Swine Escherichia coli non-toxic isolate capable of simultaneously expressing F4 and F18 pili and application of swine Escherichia coli non-toxic isolate
A technology of Escherichia coli and F418, which is applied in the field of biomedicine, can solve problems such as instability, weak colonization ability, and strong virulence, and achieve the effect of exerting immune efficacy and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
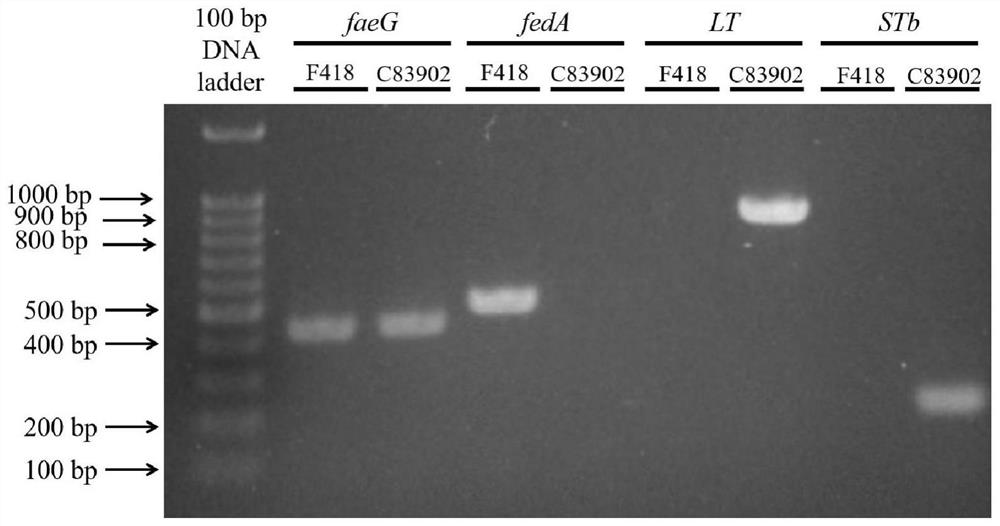
[0028]Example 1 Isolation and identification of porcine Escherichia coli avirulent isolate F418
[0029] On April 16, 2019, 30 fecal samples of 45-day-old weaned piglets were collected from Liu Jiaqi, and bacteriological detection was carried out according to the national standard method (GB 4789.6-2016). The samples were retained in the laboratory, and about 25 g of the samples were taken and placed in a sterile beaker containing 225 mL of sterile liquid LB medium, fully stirred and then placed in a sterile conical flask for 6 hours with shaking at 180 r / min. Take 10 μL of the culture and inoculate it into 30 mL of intestinal bacteria enrichment medium (the ingredients are 1 g of gelatin trypsin hydrolyzate per 100 mL of water, 0.8 g of disodium hydrogen phosphate dihydrate, 0.2 g of bovine bile salt, 0.0015 g of bright green, and 0.5 g of glucose. g, potassium dihydrogen phosphate 0.2g, pH 7.2±0.2) was placed at 42°C for 18h enrichment. The enrichment solution was streaked ...
Embodiment 2
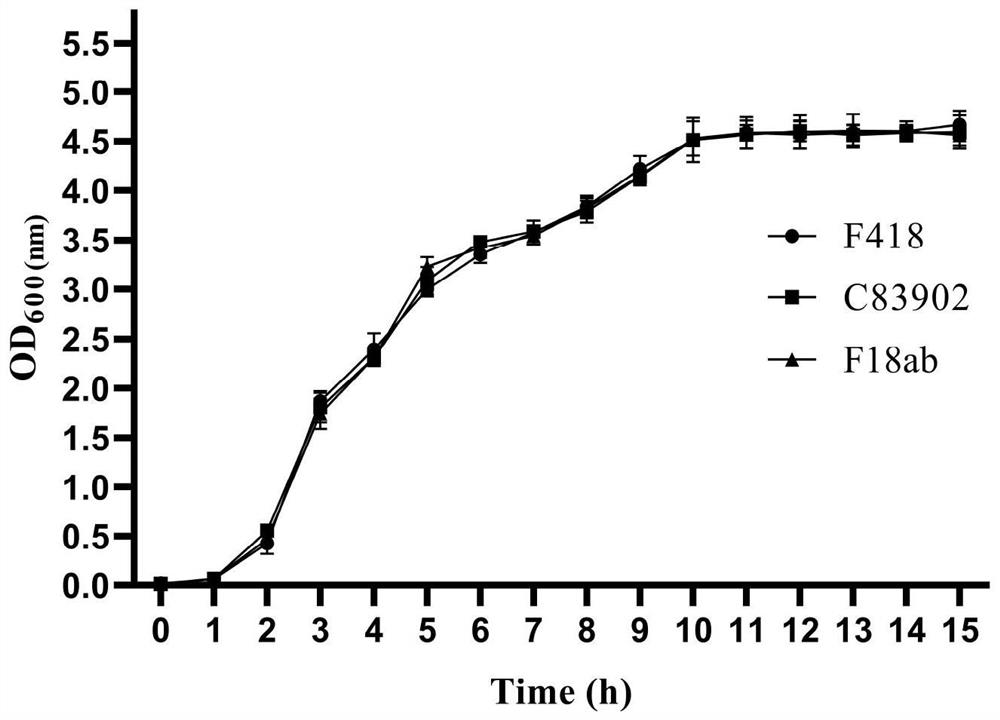
[0044] Example 2 Biological study of pig-derived Escherichia coli avirulent isolate F418
[0045] Single colonies of the above-mentioned isolates F418, F4 virulent standard strain C83902 and F18ab standard strain E. coli 107 / 86 were inoculated in liquid LB at 37°C for overnight shaking culture. The next day, the bacterial liquid was drawn and streaked on MacConkey plates for comparison of culture characteristics. At the same time, the three strains were subjected to sucrose, lactose, glucose, raffinose, maltose, mannitol, indigo matrix, mannose, citric acid, dulcitol, ornithine, lysine, potassium cyanide, hydrogen sulfide, Trace biochemical reactions such as urea, ONPG, MR test, V-P test, semi-solid agar, Calendula officinalis, nitrate reduction (shown in Table 4).
[0046] On the MacConkey plate, isolates F418, F4 virulent standard strain C83902 and F18ab standard strain E. coli 107 / 86 all grew pink round smooth colonies.
[0047] Table 4 Comparison of biochemical properties...
Embodiment 3
[0051] Example 3 Infection test of porcine-derived Escherichia coli avirulent isolate F418 to 30-day-old weaned piglets
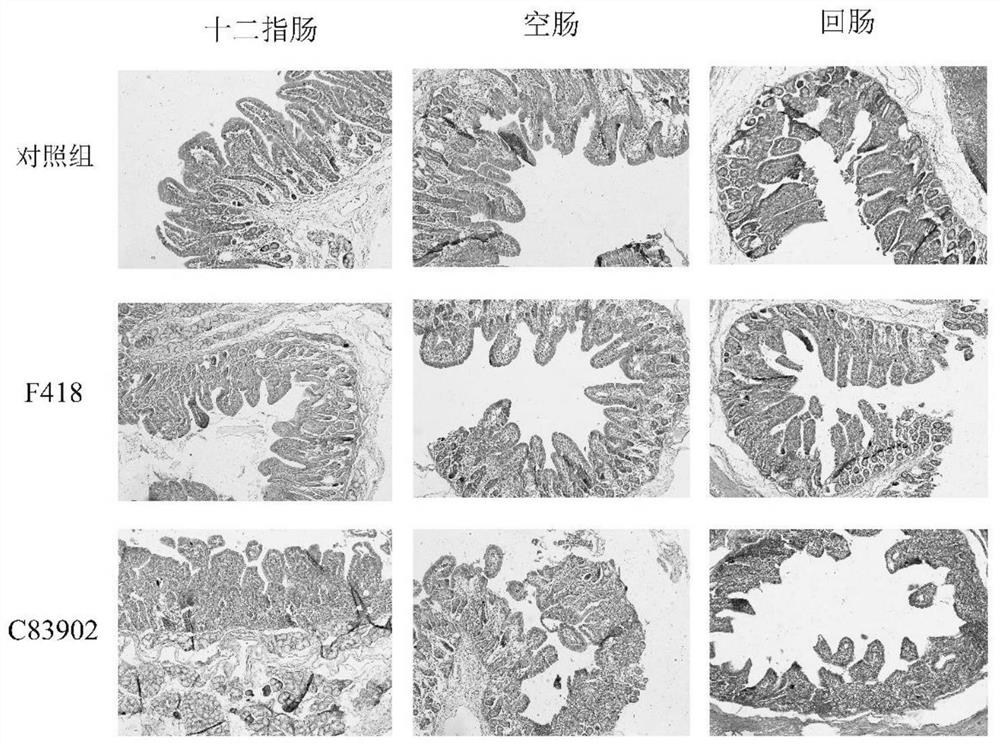
[0052] Nine 5-day-old weaned piglets were randomly divided into 3 groups with 3 piglets in each group. 9 CFU / mL was orally administered orally, and a balanced salt buffer control group was set up, and the oral dose was 1 mL. Each group was reared in isolation, fed with antibiotic-free feed, and carefully observed for a week. Diarrhea symptoms and other clinical symptoms (such as mental state, feeding situation, etc.) of all experimental piglets were observed every day. At the same time, the mortality was recorded, and the organ lesions were observed by autopsy one week later. The duodenum, jejunum and ileum of piglets in each group were collected and sliced to observe the pathological changes.
[0053] The results of the one-week test showed that the piglets in the isolated strain F418 infection group did not die, and the piglets were in good condition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com