RECOMBINANT BCG OVEREXPRESSING phoP-phoR
A technology of overexpression and composition, applied in the field of recombinant BCG, can solve the problems such as the improvement of no observed protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Cloning vector pME
[0082] The kanamycin-resistant shuttle vector containing the T7 promoter was obtained as follows. use EcoR I cut the pDrive cloning vector (from Qiagen) and self-ligated to generate pDRI. use Ssp I digested pDRI and isolated the 1903 bp fragment product. use Ssp I digested the pMD31 shuttle vector (Wu et al ., 1993, Molecular Microbiology , 7, 407-417) and isolated a 3379 bp fragment. put two Ssp The resulting fragments were ligated to generate pME (5282 bp), which contained the original T7 promoter of pDRIVE. Cloning vector pME such as figure 2 shown.
Embodiment 2
[0083] Embodiment 2: for phoP with phoP-phoR Construction of the expression vector
[0084] use respectively contain KpnI with PstI Restriction site (underlined) forward primer phoP-F (5'-AAAAA GGTACC GCTTGTTTGGCCATGTCAAC-3') and reverse primer phoP-R (5'-AAAAA CTGCAG GCTGCCGATCCGATTAACTAC-3'), amplified by PCR containing phoP as well as phoP 1028 bp product of the 257 bp upstream region of the translation initiation site. Using these restriction sites, the PCR product was ligated into the shuttle vector pME (pME-PhoP). Similarly, by adding phoP with pho (and the intergenic region between the two genes, phoP 177bp upstream of the start codon and phoThe 2501 bp PCR product 78 bp downstream of the stop codon was cloned into pME to construct expression phoP-phoR Vector for the operon (pME-PhoPR). use respectively contain KpnI with PstI site (underlined) of the forward primer phoPR-F (5'-AAAAA GGTACC GGTCGCAATACCCACGAG-3') and reverse primer phoPR-R...
Embodiment 3
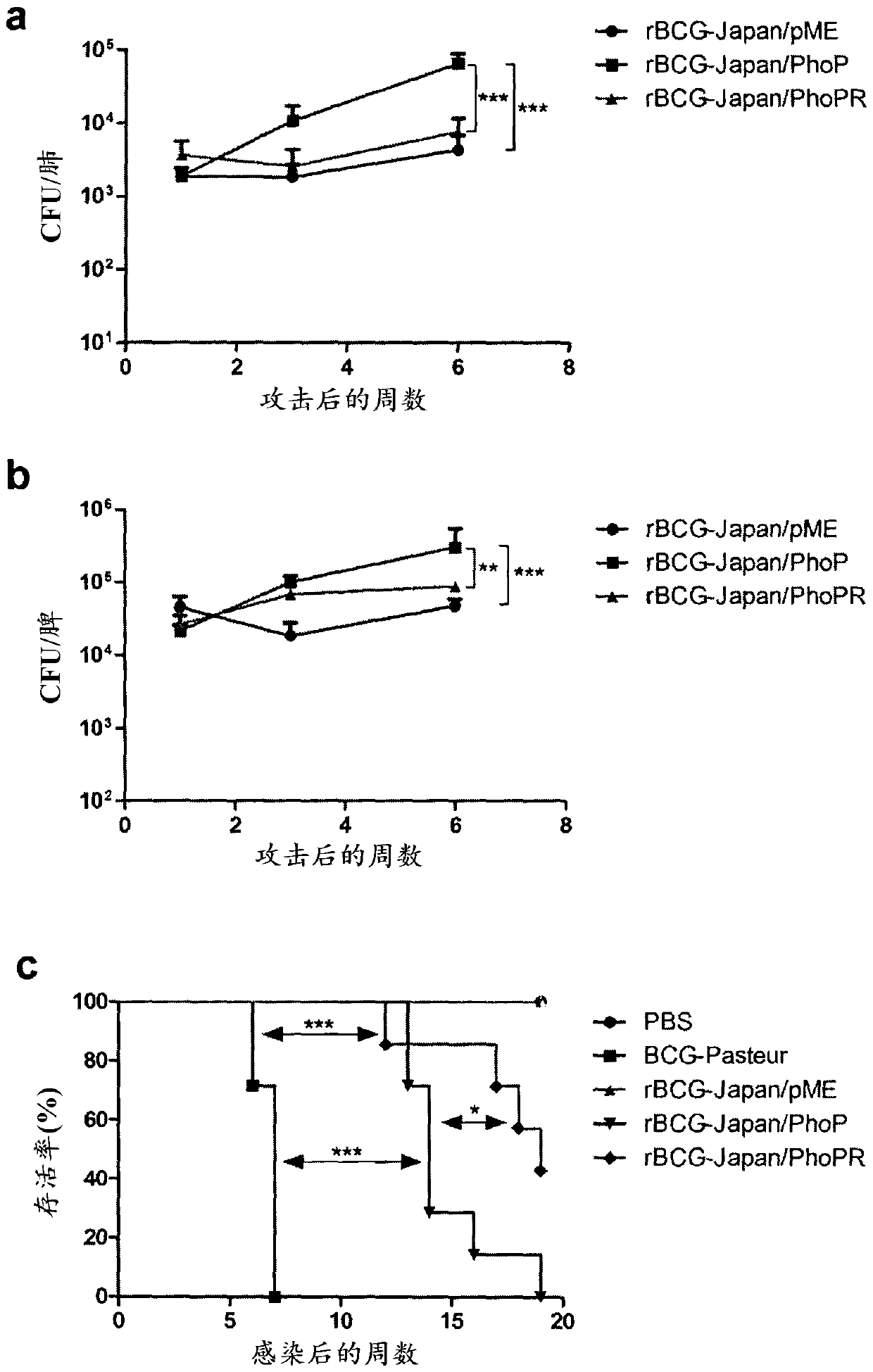
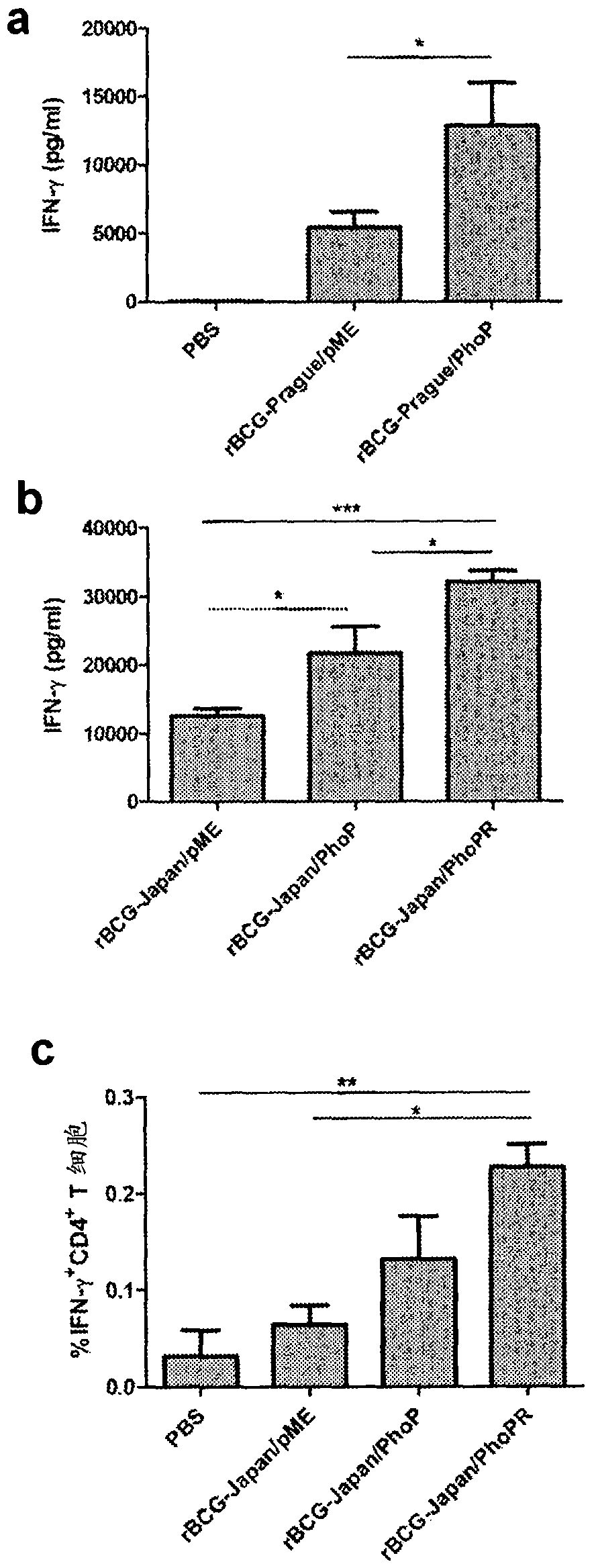
[0087] Example 3: rBCG-Japan / PhoPR induces more CD4 cells than parental BCG + IFN-γ production by T cells
[0088] Female C57BL / 6 mice were purchased from Charles River Laboratories and age-matched (6 weeks) in each experiment. For each group of 4 mice, about 5×10 in 0.2 ml PBS / 0.01% Tween 80 4 CFU of the BCG strain were inoculated subepithelially on the dorsum of the neck. Control mice were given 0.2 mL PBS / 0.01% Tween 80. After 8 weeks, the mice were euthanized and splenocytes were isolated. Quantitative measurement of IFN-γ production was determined using an enzyme-linked immunosorbent assay (ELISA) using the OptEIA mouse IFN-γ ELISA kit (BD Biosciences). The sample used for ELISA was the supernatant from splenocytes stimulated with PPD (10 μg / ml) for 72 hours. Briefly, samples were added in triplicate to 96-well flat-bottomed plates (Nunc MaxiSorp) pre-coated with capture antibody and measured following the manufacturer's protocol. Absorbance was read at 450 nm on a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com