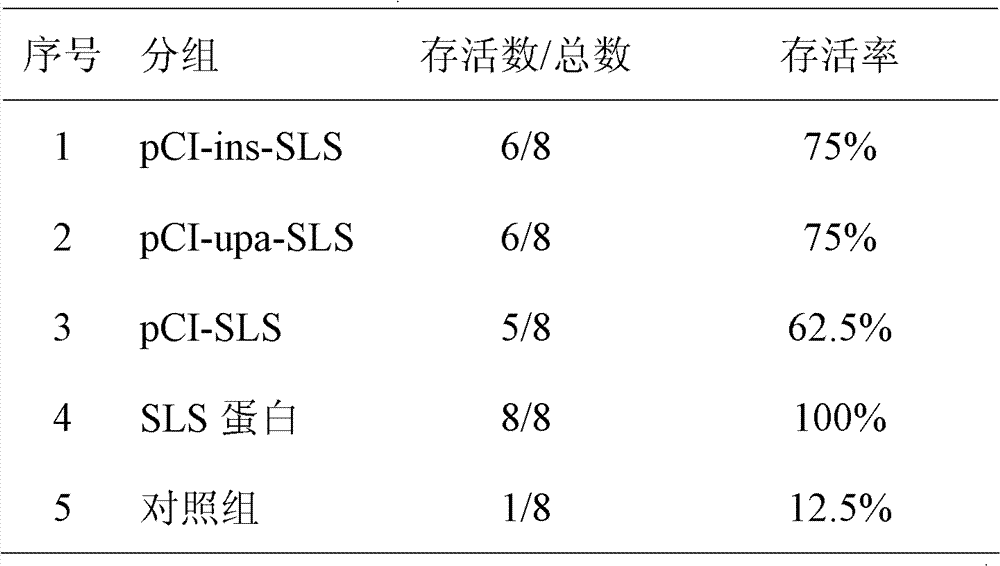
Patents
Literature
32 results about "Enterotoxin gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
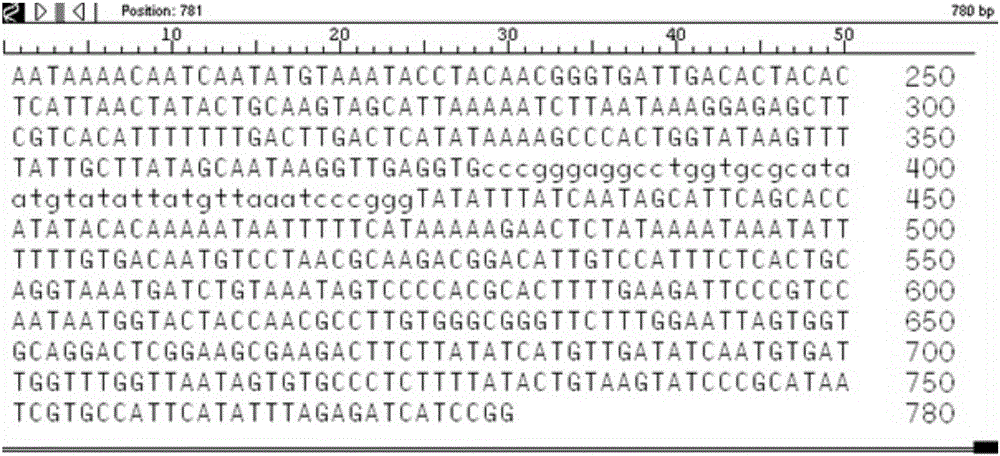
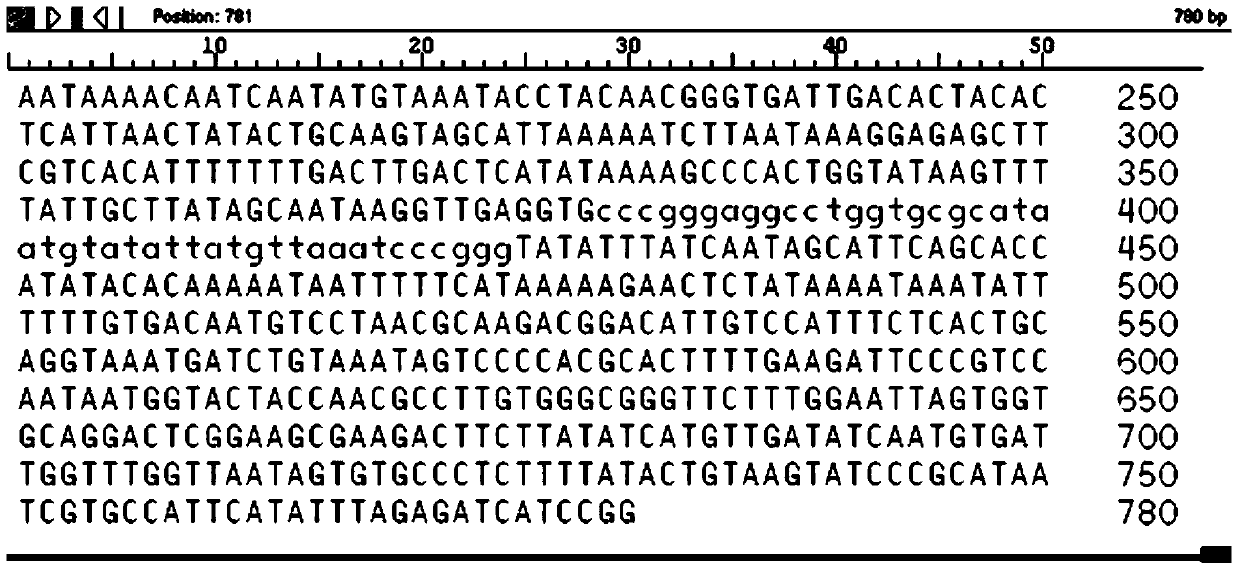
We determined the nucleotide sequence of the gene encoding staphylococcal enterotoxin A (entA). The gene, composed of 771 base pairs, encodes an enterotoxin A precursor of 257 amino acid residues.
Bacteroides fragilis and applications thereof
ActiveCN106399141AEffective for diarrheaGood probiotic propertiesBacteriaBacteria material medical ingredientsAntibiotic-associated diarrhoeaFood additive
The invention relates to Bacteroides fragilis and applications thereof, particularly to Bacteroides fragilis ZY-312 having the preservation number of CGMCC No.10685, and applications of the Bacteroides fragilis ZY-312 in preparation of medicines, pharmaceutical compositions, foods, health products and food additives for prevention and / or treatment of antibiotic-associated diarrhea, wherein the Bacteroides fragilis ZY-312 is preserved in the China general microbiological culture collection center on April 2, 2015, has the preservation number of CGMCC No.10685, does not contain enterotoxin gene bft, has characteristics of significantly-improved cholate resistance and significantly-improved gastric acid resistance compared to the existing Bacteroides fragilis, and can effectively prevent and / or treat antibiotic-associated diarrhea.
Owner:GUANGZHOU ZHIYI PHARMA INC
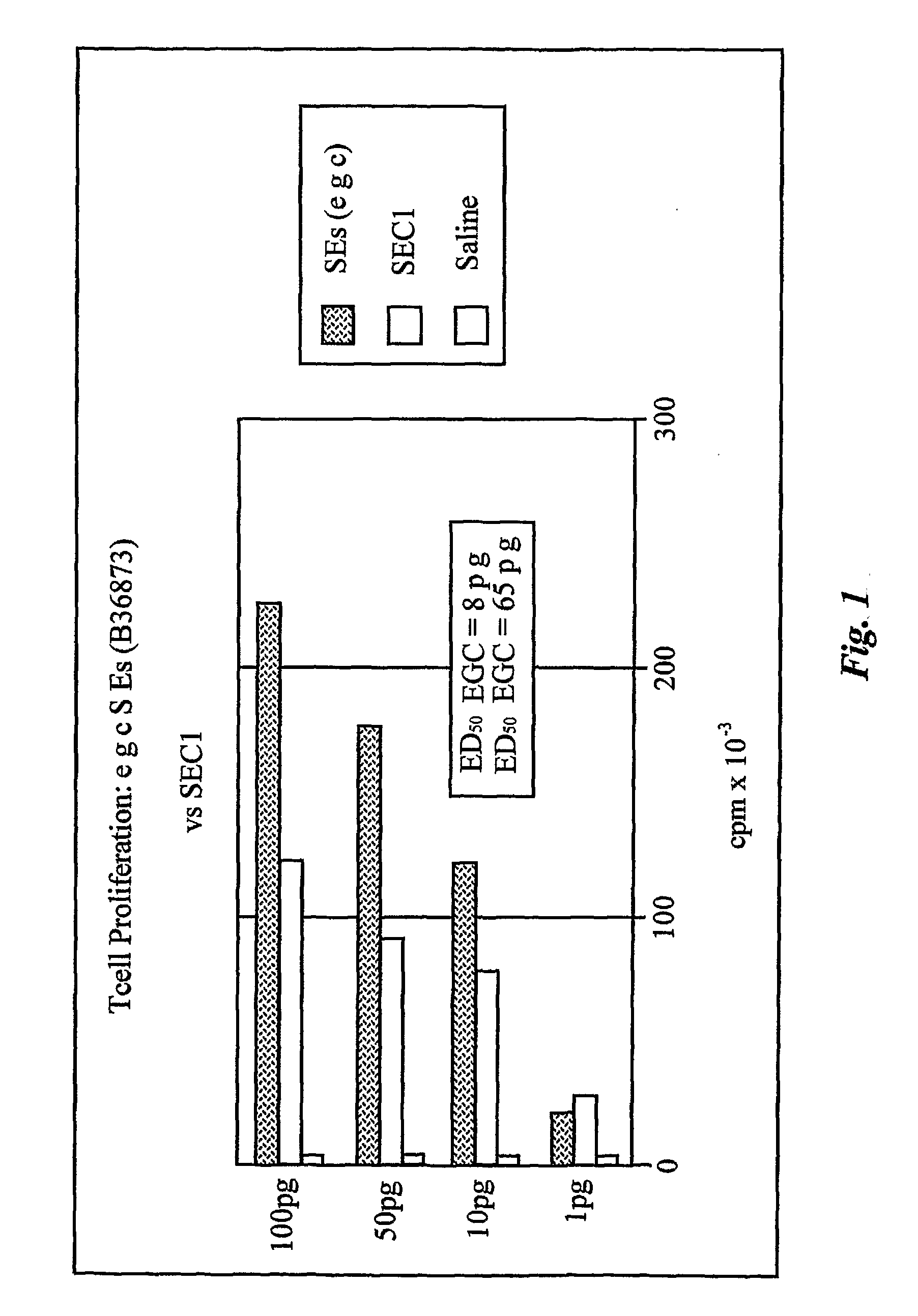
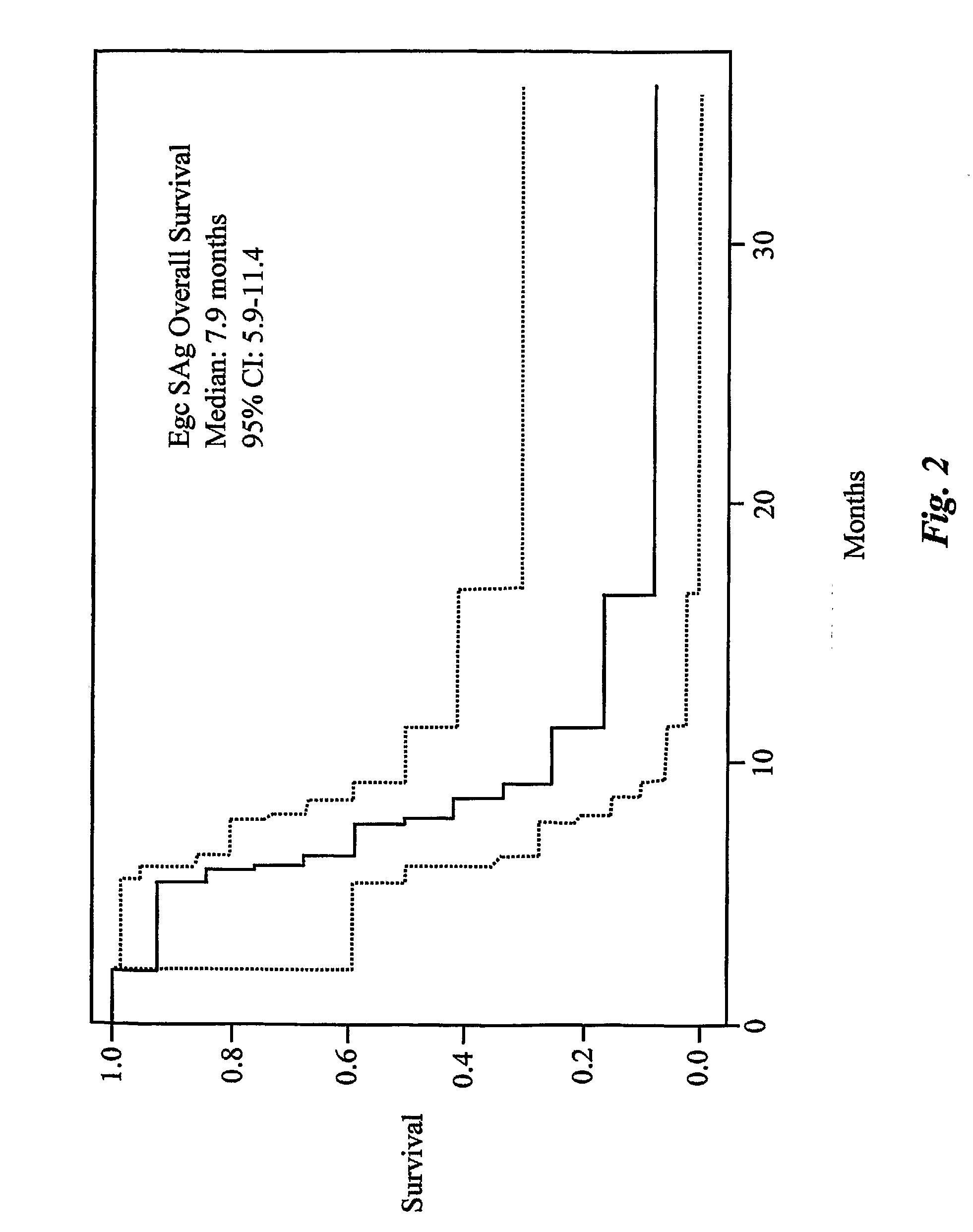
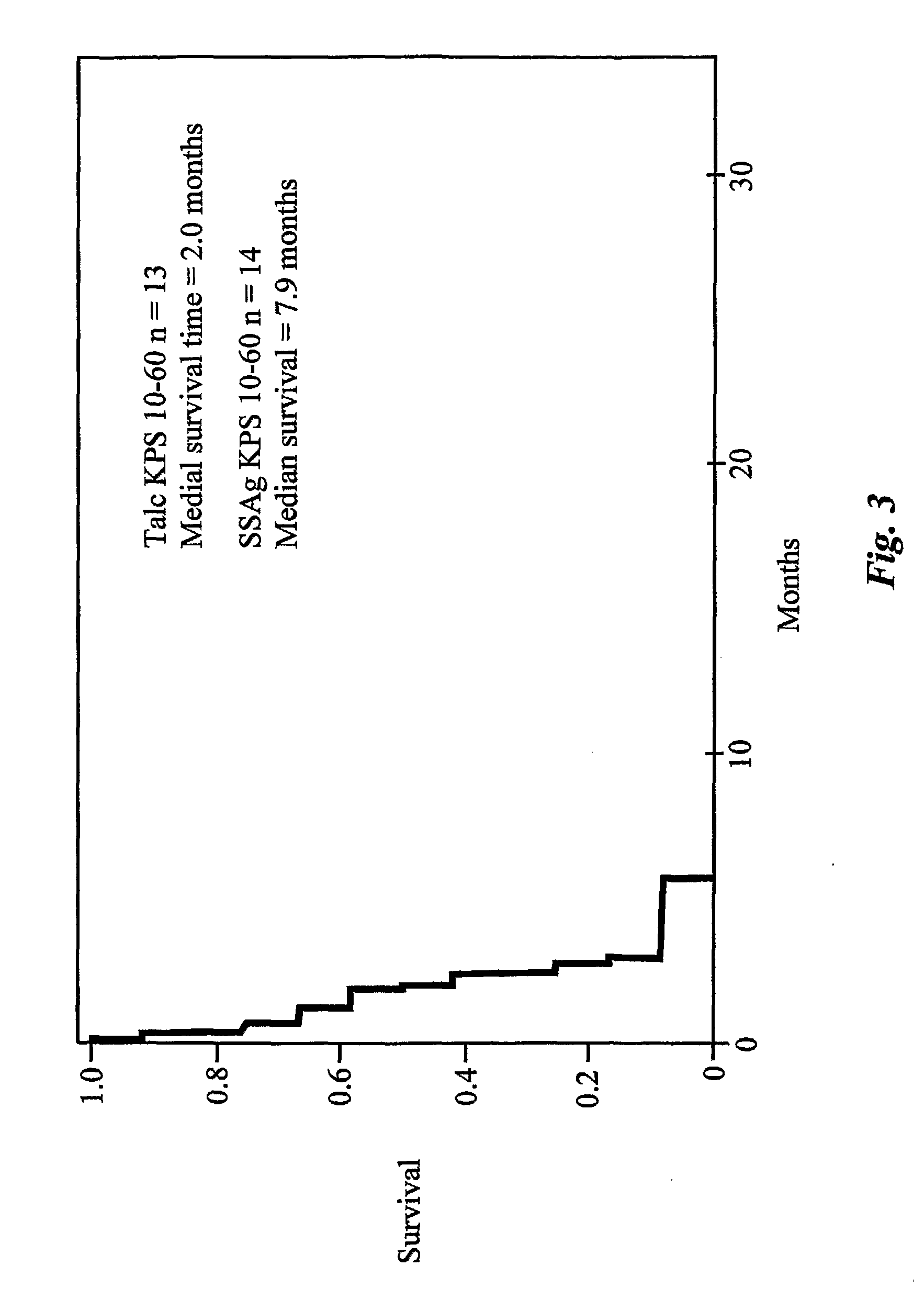
Enterotoxin gene cluster (egc) superantigens to treat malignant disease
InactiveUS20090162315A1Good effectPrevent morbidityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseSystemic chemotherapy
The use of classical superantigens for treatment of cancer has resulted in a low response rates and serious toxicity in humans which is attributable, in part, to the presence of preformed superantigen specific antibodies in the plasma of treated patients. The present invention addresses this problem by providing a method for treating tumors comprising the administration of one or a plurality of egc (enterotoxin gene cluster) staphylococcal enterotoxins comprising staphylococcal enterotoxins G, I, M, N, O. These superantigens in native unmodified form can be administered intrathecally, intratumorally, intravenously to humans with advanced lung cancer while resolving pleural effusions and prolonging survival to 300% above control patients treated with talc pleurodesis. Intratumoral egc superantigens induces a significant and sustained reduction of the tumor size. In contrast to classic Sags, the egc superantigens induced minimal toxicity, are rarely associated with the presence of preformed antibodies and are used as a plurality with a broad T cell Vβ profile. Useful egc superantigen compositions for parenteral administration native egc enterotoxins, homologues, fragments and fusion proteins of native egc enterotoxins capable of activating a broad spectrum of T cells expressing T cell receptor / α motifs. T cell survival-enhancing cytokines IL-7, Il-15, Il-23 are used. together with parenteral egc SE therapy. Also disclosed is combined therapy that includes parenteral, intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs or (iii) radiation therapy or (iv) anti-angiogenic and tyrosine kinase inhibitors.
Owner:TERMAN DAVID S +4
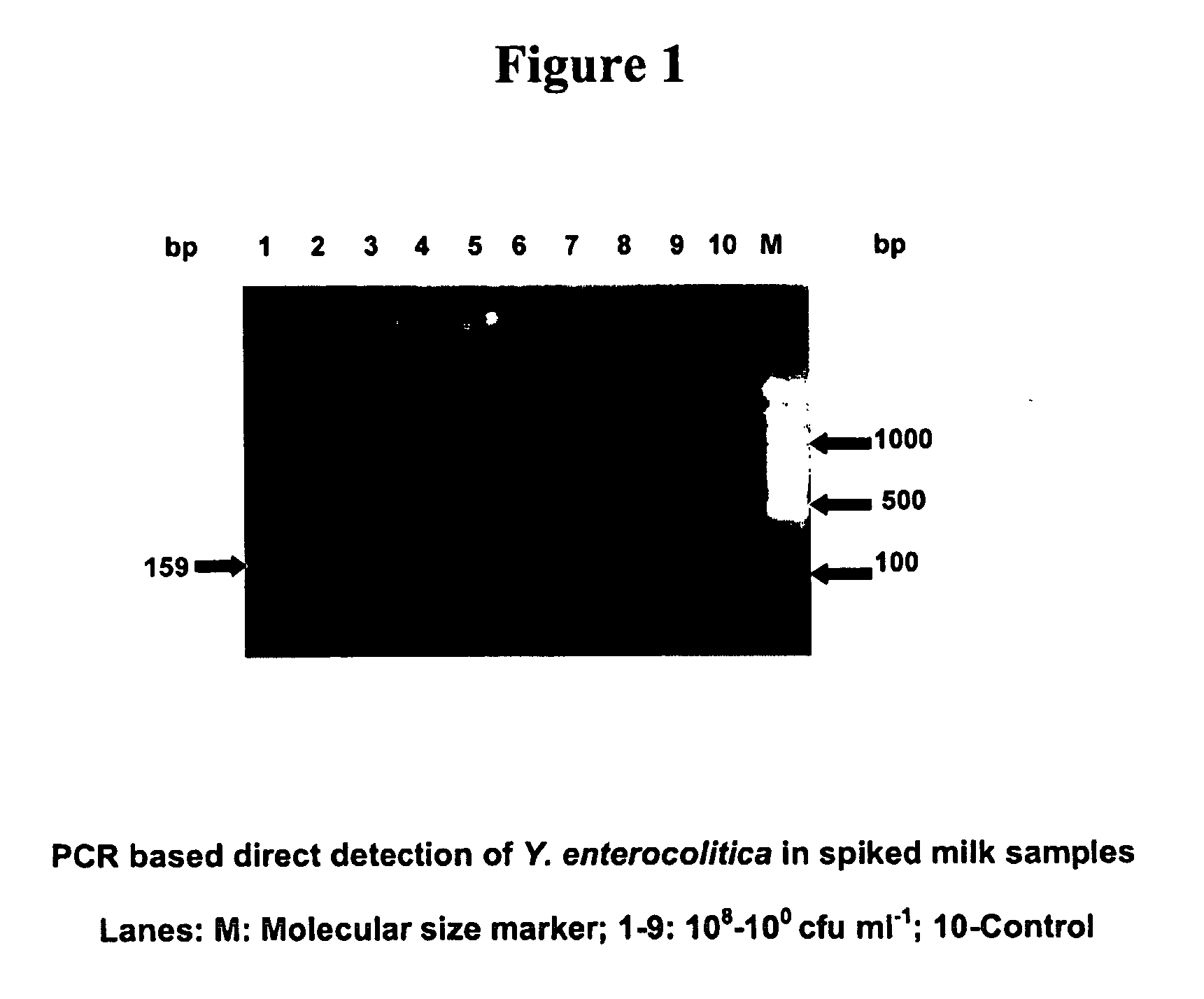
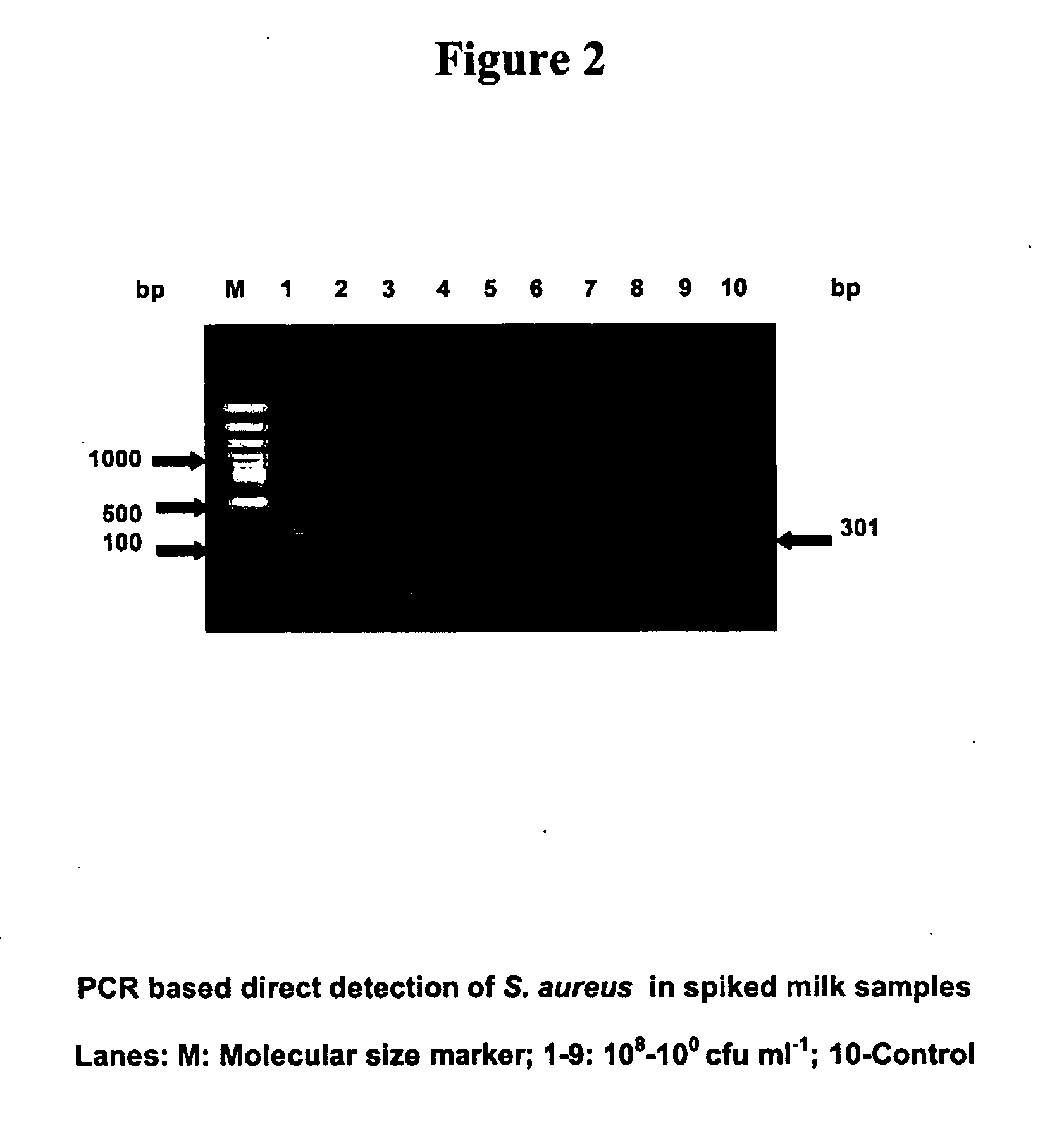
Primers for detecting food poisoning bacteria and a use thereof
InactiveUS20050233345A1Sensitive to useHighly sensitive and quick useSugar derivativesMicrobiological testing/measurementE coli heat stable toxinStaphylococcus aureus
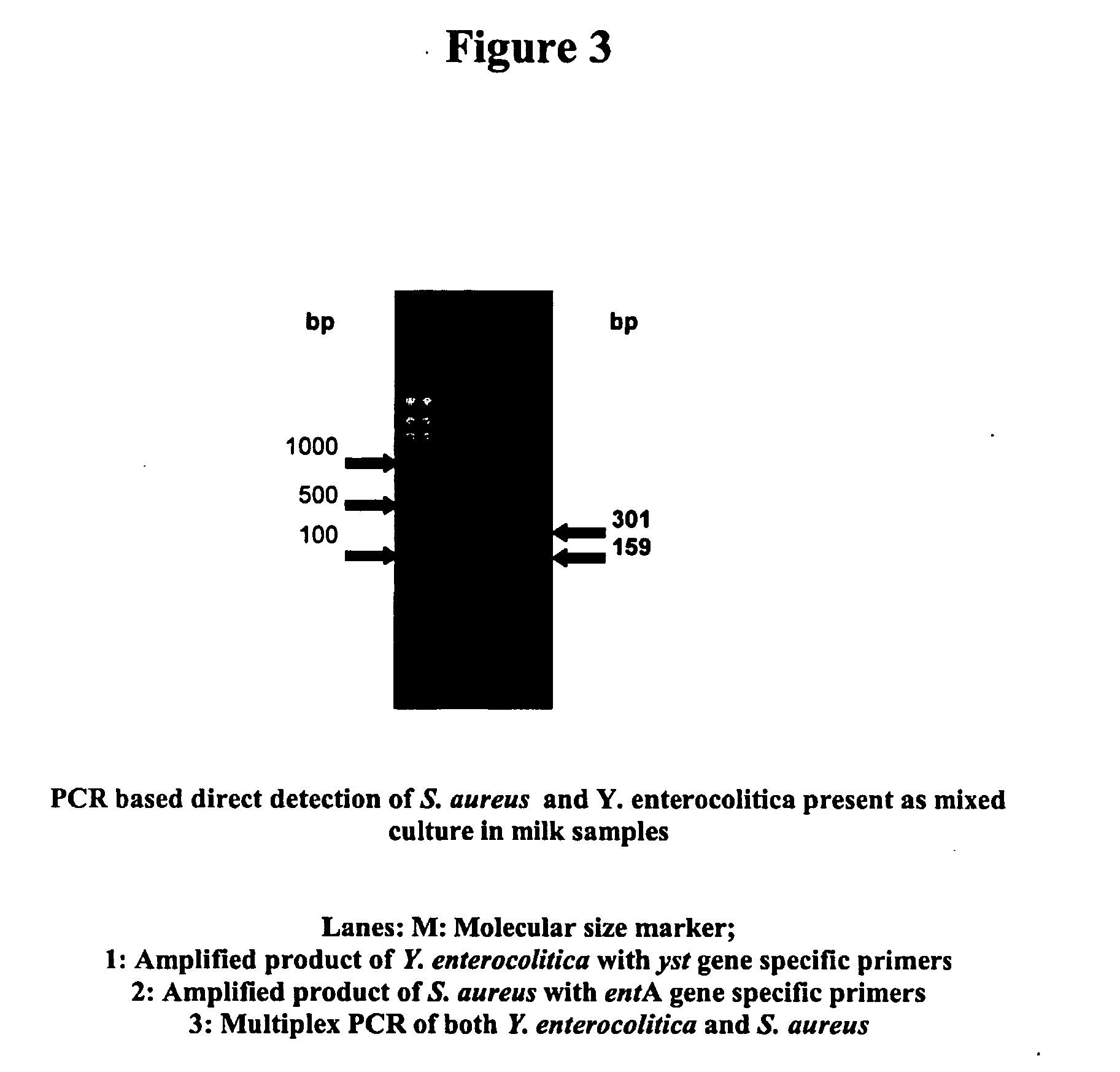
Provided are novel primers directed against enterotoxin A gene (ent A) of bacteria Staphylococcus aureus and primers directed against heat stable enterotoxin gene (yst) of bacteria Yersinia enterocolitica, for detecting poisoning in food articles. Also provided is a highly sensitive method for detecting bacterial food poisoning using the primers.
Owner:COUNCIL OF SCI & IND RES
Applications of Bacteroides fragilis in treatment and/or prevention of obesity or diabetes mellitus
ActiveCN106389478APrevent obesityPrevent diabetesMetabolism disorderUnknown materialsFood additiveBacteroides tectus
The invention relates to applications of Bacteroides fragilis in treatment and / or prevention of obesity or diabetes mellitus, particularly to applications of Bacteroides fragilis in preparation of pharmaceutical compositions, foods, health products and food additives for treatment and / or prevention of obesity or diabetes mellitus, wherein the Bacteroides fragilis is ZY-312, is preserved in the China general microbiological culture collection center on April 2, 2015, and has the preservation number of CGMCC No.10685. According to the present invention, the Bacteroides fragilis provides good effects for treatment and / or prevention of obesity or diabetes mellitus, has characteristics of no drug resistance generation, safety, no toxicity, no enterotoxin gene bft and broad application prospects, and further has beneficial characteristics of cholate resistance, gastric acid resistance and the like compared with the existing strains.
Owner:GUANGZHOU ZHIYI PHARMA INC
Fusion of multiple enterotoxin genes of escherichia coli and application thereof
ActiveCN101914564AImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInfant animal
The invention relates to an escherichia coli (E. coli) trivalent enterotoxin fusion gene and application thereof, and belongs to the field of genetic engineering subunit vaccines. The enterotoxigenic E. coli (ETEC) is a main pathogen causing baby-animal and infantile diarrhea. Aiming at the defects that the conventional TEC vaccine has narrow protection range and cannot induce high-level antitoxin, the invention designs a trivalent E. coli enterotoxin fusion with the fusion mode of 5'-STa-LT-STb-3' or 5'-STb-LT-STa-3'. After the multivalent enterotoxin gene or protein immunizes animals, high-level STa, STb and LT resistant antibodies can be induced to be generated. Therefore, the escherichia coli (E. coli) trivalent enterotoxin fusion gene can neutralize the toxicity of a key pathogenic factor enterotoxin, improve the immunity protection rate and widen the protection range without being limited by E. coli pilus types so as to effectively control ETEC diarrhea.
Owner:DALIAN UNIV OF TECH
Recombinant superantigen SEB mutant, preparation method and applications thereof
ActiveCN103965302AHigh activityImprove anti-tumor effectBacteriaPeptide/protein ingredientsAntigenAntineoplastic Immunotherapeutic
The present invention discloses a recombinant superantigen staphylococcal enterotoxin B (SEB) mutant, and a preparation method and applications thereof. According to the present invention, a SEB genome derived from natural staphylococcus aureus is extracted, and after the SEB genome is obtained, site-specific mutagenesis at toxicity-related amino acid sites is performed to obtain the SEB mutant. The SEB mutant has characteristics of both enhanced activity and weakened toxicity, and can be used as anti-tumor immune therapy drugs or immune system modulating drugs. The recombinant superantigen SEB mutant has good market prospects.
Owner:军事科学院军事医学研究院微生物流行病研究所
Method for rapidly detecting and parting staphylococcal enterotoxin in food by fluorescent polymerase chain reaction (PCR) method
InactiveCN102061336AInnovativeHigh speedMicrobiological testing/measurementMicroorganismStaphylococcal Enterotoxins
The invention belongs to the field of molecular detection for microbial toxin in food science and technology, in particular to rapid detection and parting of 11 serotypes of staphylococcal enterotoxin in food by applying the flows such as total DNA component extraction and SYBRGreen fluorescent quantitative PCR (polymerase chain reaction) rapid detection. Results show that the staphylococcal enterotoxin in food can be effectively typed according to the situation whether marked amplification curves appear in 35 PCR cycles by utilizing the method, thus the method has the characteristics of specificity aiming at enterotoxin genes of different serotypes, sensitivity, accuracy, rapidness and convenience and the like; and compared with the existing method, the method provided by the invention has the advantages of greatly improving the detection and parting efficiency and providing a new way for risk analysis of food pollution caused by the staphylococcal enterotoxin of different types.
Owner:NANJING INST OF PROD QUALITY INSPECTION
Method for detecting staphylococcus aureus and enterotoxin genotypes thereof through multiple PCR (polymerase chain reaction)
ActiveCN102653792ASave testing timeSave testing costMicrobiological testing/measurementMicroorganism based processesFood poisoningStaphyloccocus aureus
The invention provides a method for detecting staphylococcus aureus and enterotoxin genotypes of the staphylococcus aureus by utilizing multiple PCR (polymerase chain reaction) technology. The main technical scheme is as follows: designing primer sequences and optimizing a multiple PCR system and conditions. Enterotoxins and invasive enzymes of staphylococcus aureus affect the pathogenicity of staphylococcus aureus and the enterotoxins which have been identified already are A, B, C1-C3, D, E, G, H, I, J, L, M and N. Generally speaking, food poisoning is commonly caused by A and D and less commonly caused by B and C, the occurrence rate of food poisoning related to the toxin E is the lowest, and the enterotoxins have different toxicity, wherein the toxin A has stronger toxicity and D has weaker toxicity. Aiming at the common enterotoxin genotypes of staphylococcus aureus, the invention overcomes the defects in the prior art and provides the method for detecting staphylococcus aureus and enterotoxin genotypes of the staphylococcus aureus in food by utilizing the multiple PCR technology. The method can be used for simultaneously detecting staphylococcus aureus generating enterotoxins A, B, C and D in the same reaction system.
Owner:嘉兴实践医学科技有限公司
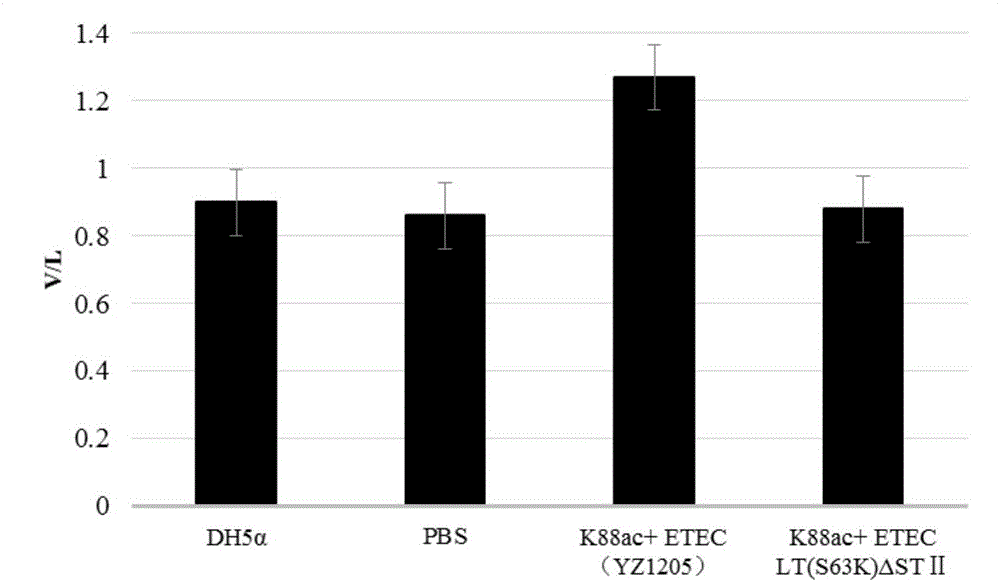
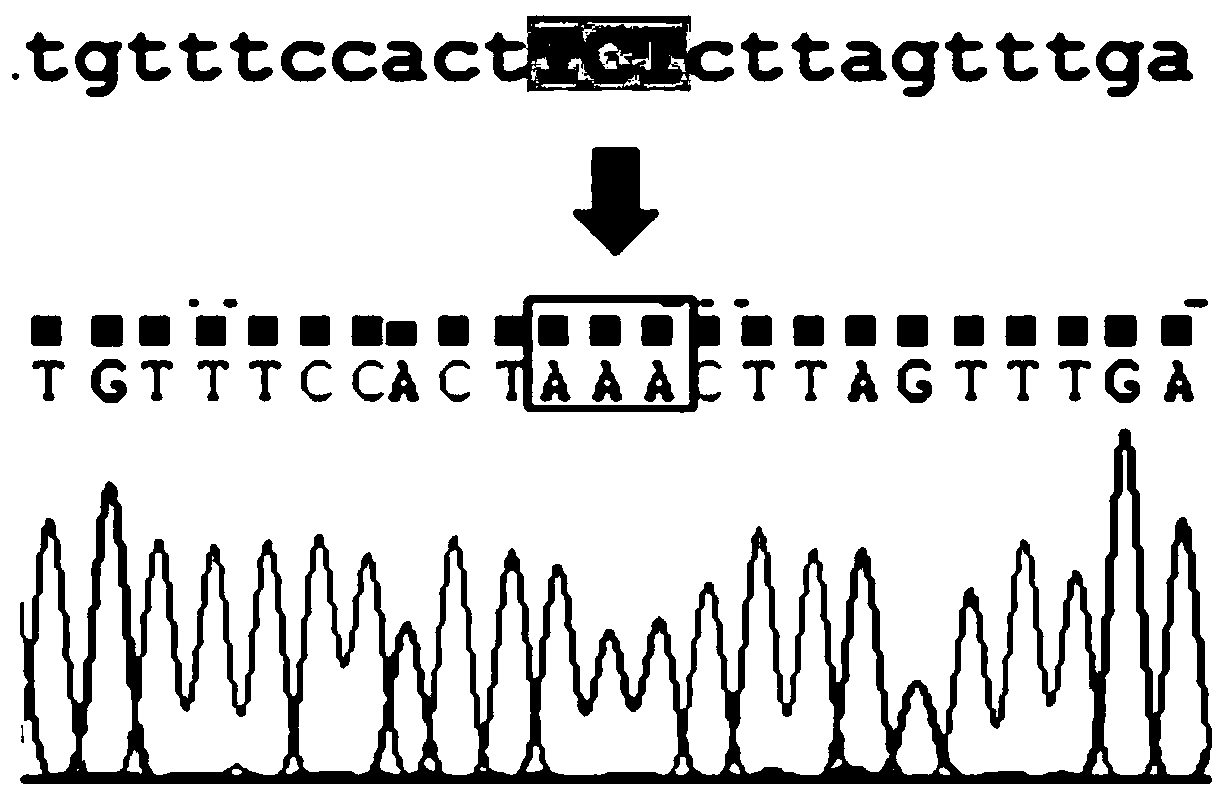
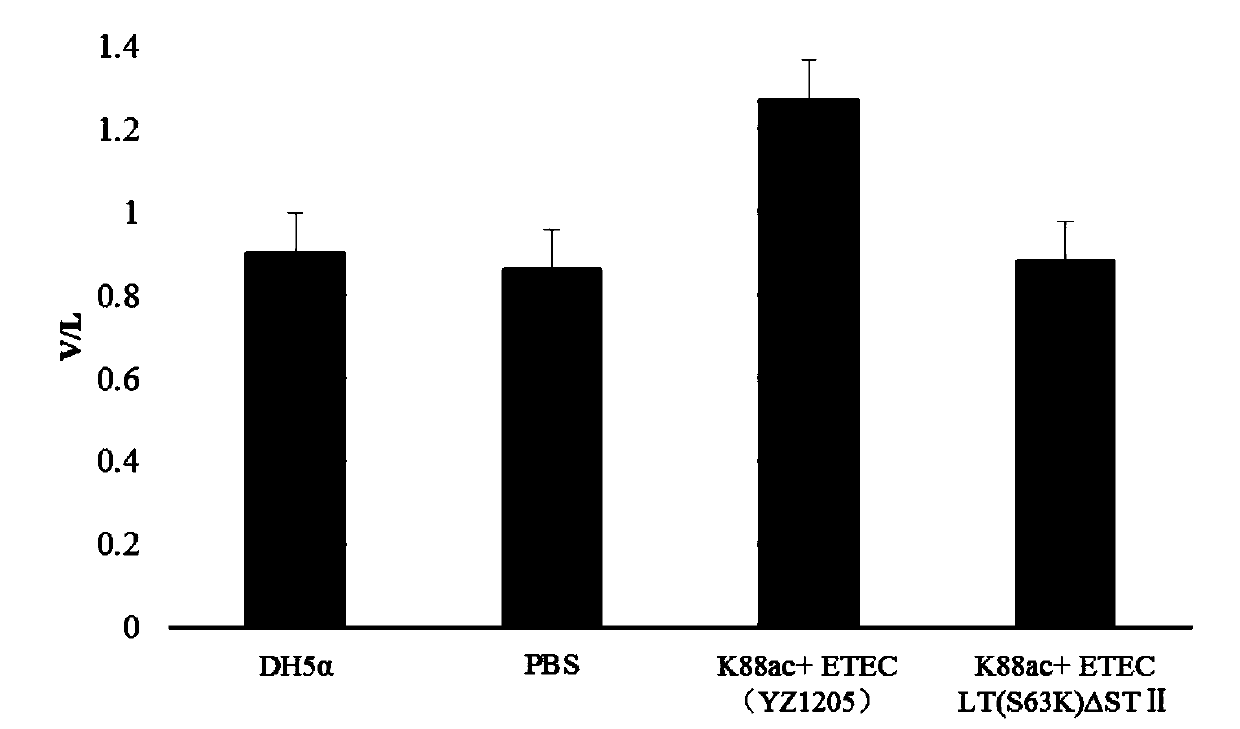
Method for constructing K88ac<+> enterotoxigenic escherichia coli attenuated virus strain and application
ActiveCN106148376AAvoid Easy Lost SituationsDoes not affect treatmentAntibacterial agentsBacteria material medical ingredientsEscherichia coliAntibiotic Y
The invention provides a method for constructing a K88ac<+> enterotoxigenic escherichia coli attenuated virus strain. The method includes the following steps that 1, K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are subjected to traceless point mutation, wherein TCT on the LT I gene of K88ac<+> enterotoxigenic escherichia coli without antibiotics resistance genes is mutated into AAA, and correspondingly-encoded No. 63 serine is mutated into lysine (K); 2, the K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are knocked out, wherein on the basis of the first step, the ST II genes in the K88ac<+> enterotoxigenic escherichia coli are knocked out, and therefore the aim of attenuating the K88ac<+> enterotoxigenic escherichia coili is achieved accordingly. The attenuated virus strain constructed with the method is high in adhesivity and long in in-vivo planting time, infection of the pathopoiesia stain can be prevented, and the method can be used for biological preventing of colibacillosis caused by K88ac<+> ETEC.
Owner:YANGZHOU UNIV
Stn gene oligonucleotide primers for detecting salmonella species and detection process using the same
InactiveCN1771331BMicroencapsulation basedMicrobiological testing/measurementSpecific detectionSalmonella chandans
Owner:科学和工业研究委员会
Vibrio cholerae multiplex fluorescence PCR detection kit as well as preparation and application thereof
ActiveCN105603091AShort experiment cycleImprove detection efficiencyMicrobiological testing/measurementAgainst vector-borne diseasesAntigenFluorescence
The invention belongs to the field of biotechnological detection and particularly relates to a vibrio cholerae multiplex fluorescence PCR detection kit as well as preparation and application thereof. The kit provided by the invention comprises a vibrio cholerae hemolysin gene detection primer and a probe, a vibrio cholerae O1 antigen gene detection primer and a probe, vibrio cholerae O139 antigen gene detection primer and a probe and a vibrio cholerae enterotoxin gene detection primer and a probe. The kit has high detection sensitivity, the lowest detection limit is 1*10<3>copy / ml, and the accuracy and positive rate both reach 100%.
Owner:广东省疾病预防控制中心 +1
Applications of Bacteroides fragilis in animal breeding
ActiveCN106387314AOvercome disadvantages such as easy deactivationHas probiotic propertiesBacteriaDigestive systemDiseaseEcological environment
The invention relates to applications of Bacteroides fragilis in animal breeding, particularly to applications of Bacteroides fragilis in prevention and / or treatment of animal gastrointestinal diseases and in pharmaceutical compositions and feed additives, wherein the Bacteroides fragilis is ZY-312 and has the preservation number of CGMCC No.10685. According to the present invention, the Bacteroides fragilis ZY-312 does not contain the enterotoxin gene bft, has significant and high capabilities of cholate resistance and gastric acid resistance compared with the existing Bacteroides fragilis, can effectively prevent and / or treat animal gastrointestinal diseases, improves animal immunity and disease resistance, reduces animal prevalence, improves animal feed conversion rate, increases animal weight, promotes growth and development and production performance, can reduce the use of antibiotics, does not produce drug resistance, further has characteristics of safety and no toxicity, can improve the quality of products such as meat, eggs, milk and the like, can eliminate antibiotic drug residues, purify mew environment, and protects ecological environment.
Owner:GUANGZHOU ZHIYI PHARMA INC
Use of Bacteroides fragilis in the treatment and/or prevention of obesity or diabetes
ActiveCN106389478BPrevent obesityPrevent diabetesMetabolism disorderUnknown materialsFood additiveDiabetes mellitus
Provided is an application of bacteroides fragilis in treating and / or preventing obesity or diabetes, and also provided is an application of the bacteroides fragilis in preparing pharmaceutical compositions, food, healthcare products, and food additives for treating and / or preventing obesity or diabetes. The bacteroides fragilis is bacteroides fragilis ZY-312.
Owner:GUANGZHOU ZHIYI PHARMA INC
Applications of Bacteroides fragilis in prevention and/or treatment of meningitis
ActiveCN106389475AHas probiotic propertiesBile saltAntibacterial agentsNervous disorderFood additiveBacteroides tectus
The invention relates to applications of Bacteroides fragilis in prevention and / or treatment of meningitis, particularly to applications of Bacteroides fragilis in preparation of drugs, pharmaceutical compositions, foods, health products and food additives for prevention and / or treatment of meningitis, wherein the Bacteroides fragilis is ZY-312, is preserved in the China general microbiological culture collection center on April 2, 2015, and has the preservation number of CGMCC No.10685. According to the present invention, the Bacteroides fragilis provides good effects for prevention and / or treatment of meningitis, has characteristics of no drug resistance generation, safety, no toxicity, no enterotoxin gene bft and broad application prospects, and further has beneficial characteristics of cholate resistance, gastric acid resistance and the like compared with the existing strains.
Owner:石家庄普维生物科技有限公司
Stn gene oligonucleotide primers for detecting salmonella species and detection process using the same
InactiveCN1771331AMicroencapsulation basedMicrobiological testing/measurementSpecific detectionA-DNA
The present invention relates to oligonucleotide primers having SEQ ID NOs. 1 to 21 specific for Salmonella enterotoxin gene (stn) gene, useful for rapid and specific screening of Salmonella. The present invention relates to a process for the rapid and specific detection of Salmonella enterotoxin gene (stn) gene in a subject for the presence of parasite Salmonella using oligonucleotide primers having SEQ ID NOs. 1 to 21 for Polymerase Chain Reaction (PCR), said process comprising the steps of preparing a DNA template, amplifying the template using the primers by PCR, running the PCR products on gel, and detecting the Salmonella.
Owner:科学和工业研究委员会
Oligonucleotide primers having SEQ ID NOs. 1 to 21 and a process for detection of parasite Salmonella using oligonucleotide primers
ActiveUS7041482B2Efficient and rapid detectionSugar derivativesMicrobiological testing/measurementSpecific detectionA-DNA
The present invention relates to oligonucleotide primers having SEQ ID NOs. 1 to 21 specific for Salmonella enterotoxin gene (stn) gene, useful for rapid and specific screening of Salmonella. The present invention relates to a process for the rapid and specific detection of Salmonella enterotoxin gene (stn) gene in a subject for the presence of parasite Salmonella using oligonucleotide primers having SEQ ID NOs. 1 to 21 for Polymerase Chain Reaction (PCR), said process comprising the steps of preparing a DNA template, amplifying the template using the primers by PCR, running the PCR products on gel, and detecting the Salmonella.
Owner:COUNCIL OF SCI & IND RES
Oligonucleotide primers of SEQ ID NOs. 1 to 21 and a process for detection of a parasite Salmonella using oligonucleotide primers
The present invention relates to oligonucleotide primers having SEQ ID NOs. 1 to 21 specific for Salmonella enterotoxin gene (stn) gene, useful for rapid and specific screening of Salmonella. The present invention relates to a process for the rapid and specific detection of Salmonella enterotoxin gene (stn) gene in a subject for the presence of parasite Salmonella using oligonucleotide primers having SEQ ID NOs. 1 to 21 for Polymerase Chain Reaction (PCR), said process comprising the steps of preparing a DNA template, amplifying the template using the primers by PCR, running the PCR products on gel, and detecting the Salmonella.
Owner:QAZI GHULAM NABI +2
Construction method, expression system and application of multi-union fusion recombinant protein capable of preventing piglet diarrhea
ActiveCN113683706AStrong specificityGood effectAntibacterial agentsBacterial antigen ingredientsTGE VACCINETitin Antibody
The invention provides a construction method, an expression system and application of a multi-union fusion recombinant protein capable of preventing piglet diarrhea, and relates to the technical field of biological medicines. According to the construction method, the expression system and application of the multi-union fusion recombinant protein capable of preventing piglet diarrhea, heat-resistant enterotoxin and heat-labile enterotoxin genes of ETEC and clostridium welchii alpha toxin and beta 1 toxin genes are cloned, functional areas of part of the genes are connected in series by using an SOE-PCR method, an LTI-LTII-ST-Cp recombinant sequence is amplified, an expression vector is successfully constructed, and the recombinant protein LTI-LTII-ST-Cp is successfully expressed. Each segment of the recombinant protein LTI-LTII-ST-Cp is good in specificity, the recombinant protein is non-toxic after immunizing mice, the antibody titer is relatively high, and the recombinant protein has a very good protection effect on diarrhea. According to the construction method, the expression system and application of the multi-union fusion recombinant protein capable of preventing the piglet diarrhea, the protection rate of the prepared vaccine on piglet red scour and piglet yellow scour can reach 80% or above, the effect is good, and the vaccine has very high protection capacity.
Owner:潍坊峡山维泰生物科技有限公司
Fusion of multiple enterotoxin genes of escherichia coli and application thereof
ActiveCN101914564BImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsAntitoxinGenetic engineering
The invention relates to an escherichia coli (E. coli) trivalent enterotoxin fusion gene and application thereof, and belongs to the field of genetic engineering subunit vaccines. The enterotoxigenic E. coli (ETEC) is a main pathogen causing baby-animal and infantile diarrhea. Aiming at the defects that the conventional TEC vaccine has narrow protection range and cannot induce high-level antitoxin, the invention designs a trivalent E. coli enterotoxin fusion with the fusion mode of 5'-STa-LT-STb-3' or 5'-STb-LT-STa-3'. After the multivalent enterotoxin gene or protein immunizes animals, high-level STa, STb and LT resistant antibodies can be induced to be generated. Therefore, the escherichia coli (E. coli) trivalent enterotoxin fusion gene can neutralize the toxicity of a key pathogenic factor enterotoxin, improve the immunity protection rate and widen the protection range without being limited by E. coli pilus types so as to effectively control ETEC diarrhea.
Owner:DALIAN UNIV OF TECH
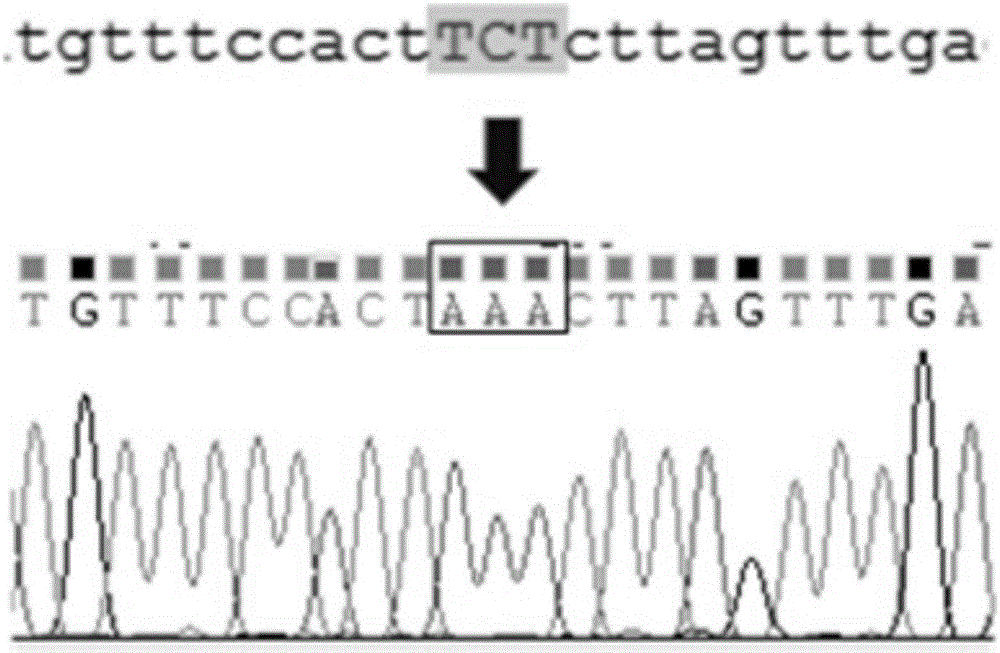
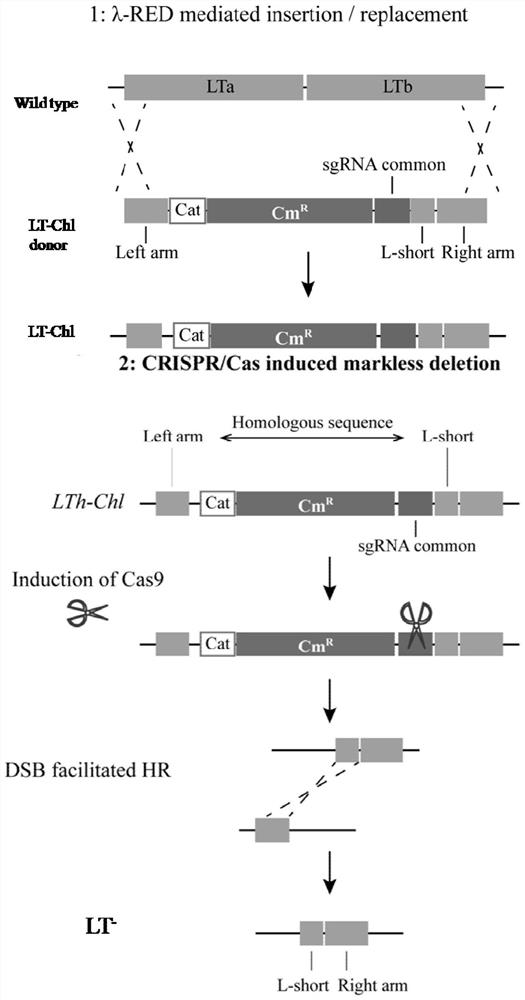
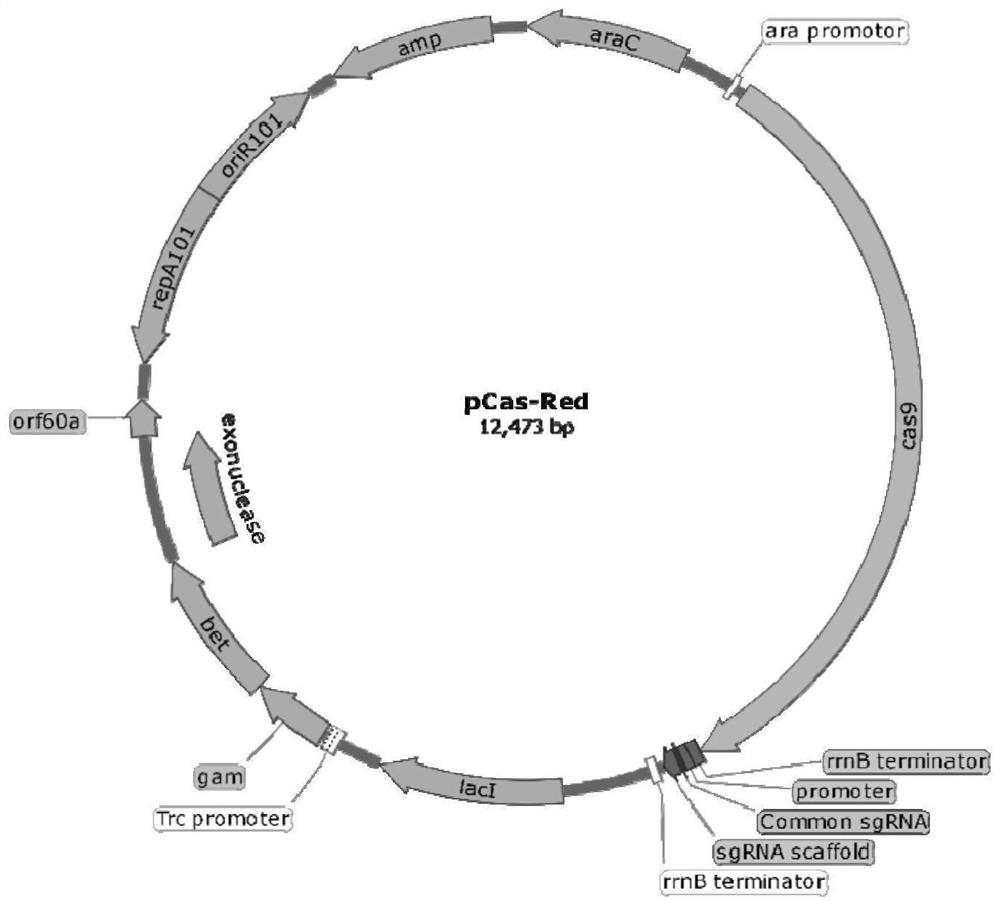
A kind of enterotoxin gene lt knockout Escherichia coli and its construction method
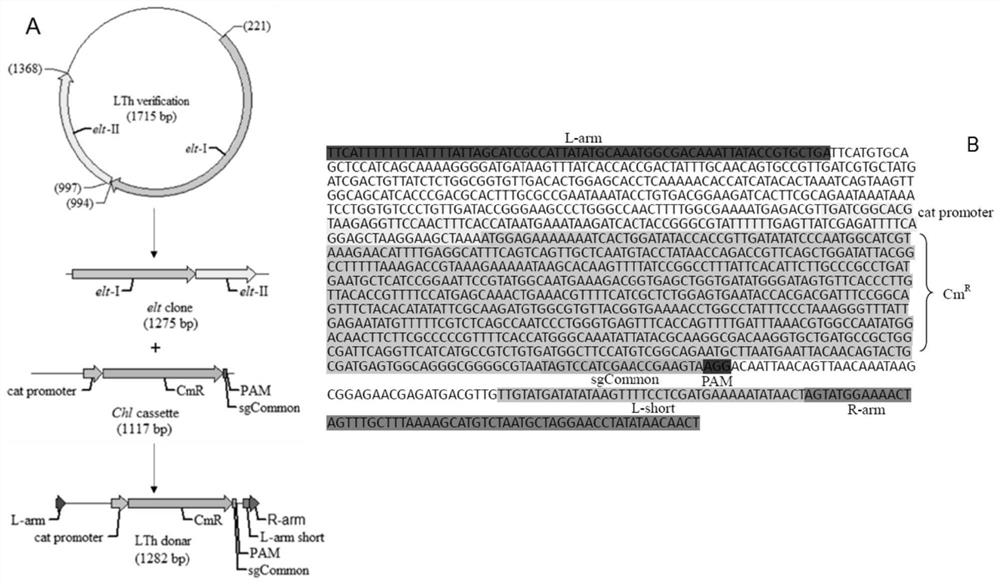
ActiveCN110551668BFast and seamless knockoutConvenient and seamless knockoutBacteriaStable introduction of DNAEscherichia coliBase J
The invention discloses a method for constructing Escherichia coli with enterotoxin gene LT knockout, comprising the following steps: 1) Constructing LT gene homologous recombination donor LT donar, the base sequence of LT donar includes L-arm, cat promoter in sequence , CmR, sgCommon, PAM, L-short, R-arm polynucleotide fragments; and 2) prepare competent cells containing pCas-Red plasmid, add step 1) LT donar to transform, and then screen positive clones to obtain Escherichia coli with traceless knockout of the heat-labile enterotoxin gene LT. The present invention combines Crispr / Cas9 with the λ-Red homologous recombination system to successfully knock out the LT gene of enterotoxigenic Escherichia coli K88, and the mutant strain loses hemolytic ability and slows down growth; , Convenient and seamless knockout of enterotoxigenic Escherichia coli K88 gene, which provides a solid research basis for the study of LT function.
Owner:ANIMAL SCI RES INST GUANGDONG ACADEMY OF AGRI SCI
Swine Escherichia coli non-toxic isolate capable of simultaneously expressing F4 and F18 pili and application of swine Escherichia coli non-toxic isolate
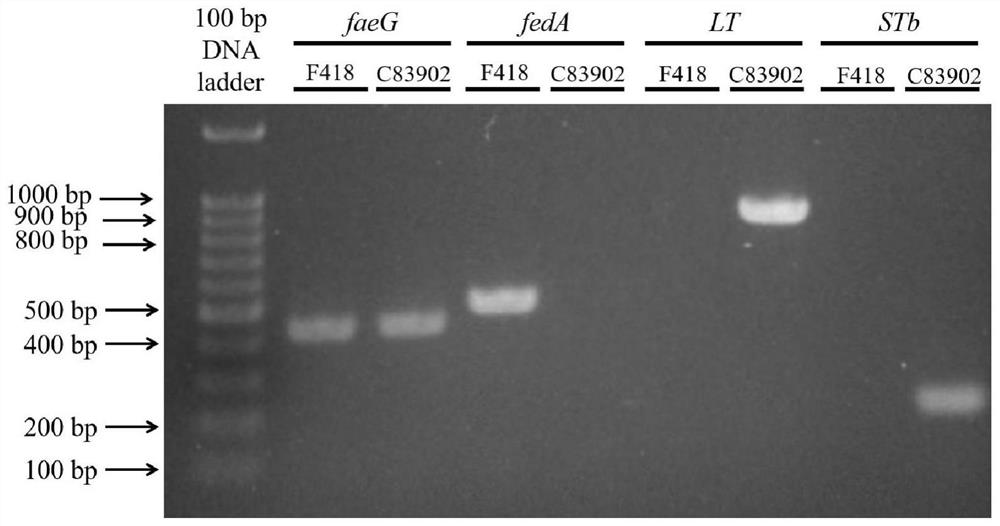
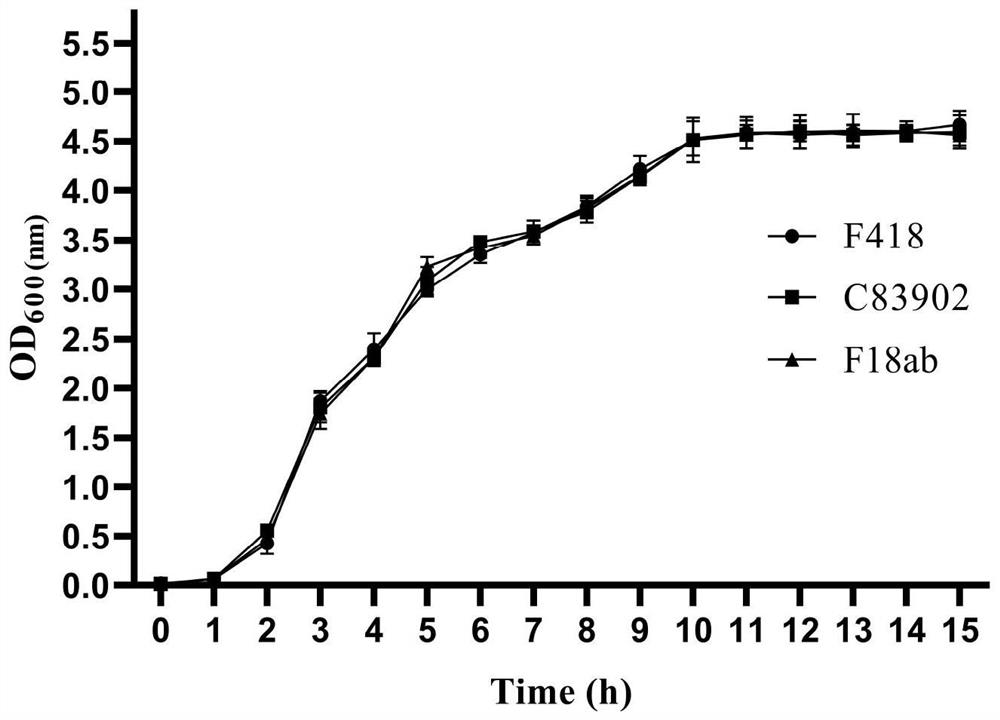
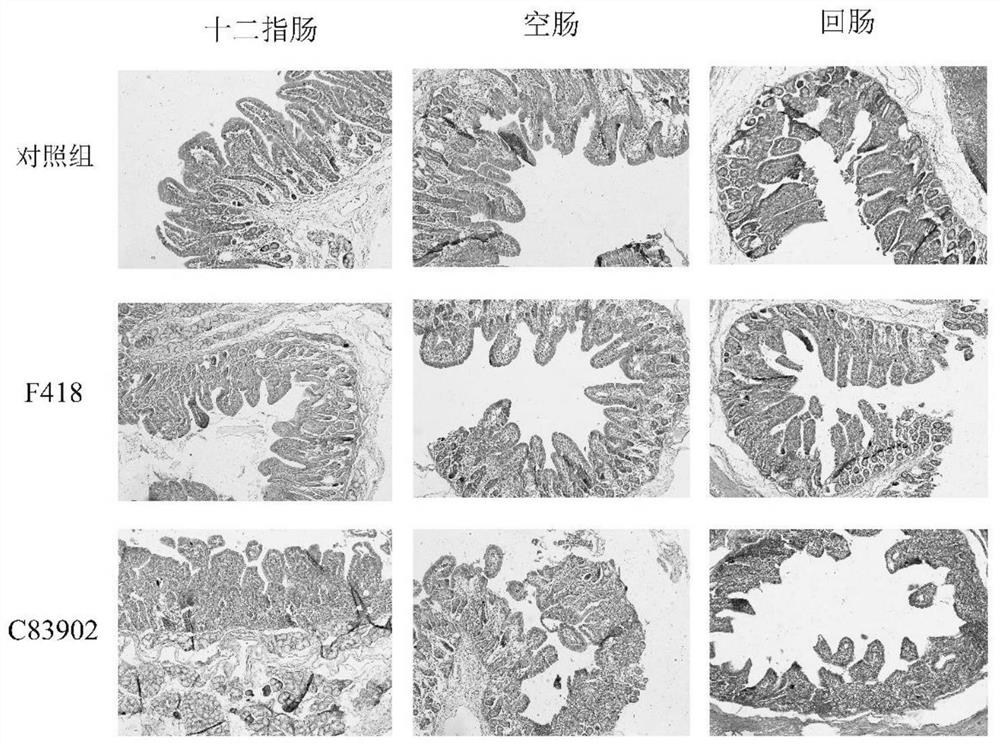
ActiveCN114752531AImprove adhesionGood adhesion colonizationAntibacterial agentsBacteriaAntigenPilus
The invention relates to a swine Escherichia coli non-toxic isolated strain capable of simultaneously expressing F4 and F18 pili and application of the swine Escherichia coli non-toxic isolated strain, the preservation number of the swine Escherichia coli non-toxic isolated strain F418 is CCTCC M 2022089, and the preservation date is January 18, 2022. The isolated strain is escherichia coli, can simultaneously express F4 and F18 fimbriae antigens and is a swine-derived natural isolated strain, PCR (Polymerase Chain Reaction) tests show that the strain lacks LT and STb enterotoxin genes, does not generate enterotoxin, can be well adhered and colonized to a host pig, has the potential of being developed into a bacterial non-toxic live vaccine candidate strain, and can be used for preparing a bacterial non-toxic live vaccine candidate strain. The strain can be well adhered and colonized to a host pig by oral administration, a piglet is induced to generate immune response for resisting F4 and F18 pilus antigens, a certain immune efficacy is exerted, the safety is guaranteed, and the strain is expected to become a potential live vaccine candidate strain for preventing and controlling F4 and F18 escherichia coli infection.
Owner:YANGZHOU UNIV
Construction method and application of a k88ac+ attenuated strain of enterotoxigenic Escherichia coli
ActiveCN106148376BAvoid Easy Lost SituationsDoes not affect treatmentAntibacterial agentsBacteria material medical ingredientsEscherichia coliVirus strain
The invention provides a method for constructing a K88ac<+> enterotoxigenic escherichia coli attenuated virus strain. The method includes the following steps that 1, K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are subjected to traceless point mutation, wherein TCT on the LT I gene of K88ac<+> enterotoxigenic escherichia coli without antibiotics resistance genes is mutated into AAA, and correspondingly-encoded No. 63 serine is mutated into lysine (K); 2, the K88ac<+> enterotoxigenic escherichia coli enterotoxin genes are knocked out, wherein on the basis of the first step, the ST II genes in the K88ac<+> enterotoxigenic escherichia coli are knocked out, and therefore the aim of attenuating the K88ac<+> enterotoxigenic escherichia coili is achieved accordingly. The attenuated virus strain constructed with the method is high in adhesivity and long in in-vivo planting time, infection of the pathopoiesia stain can be prevented, and the method can be used for biological preventing of colibacillosis caused by K88ac<+> ETEC.
Owner:YANGZHOU UNIV
Use of Bacteroides fragilis in the prevention and/or treatment of meningitis
ActiveCN106389475BHas probiotic propertiesBile saltBacteriaBacteria material medical ingredientsMicroorganismFood additive
The application of Bacteroides fragilis in the prevention and / or treatment of meningitis, specifically relates to the application of Bacteroides fragilis in the preparation of medicines, pharmaceutical compositions, food, health products and food additives for the prevention and / or treatment of meningitis, wherein , said Bacteroides fragilis is Bacteroides fragilis ZY‑312, which was deposited in China General Microorganism Culture Collection and Management Center on April 2, 2015, and its preservation number is CGMCC No.10685. The Bacteroides fragilis of the present invention has a good effect on the prevention and / or treatment of meningitis, does not produce drug resistance, is safe and non-toxic, does not contain enterotoxin gene bft, and has bile salt resistance, It has probiotic properties such as gastric acid resistance and has broad application prospects.
Owner:石家庄普维生物科技有限公司
CPA detection primers, detection kit and detection method for enterotoxin B-producing staphylococcus aureus
PendingCN113667766AShort cycleImprove diagnosis rateMicrobiological testing/measurementMicroorganism based processesStaphylococcus aureus enterotoxin BNucleotide
The invention discloses CPA detection primers, a detection kit and a detection method for enterotoxin B-producing staphylococcus aureus. The CPA primers comprise stripping primers 4s and 5a, a cross amplification primer 2a1s, and specific primers 2a and 3a, wherein the nucleotide sequences of the above primers are respectively as shown in SEQ ID NO. 1 to SEQ ID NO. 5. A cross primer isothermal amplification reaction detection and identification system designed for the gene sequence of staphylococcus aureus enterotoxin B in by the invention overcomes the defects of long period, low sensitivity, high cost, difficulty in field application and the like of methods in the prior art. By selecting a conserved region of an enterotoxin B gene sequence of a target strain, a pair of the stripping primers, the cross primer and the specific primers are designed to construct a cross primer isothermal amplification reaction system, and a detection result is obtained within about 60 minutes, so the period of detecting enterotoxin B-producing staphylococcus aureus in the prior art is shortened.
Owner:SOUTH CHINA UNIV OF TECH
Method for detecting staphylococcus aureus and enterotoxin genotypes thereof through multiple PCR (polymerase chain reaction)
ActiveCN102653792BImprove accuracyStrong specificityMicrobiological testing/measurementMicroorganism based processesFood poisoningStaphyloccocus aureus
The invention provides a method for detecting staphylococcus aureus and enterotoxin genotypes of the staphylococcus aureus by utilizing multiple PCR (polymerase chain reaction) technology. The main technical scheme is as follows: designing primer sequences and optimizing a multiple PCR system and conditions. Enterotoxins and invasive enzymes of staphylococcus aureus affect the pathogenicity of staphylococcus aureus and the enterotoxins which have been identified already are A, B, C1-C3, D, E, G, H, I, J, L, M and N. Generally speaking, food poisoning is commonly caused by A and D and less commonly caused by B and C, the occurrence rate of food poisoning related to the toxin E is the lowest, and the enterotoxins have different toxicity, wherein the toxin A has stronger toxicity and D has weaker toxicity. Aiming at the common enterotoxin genotypes of staphylococcus aureus, the invention overcomes the defects in the prior art and provides the method for detecting staphylococcus aureus and enterotoxin genotypes of the staphylococcus aureus in food by utilizing the multiple PCR technology. The method can be used for simultaneously detecting staphylococcus aureus generating enterotoxins A, B, C and D in the same reaction system.
Owner:嘉兴实践医学科技有限公司
Camp detection primer set and kit for Bacillus cereus enterotoxin gene
ActiveCN110257541BQuick checkAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesBacillus cereusBacilli
The present invention relates to a CAMP detection primer set and kit for Bacillus cereus enterotoxin gene. The primer set includes two sets of primers, namely, primers for amplifying the entFM region fragment of the Bacillus cereus enterotoxin gene and primers for amplifying the bceT region fragment of the Bacillus cereus enterotoxin gene. The primer set provided by the invention has high sensitivity and strong specificity, and the kit prepared by the primer set can quickly and accurately detect whether the sample contains Bacillus cereus, and at the same time judge whether it contains the toxin-producing genes entFM and bceT. Moreover, because the primer set provided by the present invention has extremely high specificity, the time required for CAMP amplification is short, which further shortens the detection time and simplifies the operation.
Owner:SHENYANG AGRI UNIV
A kind of recombinant superantigen seb mutant, its preparation method and application
ActiveCN103965302BHigh activityImprove anti-tumor effectBacteriaPeptide/protein ingredientsAntigenAntineoplastic Immunotherapeutic
The present invention discloses a recombinant superantigen staphylococcal enterotoxin B (SEB) mutant, and a preparation method and applications thereof. According to the present invention, a SEB genome derived from natural staphylococcus aureus is extracted, and after the SEB genome is obtained, site-specific mutagenesis at toxicity-related amino acid sites is performed to obtain the SEB mutant. The SEB mutant has characteristics of both enhanced activity and weakened toxicity, and can be used as anti-tumor immune therapy drugs or immune system modulating drugs. The recombinant superantigen SEB mutant has good market prospects.
Owner:军事科学院军事医学研究院微生物流行病研究所
CAMP detection primer group of bacillus cereus enterotoxin gene and kit
ActiveCN110257541AQuick checkAccurate detectionMicrobiological testing/measurementAgainst vector-borne diseasesBacillus cereusBacillus aryabhattai
The invention relates to a CAMP detection primer group of a bacillus cereus enterotoxin gene and a kit. The primer group comprises two primers which are respectively a primer for amplifying the entFM region fragment of the bacillus cereus enterotoxin gene and a primer for amplifying the bceT region fragment of the bacillus cereus enterotoxin gene. The primer group provided by the invention is high in sensitivity and high in specificity, and the kit prepared by the primer group can quickly and accurately detect whether the sample contains bacillus cereus or not, and simultaneously judge whether the sample contains toxigenic genes entFM and bceT. Moreover, because the primer group provided by the invention has extremely high specificity, the time required for CAMP amplification is short, the detection time is further shortened, and operation is simplified.
Owner:SHENYANG AGRI UNIV
Treble fluorescent quantitation PCR reagent kit for detecting sea, seb and sec genes of staphylococcus aureus enterotoxins
PendingCN111378727AValidation of Assay SensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFood poisoningStaphylococcus aureus enterotoxin
The invention provides a fluorescent quantitation PCR reagent kit for detecting enterotoxin genes sea, seb and sec. The fluorescent quantitation PCR reagent kit comprises Premix ExTaq, a primer, a probe, positive plasmids and sterilization water, wherein the sequences of the primer and the probe are as shown in SEQID No.1-9, a report fluorophore is marked at a 5' terminal of the probe, and a quenching fluorophore is marked at a 3' end of the probe. The invention also provides a method for detecting the enterotoxin genes sea, seb and sec through the reagent kit. Through the reagent kit disclosed by the invention, staphylococcus aureus enterotoxin genes sea, seb and sec can be rapidly detected, enterotoxins are subtyped, and a basis is provided for diagnosis of food poisoning.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
A kind of Bacteroides fragilis and its application
ActiveCN106399141BEffective for diarrheaGood probiotic propertiesBacteriaBacteria material medical ingredientsFood additiveAntibiotic-associated diarrhoea
A kind of Bacteroides fragilis and its application, specifically related to Bacteroides fragilis ZY-312 with the preservation number of CGMCC No.10685, and the preparation of Bacteroides fragilis ZY‑312 for preventing and / or treating antibiotic-associated diarrhea, and a pharmaceutical composition , food, health products and food additives, wherein, the Bacteroides fragilis ZY‑312 was deposited in the China General Microorganism Culture Collection Management Center on April 2, 2015, and its preservation number is CGMCC No.10685. The Bacteroides fragilis ZY‑312 of the present invention does not contain the enterotoxin gene bft, has significantly higher tolerance to bile salts and gastric acid than the existing Bacteroides fragilis, and can effectively prevent and / or treat antibiotic-related diarrhea.
Owner:GUANGZHOU ZHIYI PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com