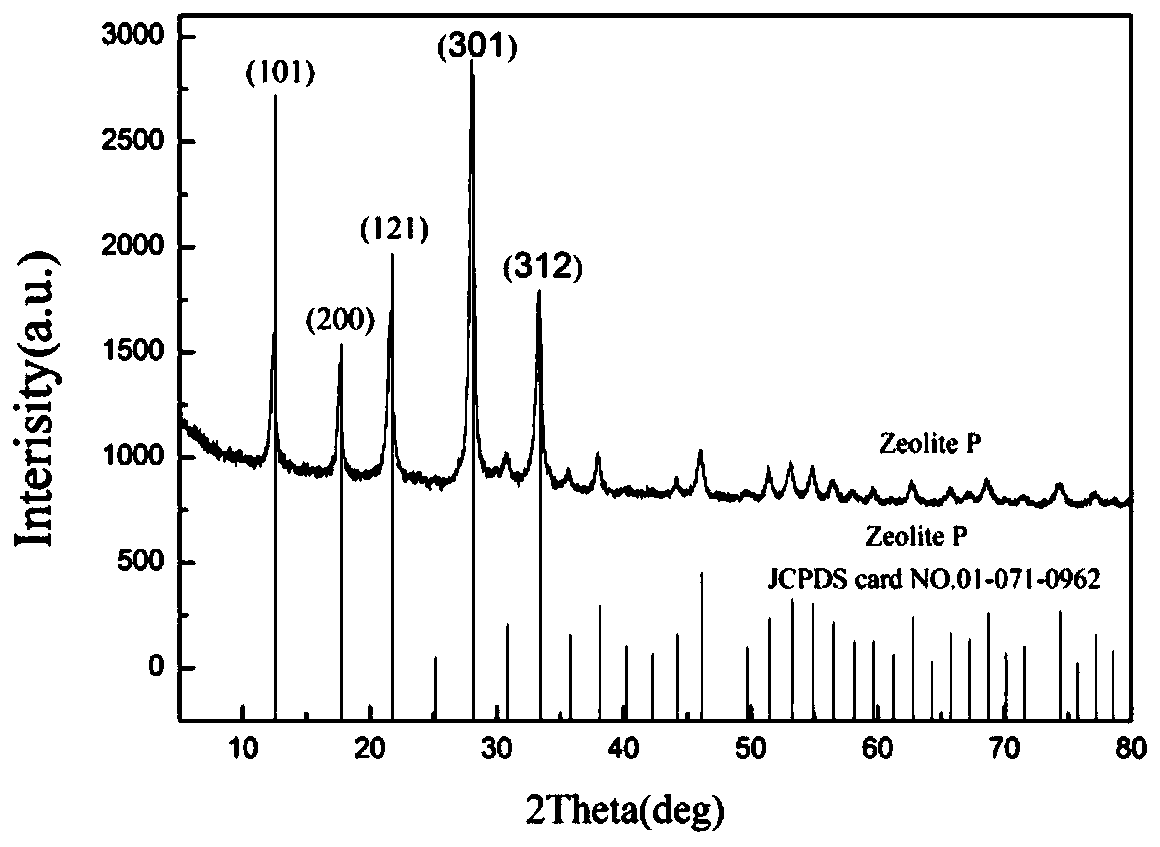
Preparation method of P-type molecular sieve-chitosan-sodium alga acid-potassium diformate slow-release antibacterial agent
ActiveCN109731103AGood prospects for development and utilizationAntibacterial agentsPharmaceutical non-active ingredientsEscherichia coliDiarrheal diseases
The invention discloses a P-type molecular sieve-chitosan-sodium alga acid-potassium diformate compound and a method for forming a livestock antibacterial agent with a slow-release effect. The defectsare overcome that heavy metal in a silver zeolite antibacterial agent easily deposits in an organism and cannot be applied to fodder, and potassium diformate as an acidifying agent cannot exert effects in the intestinal tract. With a P-type molecular sieve being a carrier, chitosan and sodium alga acid are sued a bridge. Potassium diformate as the acidifying agent is adsorbed, and the livestock slow-release antibacterial agent is prepared. Acidity detection and in-vitro antibacterial testing show that the slow-release antibacterial agent has slow-release performance, has an excellent antibacterial effect on escherichia coli, and can prevent and treat a diarrheal disease caused by escherichia coli.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Construction method of IPEC-J2 cell with APN gene knockout
InactiveCN108220338AKnockout worksAids in functional studiesHydrolasesStable introduction of DNAAgricultural scienceDiarrheal diseases
The invention discloses a construction method of an IPEC-J2 cell with APN gene knockout and belongs to the technical field of gene engineering. According to the construction method, a CRISPR / Cas9 technology is adopted to knock out an APN gene sequence in an IPEC-J2 cell line genome, and an established intestinal epithelial cell with APN gene knockout can provide more direct and effective study model to deeply reveal a diarrhea pathogen pathogenic mechanism and to cultivation and application of a diarrheal disease resistance transgenic pig.
Owner:YANGZHOU UNIV
Method of using IL-11 for inflammation associated with acute pancreatitis
Provided by the present invention are methods of treating a variety of disorders including AIDS, arthritis (rheumatoid arthritis, osteoarthritis, spondyloarthropathies), antibiotic induced diarrheal diseases (Closbidium difficile), multiple sclerosis, osteoporosis, gingivitis, peptic ulcer disease, esophagitis, diabetes, retinitis, uveitis, reperfusion injury after myocardial infarction (MI) or cerebral vascular accident (CVA), aphthous ulcers (oral), atherosclerosis (plaque rupture), prevention of tumor metastases, asthma, preeclampsia, acute pancreatitis, psoriasis, infertility and allergic disorders such as rhinitis, conjunctivitis, and urticaria.
Owner:GENETICS INST INC
Kit capable of simultaneously detecting 12 diarrhea pathogenic bacteria and application thereof
InactiveCN105441539AReduce the effect of amplification biasAvoid mismatchMicrobiological testing/measurementAgainst vector-borne diseasesAgarose electrophoresisMicrobiology
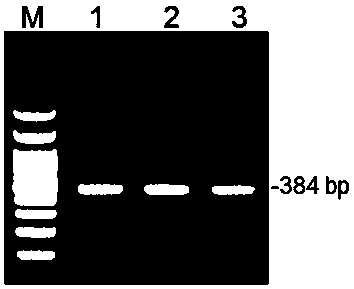
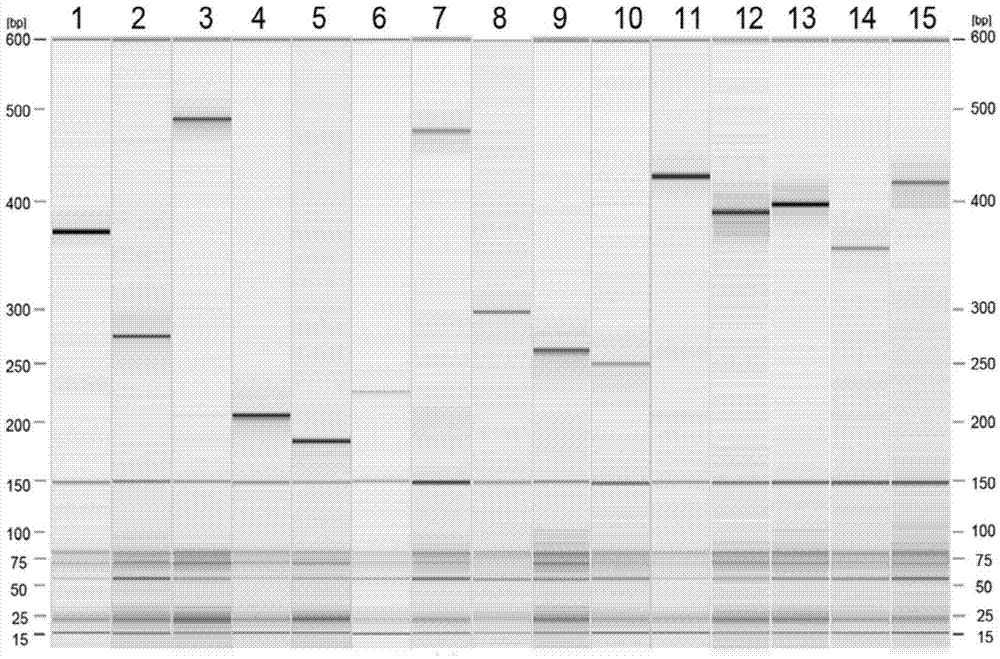
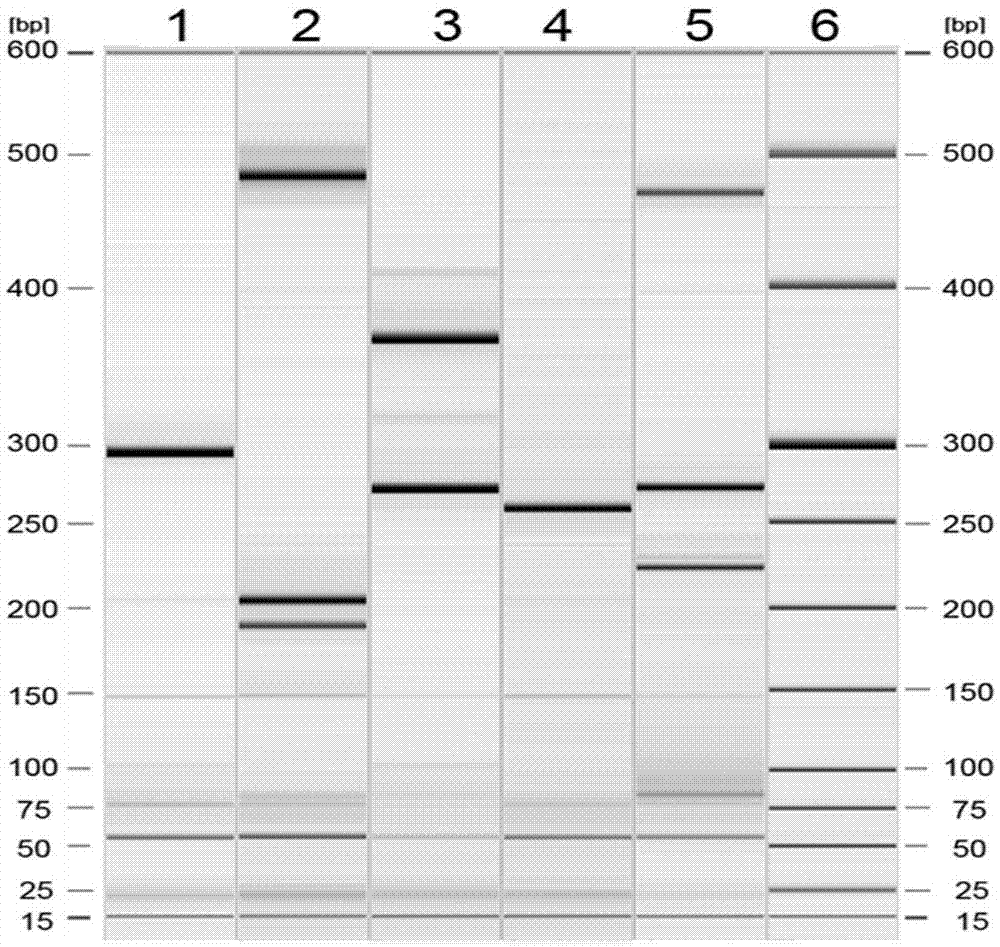
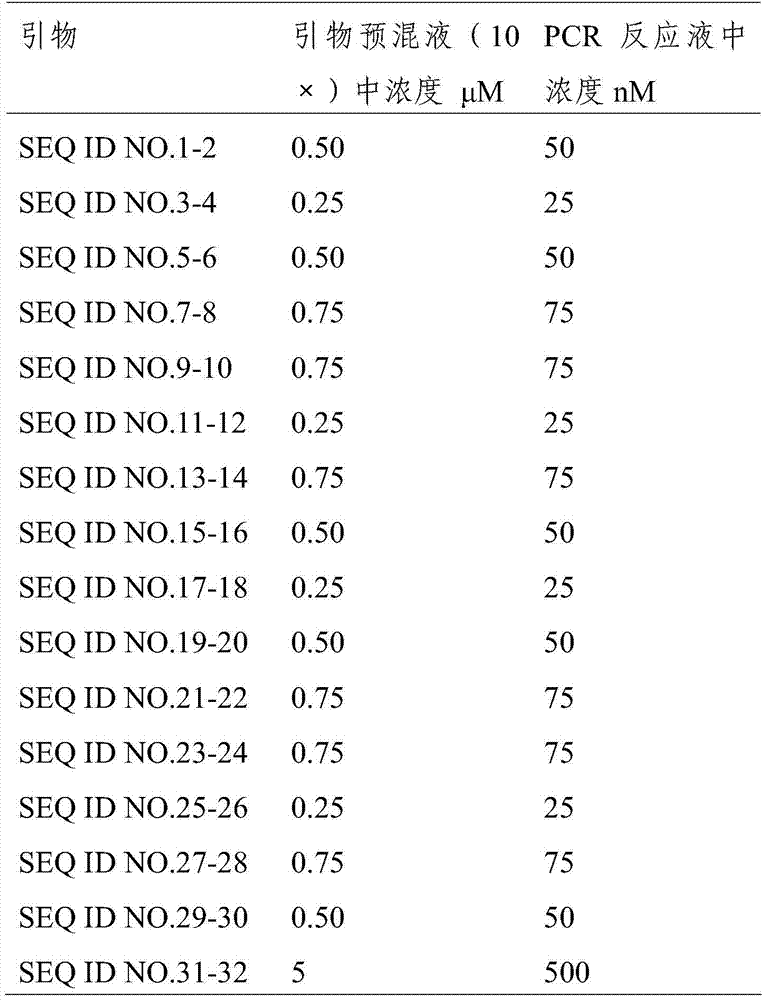
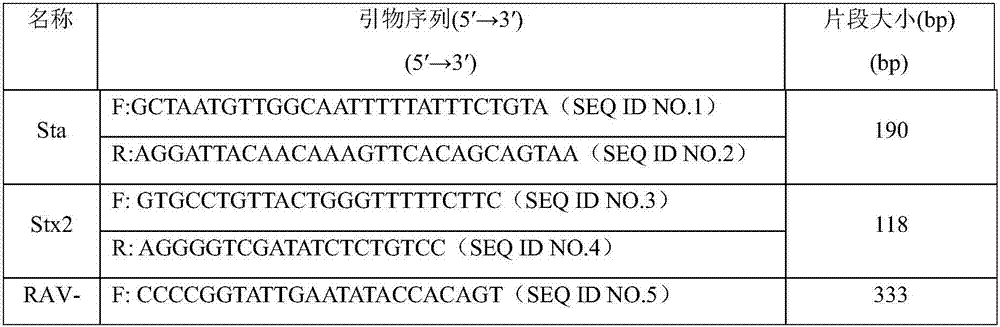
The invention provides a kit capable of simultaneously detecting 12 diarrhea pathogenic bacteria and application thereof, and belongs to the technical field of multiplex PCR detection. The kit comprises specific chimeric primers corresponding to the 12 common diarrhea pathogenic bacteria and a pair of internal reference primers, and the nucleotide sequences of the specific chimeric primer pairs are shown in SEQ ID NO.1-32 respectively. According to the kit, reaction conditions are optimized by arranging a multiplex PCR system, and accurate detection on the 12 diarrhea pathogenic bacteria is achieved by analyzing the length of a product through single-tube one-time PCR and agarose electrophoresis. The kit has the advantages of being accurate in detection, high in sensitivity, strong in specificity, simple, convenient and rapid and suitable for preliminary screening of common pathogenic bacteria in clinical diarrhea samples and epidemiological investigation of diarrhea, and a good application prospect is achieved.
Owner:ICDC CHINA CDC +1
Traditional Chinese medicinal composition for pigs and application of traditional Chinese medicinal composition
ActiveCN103432267AImprove survival rateLow priceAntibacterial agentsHeavy metal active ingredientsBiotechnologyPig farms
The invention relates to the technical field of veterinary medicines, and in particular relates to a traditional Chinese medicinal composition for pigs. The traditional Chinese medicinal composition consists of 6-14 parts of smoked plum, 6-14 parts of myrobalan, 4-8 parts of rhizoma picrorhizae, 2-6 parts of sanguisorba carbon, 2-6 parts of astragalus membranaceus, 8-12 parts of halloysitum rubrum, 6-10 parts of anemone chinensis, 2-6 parts of rhizoma atractylodis and 2-6 parts of liquorice. The traditional Chinese medicinal composition can be powder, granules or oral administration liquid. The traditional Chinese medicinal composition for the pigs, which is disclosed by the invention, is a pure traditional Chinese medicinal composition, does not contain any chemical medicaments, has no residue and no toxic or side effect, is high in safety and difficultly generates drug resistance; an application test in a pig farm shows that the traditional Chinese medicinal composition has good treating effects of bacterial diarrhea, viral diarrhea, nutritional diarrhea and diarrhea caused by other various reasons of piglets; the pharmaceutical effect is quick to take, a quick respond is realized; the using amount of medicines is small; the cost is reduced; the survival rate of piglets is obviously improved; the treatment rate can be over 95 percent; the price is low; the medication safety is realized; the drug resistance can be avoided; the traditional Chinese medicinal composition can be popularized and applied.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Breeding method of new disease-resistance large white pig strain
InactiveCN106973854AEnhance immune responseReduce morbidityAnimal feeding stuffBiological testingInterleukin 6Lean meat
The invention discloses a breeding method of a new disease-resistance large white pig strain. The method includes the following steps that 1, a feed adding method is adopted to conduct an Escherichia coli infection experiment on weaned piglets of a core group in large white pigs, and an Escherichia coli resistant breeding basic group in the large white pigs is established; 2, concentration determination is conducted on cytokines of interleukin 1beta, interleukin 4, interleukin 6, interleukin 8, interleukin 10, transforming growth factor beta, tumor necrosis factor alpha and interferon gamma of an Escherichia coli resistant population in the basic group; reproductive performances are detected and selected, growth speeds, lean meat ratios and carcass quality characters, and a first generation is established; 3, a group subculture selective breeding method and a molecular-marker-assisted selection method are adopted. In this way, by the adoption of the breeding method of the new disease-resistance large white pig strain, after four to five generations, a vested breeding target is achieved gradually, a new-strain core group is established, and the morbidity of piglet diarrheal diseases is expected to be reduced.
Owner:TAICANG JINZHU AGRI DEV
Pharmaceutical composition for preventing and treating animal diarrheal diseases and preparation method of pharmaceutical composition
ActiveCN104523822AImprove immunityPromote growthAntibacterial agentsDigestive systemDiseaseORIGANUM OIL
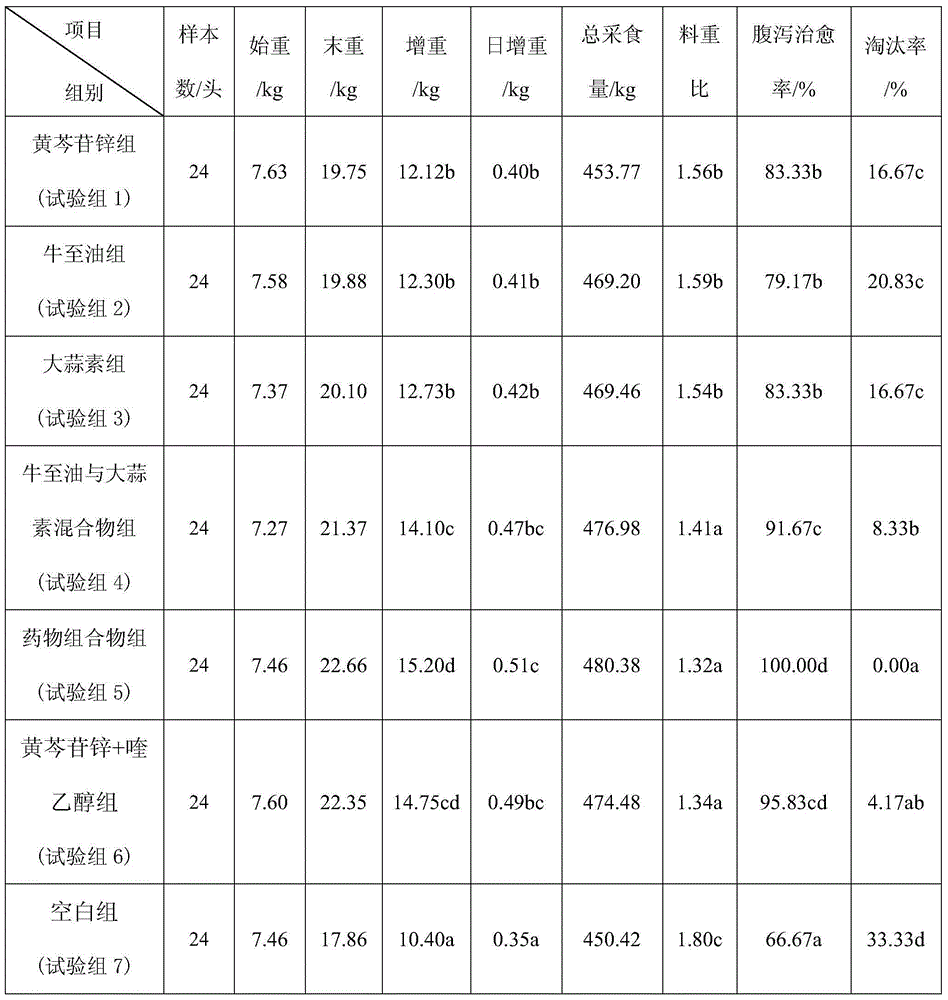
The invention provides a pharmaceutical composition for preventing and treating animal diarrheal diseases and a preparation method of the pharmaceutical composition. The pharmaceutical composition is prepared by mixing the following components in parts by weight: 2.5-30 parts of baicalin-Zn, 2.5-30 parts of origanum oil and 5-30 parts of allicin. The pharmaceutical composition has favorable preventing and treating effects on animal diarrheal diseases, has the effects of trapping and improving the intestinal florae of animals, and can be used for effectively improving the immunities of the animals, promoting the treatment of the diseases and promoting the growth of the animals if being added for a long term.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Establishment of methodology for carrying out joint detection on bacterial genus genes and toxin genes of clostridium difficile by using TaqMan-MGB probe real-time fluorescent quantitative PCR (polymerase chain reaction) technology
InactiveCN103215362AImprove throughputMicrobiological testing/measurementMicroorganism based processesFecesClostridium difficile toxin B
A TaqMan / MGB probe PCR (polymerase chain reaction) technology can carry out bacterial genus identification on clostridium difficile in fecal genomes, simultaneously detect the carrying situation of toxin genes, and can judge whether a toxin A has deletion. A fecal specimen is not required to be purely cultured, and the strain identification and the toxin detection are completed in a reaction system. The method has the characteristics of simple operation, good repeatability, high flux detection specimens, short report time, and the like, and is applicable to the screening of pathogenesis of patients with diarrhea caused by clinically unexplained causes. The invention solves the technical problems that fecal specimens can be subjected to strain identification and strain toxin carrying situation screening simultaneously without being purely cultured, and a DNA (deoxyribonucleic acid) detection method for fecal genomes, which is high in sensibility, is provided. Because traditional anaerobic culture is uneasy to perform, and an enzyme immunoassay has methodology defects, according to the invention, the diagnosis rate of clostridium difficile associated diarrhea is significantly increased. Meanwhile, the invention also can be applied to the monitoring of drug used in the process of treating the diseases.
Owner:AEROSPACE CENT HOSPITAL
Chinese herbal medicine feed additive to improve immunity of rex rabbit and prevent diarrheal disease and feed containing the same
InactiveCN105233094AEnhance physical fitnessImprove the body's immunityDigestive systemInanimate material medical ingredientsDiseaseBetel
A Chinese herbal medicine feed additive to improve immunity of rex rabbit and prevent diarrheal diseases is composed of, by weight, 30-50g of Polygonum multiflorum, 15-30g of dandelion, 15-45g of Radix Codonopsis, 15-45g of Poria cocos, 5-15g of Eucommia, 5-15g of Phellodendron, 5-15g of Scutellaria baicalensis, 5-15g of Chinese angelica, 5-15g of Chinese herbaceous peony, 5-15g of radices saussureae, 15-45g of scorch-fried medicated leaven, 15-45g of scorch-fried malt, 15-45g of scorch-fried sanxian, 5-8g of aloe, 6-10g of isatis root, 30-90g of fossil fragments, 10-30g of lily, 30-80g of licorice, 10-20g of hawthorn and 10-20g of betel nut. According to the principle of compatibility of traditional Chinese medicine, and combined with the physiological characteristics of rex rabbit, the invention applies a medicament composed of Chinese herbal medicine as an additive to rex rabbit feed, so as to enhance constitution of rex rabbit, improve immunity, prevent and treat diarrhea, strengthen calcium and iron absorption, reduce the occurrence of malnutrition and disease.
Owner:陈红媛 +2
Fluorescent quantitative PCR detection kit for clostridium difficile toxin A/B and detection method
InactiveCN105525023AImprove responseOptimizationMicrobiological testing/measurementDNA/RNA fragmentationClostridial toxinClostridium difficile infections
The invention discloses a fluorescent quantitative PCR detection kit for clostridium difficile toxin A / B by adopting a probe method, and a detection method, which can detect clostridium difficile and also can detect and quantify toxin A and B genes. The detection kit mainly comprises specific primers and probes, wherein the specific primers and the probes are composed of primer pairs for detecting clostridium difficile, primer pairs and probes for detecting clostridium difficile toxin A, as well as primer pairs and probes for detecting clostridium difficile toxin B. According to the detection kit and the detection method, the operation is simple, the report time is short, the clostridium difficile strains can be specifically, sensitively and accurately identified for the first time, and the key toxins A and B of clostridium difficile are detected and quantified. A foundation is laid for the early diagnosis for clostridium difficile infection, and the detection kit and the detection method are suitable for screening causes of diarrhea patients with unknown causes clinically.
Owner:GUANGZHOU SAGENE BIOTECH
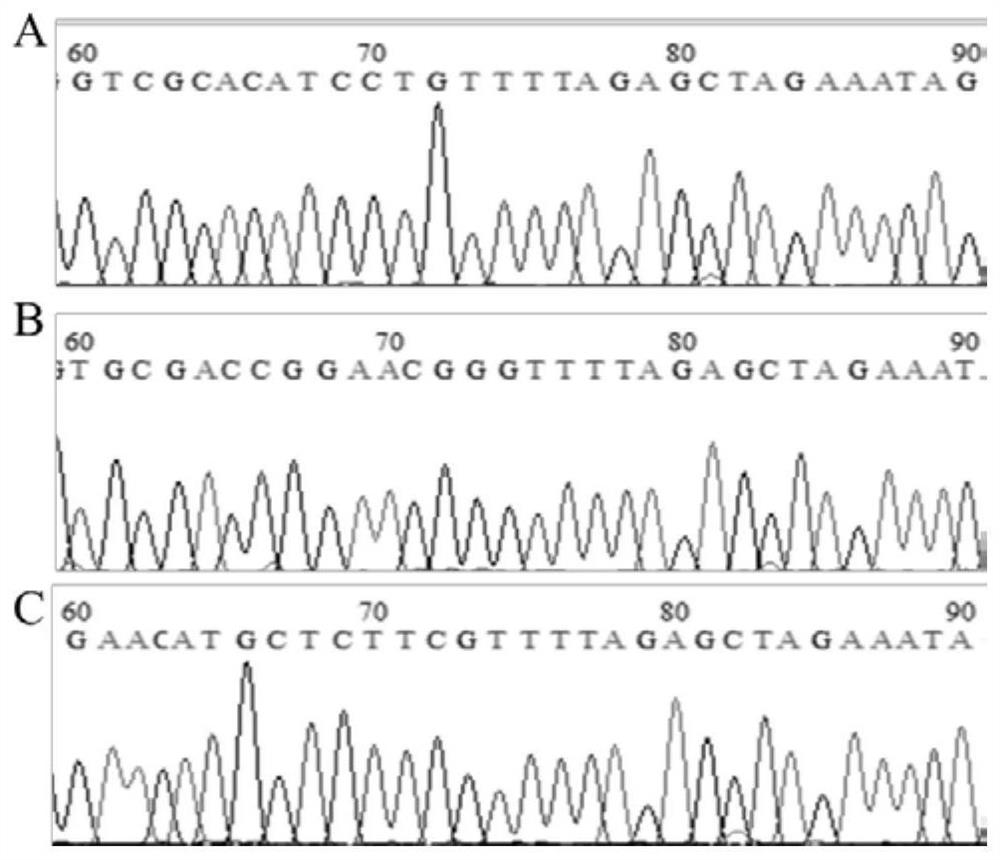
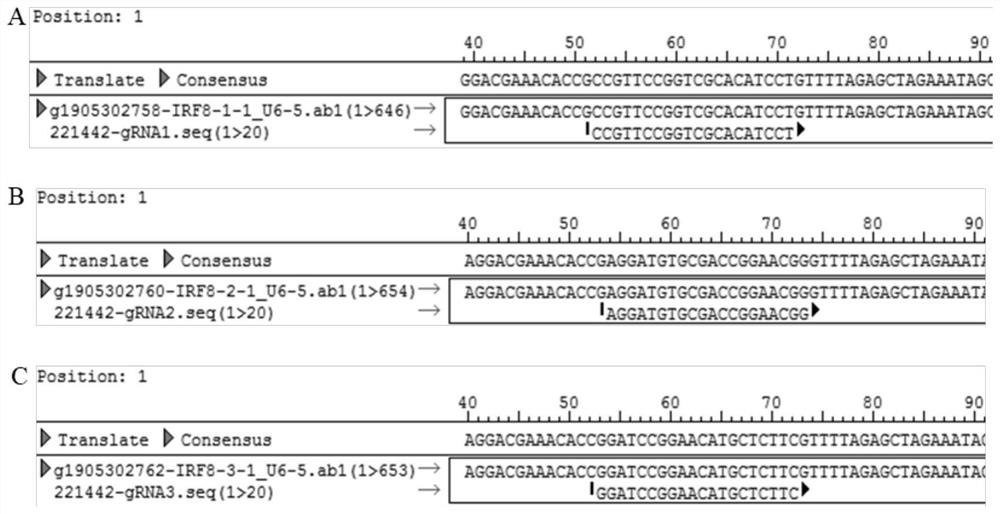
Cell line for knocking out porcine IRF8 gene based on CRISPR-Cas9 editing technology and construction method of cell line
PendingCN111607594AContribute to researchIdeal knockoutStable introduction of DNAPeptidesNegative strandDouble strand
The invention discloses a cell line for knocking out the porcine IRF8 gene based on a CRISPR-Cas9 editing technology and a construction method of the cell line. The cell line is prepared by adopting the following steps of: (1) design of a target site and synthesis of an sgRNA sequence: designing an sgRNA guide sequence according to the 5' end of a CDS region of the porcine IRF8 gene; (2) vector construction: annealing the plus-strand sgRNA sequence and the minus-strand sgRNA sequence to form double-stranded DNA; connecting the double-stranded DNA with a linearized pGK1.1 vector to obtain a positive targeting vector; and (3) cell transfection: carrying out mixed electrotransfection of the positive targeting vector into the target cell IPEC-J2 to obtain the IPEC-J2 cell with the IRF8 gene knocked out. The small intestine epithelial cell line with the IRF8 gene knocked out is established, and a more direct and effective research model can be provided for deep revealing of a diarrhea pathogen pathogenic mechanism, resistance gene mining and identification and cultivation and application of diarrhea-resistant transgenic pigs.
Owner:YANGZHOU UNIV
Human milk(containing first milk) freeze-drying powder production technique and products and use thereof
InactiveCN101422240AThe formula is scientific and reasonableTargetedMilk preparationMilk preservationRecovery periodPhysical exercise
The invention discloses a production technology of freeze-dried powder of breast milk (including colostrum) of human body, and a product and functions thereof. The production technology comprises the following steps: redundant breast milk (including colostrum) of healthy parturient women is collected; impurity removal by means of centrifugation, fat removal and pasteurization, freezing and drying at low temperature are carried out to obtain the freeze-dried powder of the breast milk (including colostrum) of human body. The freeze-dried powder can be made into tablets, capsules and oral solution; the freeze-dried powder can be adopted for supplying nutritious food required by lactation and growth for infants lack of breast feeding and teenagers in the growth stage, providing nutriments for athletes needing to create good results, personage in performing as well as film and television circles, personage succeeding in career, convalescents, subhealth crowd and crowd of various kinds of low immunity, and also can be used for curing all patients with tumors, people with AIDS, patients of organ transplantation and patients with diarrhea to speed up the recovery process of organism health, curing and avoiding exercise-induced diarrhea of athletes and improving the performance of sports; and the freeze-dried powder can further be used for anti-inflammation, sterilization and antivirus and providing special nutriments for cosmonauts, aviators and special forces.
Owner:蒋来高 +1
Functional additive for preventing milk accumulation diarrhea of calves and lambs and preparation method of the functional additive
PendingCN111557382ATimely and fast energyReduce usageFood processingDigestive systemBiotechnologyEscherichia coli
The invention discloses a functional additive for preventing milk accumulation diarrhea of calves and lambs and a preparation method of the functional additive. The invention particularly relates to preparation of a functional additive for preventing milk accumulation diarrhea of calves and lambs. The problem caused by application of antibiotics for treating or preventing the milk accumulation diarrhea of calves and lambs is solved The functional additive is prepared from: fermented traditional Chinese medicine powder, complex enzyme, complex microorganism, medium-chain fatty acid and yeast cell walls; wherein the complex microorganisms include lactic acid bacteria, bacillus subtilis, yeast and clostridium butyricum; the traditional Chinese medicines in the fermented traditional Chinese medicine powder comprise roasted malt, hawthorn, medicated leaven, chicken's gizzard membrane, pericarpium citri reticulatae, and areca catechu. The functional additive can supply intestinal flora and digestive enzymes to calves and lambs in the lactation period; by adding the lysozyme, intestinal diseases caused by escherichia coli and salmonella are effectively controlled, the medium-chain fatty acid is added to reduce the use of antibiotic drugs; meanwhile, the medium-chain fatty acid has the advantage of quickly supplementing energy, so that the sick calves and lambs are helped to recover quickly.
Owner:开封嘉骏生物科技有限公司
Breeding method of new Berkshire pig disease-resistant variety
InactiveCN107494422AImprove reproductive performanceSimple structureAnimal husbandryDiseaseLean meat
The invention relates to a breeding method of a new Berkshire pig disease-resistant variety. The breeding method comprises the steps that firstly, weaned piglets of a Berkshire pig core group are subjected to Escherichia coli infection experiments by adopting a feed adding method, according to a phenotype identification result, a Berkshire pig Escherichia coli disease-resistant breeding base group is established; secondly, the base group is subjected to molecule breeding with general resistance to diseases by using a proprietary technology obtained in the early stage, meanwhile characters such as breeding performance, growing speed, lean meat percentage and carcass quality are detected and selected, and a first generation is built; thirdly, a group continuous progeny breeding and molecular marker assisted selection method is adopted, after four to five generations, an established breeding aim is achieved step by step, and a new variety core group is established. The new Berkshire pig disease-resistant variety has the advantages of resistance to weaned piglet diarrheal diseases, high stress resistance, high environmental adaptability and the like, and can further provide guidance for disease-resistant breeding and new variety selection and breeding of other pig varieties.
Owner:YANGZHOU UNIV
Key marker sites and applications of muc13 gene affecting susceptibility/resistance to f4ac diarrhea in piglets
ActiveCN102286480AReduce mortalityImprove resistance to diarrheal diseaseMicrobiological testing/measurementDNA/RNA fragmentationDiarrheal diseasesBOAR
The invention discloses a susceptible / resistant MUC13 (mucoprotein) gene key sign locus affecting piglet F4ac diarrhea and an application thereof in pig genetic improvement. A large scale of white Duroc*Erhualian Boars resource groups and 15 Chinese and foreign pig species distant groups are subjected to complete-genome scanning linkage position, target zone high-density SNP (Single Nucleotide Polymorphism) multi-point linkage precise positioning, breakpoint recombination analysis and distant group associativity analysis based on linkage unbalance to finally lock a coding ETEC (Enterotoxigenic escherichia coil) F4ac receptor target gene-MUC13 gene and separate and colon the MUC13 gene. 15 key mutantsites are indentified in the MUC13 gene. The 15 mutantsites and ETEC F4ac in vitro adhesive phenotype are basically mutually separated in 144 pure breed Duroc, Landrace and big white piglet from core groups of five provinces. The accuracy for the strongest relevant sign to judge susceptible / resistant individual is 97% (P=1.59*10-21). The invention provides new method for breeding for disease resistance for boar ETEC F4ac diarrheal disease.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Anti-campylobacter jejuni antibodies and uses therefor
Campylobacter jejuni is a leading cause of bacterial food-borne diseases in humans, ranging from acute diarrheal disease to neurological disorders. An isolated or purified antibody or fragment thereof specific to C. jejuni is described. The antibody or fragment thereof binds to a flagellar protein and reduces motility of C. jejuni. The antibody or fragment thereof is derived from a heavy chain IgG variable domain fragment (VHH) of a camelid animal immunized with C. jejuni flagellar protein. A multivalent form, as well as a phage format, of the antibody or fragment thereof is described. Methods of reducing presence of C. jejuni in an animal or an animal environment, methods and formulations for treating C. jejuni infection, and method of detecting C. jejuni are also described.
Owner:NAT RES COUNCIL OF CANADA
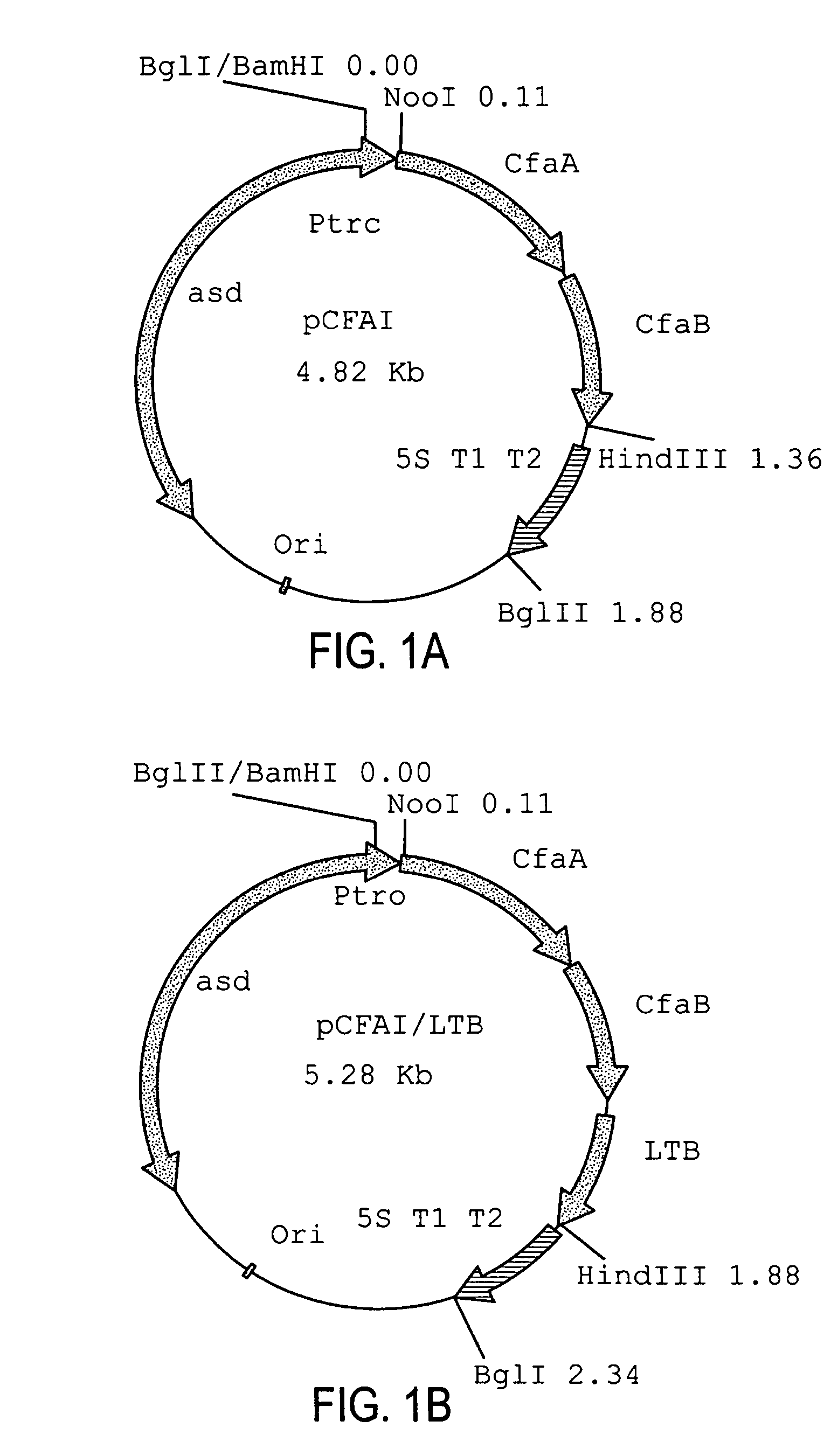
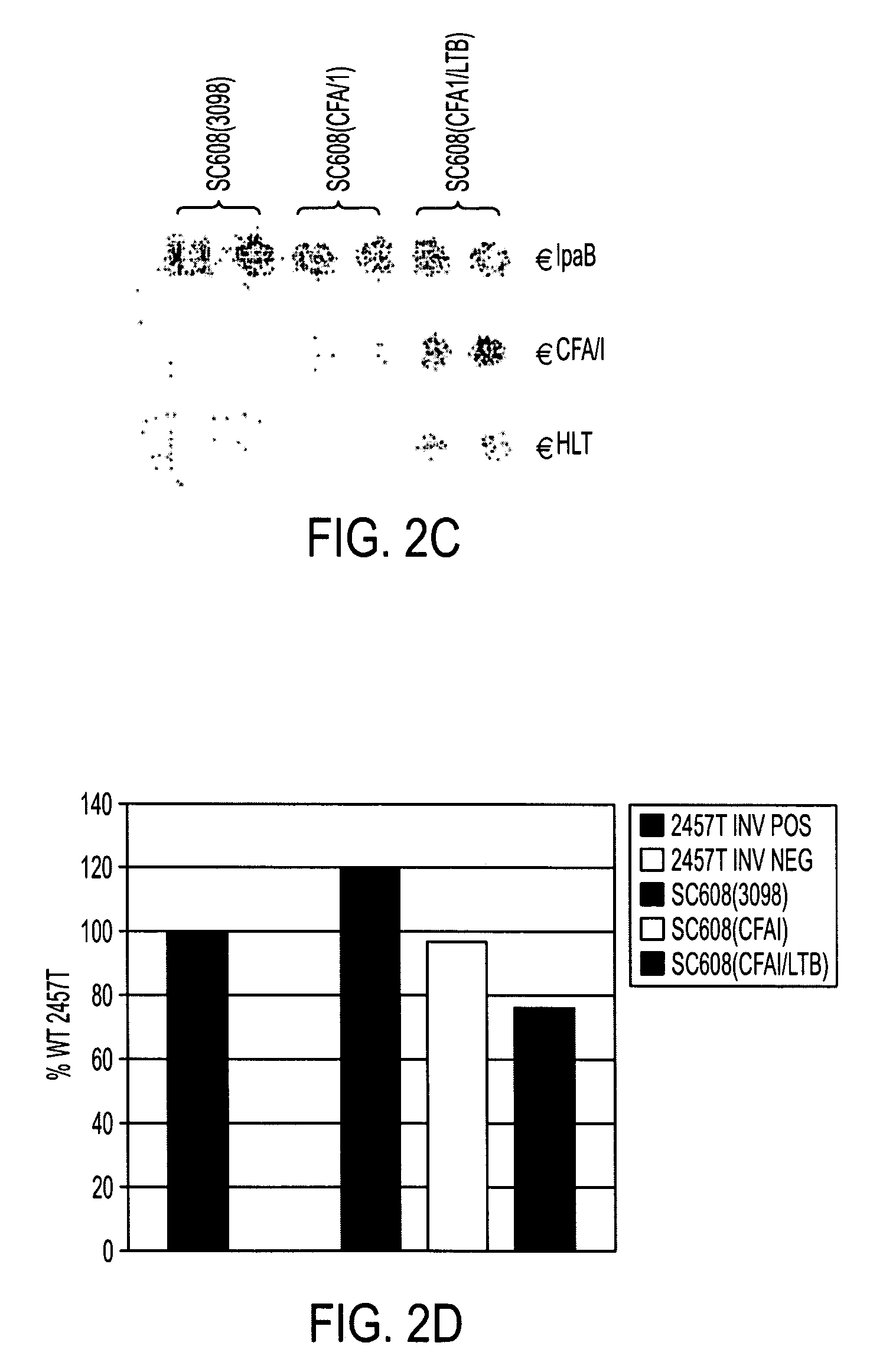
Construction of live attenuated Shigella vaccine strains that express CFA/I antigens (CfaB and CfaE) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic E. coli
ActiveUS7759106B2Reduce intrusionBacterial antigen ingredientsBacteriaHeterologousMucosal Immune Responses
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Lactobacillus johnsonii and application thereof
ActiveCN110878266AImprove securityGood in vitro probiotic potentialBacteriaDigestive systemBiotechnologyInfant animal
The invention discloses a strain of Lactobacillus johnsonii and its application in diarrhea of piglets, lambs and calves. The strain is isolated from healthy pig feces and has good in-vitro probioticpotential and safety. The Lactobacillus johnsonii of the invention and other strains are jointly applied to young animals with diarrhea, and the strain has obvious prevention and treatment effects ondiarrhea cases of different degrees.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Drug for treating enteric disease of beasts and birds
InactiveCN103169825APromote absorptionQuick resultsDigestive systemPlant ingredientsDiseaseDiarrheal diseases
The invention provides a traditional Chinese medicine preparation for treating enteric disease of beasts and birds and a preparation method of the traditional Chinese medicine preparation. The traditional Chinese medicine preparation comprises the following components by weight percent: 20-80% of spider perfume and 20-80% of grassleaf sweelflag rhizome. The preparation method comprises the following extraction technology: weighing medicinal materials according to a prescription, smashing into coarse powder, thoroughly soaking the medicinal materials by water, extracting volatile oil completely by means of steam distillation, filtering out liquid medicine, boiling dregs of a decoction by water for once, merging two filter liquor, concentrating under a reduced pressure to be proper in quantity, carrying out alcohol precipitation, leaching liquid supernatant, and recovering ethanol, i.e. the purified liquid medicine, and adding the volatile oil into the drug by a proper method when a finished product is prepared. The drug can be made into oral liquid, granules, tablets and capsules. The oral preparation of the drug is quick to absorb, and good to take effect, the granule of the drug is easy to store and take, and the tablet and the capsule of the drug are exact in dose, convenient to use, and unique in curative effect to the diarrheal diseases of the beasts and birds. Compared with an existing common drug, the traditional Chinese medicine preparation has the advantages of being high in safety, good in curative effect, free from vestigital and the like, thereby being a product for well treating the enteric disease of beasts and birds.
Owner:SHENYANG VICA ANIMAL HUSBANDRY TECH +1
Preparation method of green chitosan/carboxymethyl cellulose/P-type molecular sieve/potassium diformate slow-release antibacterial microspheres
PendingCN110787135AGood prospects for development and utilizationAntibacterial agentsInorganic non-active ingredientsBiotechnologyEscherichia coli
The invention discloses a preparation method of chitosan / carboxymethyl cellulose / P-type molecular sieve / potassium diformate slow-release antibacterial microspheres for livestock. The problems of drugresidue and drug resistance caused by antibiotics are increasingly serious, and an acidifier potassium diformate plays role in middle rear section and large intestine section of small intestine are aimed; the novel green livestock slow-release antibacterial microsphere can be prepared by taking a green chitosan / carboxymethyl cellulose / P-type molecular sieve as a drug carrier, carrying out physicalaction on the drug carrier and Ca <2+>, and taking potassium diformate as a target drug. The sustained-release and in-vitro antibacterial tests show that the antibacterial microspheres have sustained-release performance, have an excellent antibacterial effect on escherichia coli, and can prevent and treat diarrhea caused by escherichia coli.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Multi-fluorescent PCR kit and method for detecting clostridium difficile genes and toxin genes
ActiveCN110129458AAvoid false negativesPollution is fully degradedMicrobiological testing/measurementDNA/RNA fragmentationBacteroidesClostridium difficile (bacteria)
The invention belongs to the technical field of bacterial detection, and specifically discloses a multi-fluorescent PCR kit and method for detecting clostridium difficile genes and toxin genes, wherein the kit and method not only can identify clostridium difficile but also can detect toxin B genes. The kit mainly comprises a specific primer group and a probe, the primer group and the probe are composed of a primer pair and probe for detecting clostridium difficile, a primer pair and probe for detecting toxin B, and an internal primer pair and probe. By design of the specific primers and probes, the clostridium difficile genes and the toxin genes can be detected from samples with complex components. The kit has the advantages of simple operation, short reporting time, high specificity, highsensitivity and good accuracy; PCR false negativity is avoided by introducing internal standard products, false positivity caused by contamination of amplified products is reduced, and the kit is suitable for pathogenesis screening of patients with diarrhea of unknown clinical cause.
Owner:GUANGZHOU SAGENE BIOTECH
Baby rabbit healthcare sand
ActiveCN104434958AImprove survival rateEasy to prepareAntibacterial agentsSalicyclic acid active ingredientsDiseaseSalicylic acid
The invention discloses baby rabbit healthcare sand, belonging to the technical field of livestock breeding healthcare and disease prevention and control. The baby rabbit healthcare sand is composed of the following components in parts by weight: 20-30 parts of salicylic acid, 15-25 parts of compound sulfamethoxazole, 15-25 parts of calcium propionate and 920-950 parts of talcum powder. The baby rabbit healthcare sand disclosed by the invention is simple in preparation method, wide in raw material resources, capable of effectively preventing newborn rabbit yellow urine disease, septicopyemia, dermatomycoses and dysentery and also capable of effectively treating the diseases to greatly improve the survival rate of baby rabbits, the baby rabbit healthcare sand is a composition with low cost and high prevention and treatment efficiency, and the rabbit disease treatment costs of rabbit warrens are saved.
Owner:INNER MONGOLIA DONGDA BIOTECH
Chinese and Tibetan medicine composition for preventing and controlling livestock diarrhea
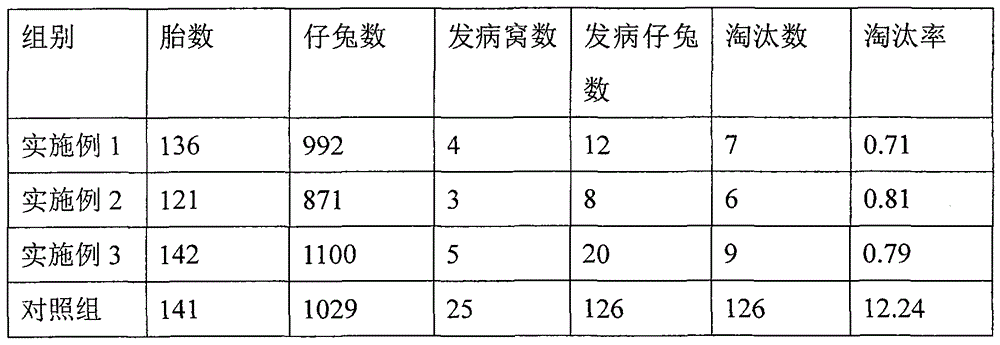
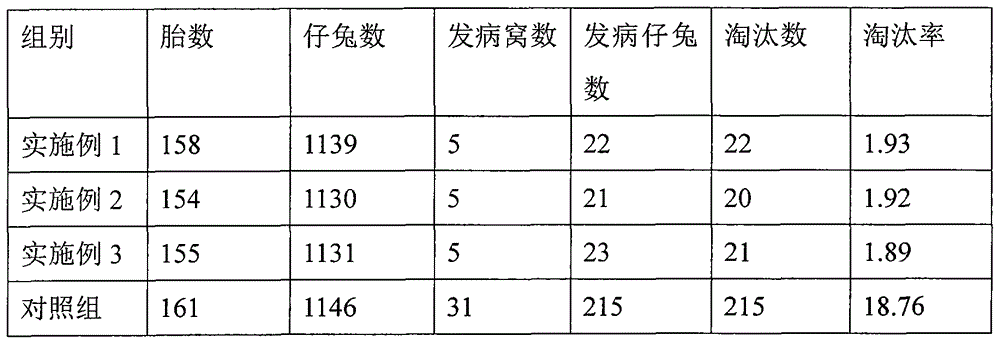
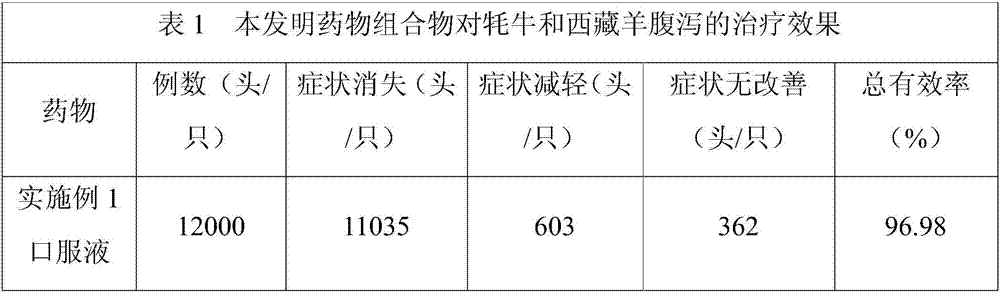
ActiveCN107233454AWill not affect milking salesSignificant effect in diarrheal diseaseAntibacterial agentsDigestive systemBetelDiarrheal diseases
The invention discloses a pharmaceutical composition for preventing and controlling livestock diarrhea. The pharmaceutical composition is an agent prepared from the following raw medicinal materials in parts by weight: 1-4 parts of Chinese herbaceous peony, 1-5 parts of scutellaria baicalensis, 1-5 parts of coptis chinensis, 1-5 parts of angelica sinensis, 1-5 parts of rheum officinale, 1-5 parts of elecampane, 1-5 parts of betel nut, 1-5 parts of lonicerae flos, 1-5 parts of purslane, 1-5 parts of rhododendron micranthum leaf, 1-5 parts of polygonum viviparum, 1-4 parts of bergenia purpurascens and 1-4 parts of liquorice. Clinical veterinary use results prove that the medicine disclosed by the invention has an obvious effect used for treating diarrheal diseases of livestock such as yak, Tibetan sheep and the like. Moreover, compared with the current Western medicine used for treating livestock diarrhea, the medicine disclosed by the invention has the advantages that milking and milk vending of animal owners are not influenced during the milking period of cattle and sheep, the advantages are obvious, and a novel medication choice is provided for clinical treatment of livestock diarrhea diseases.
Owner:阿坝藏族羌族自治州畜牧科学技术研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com