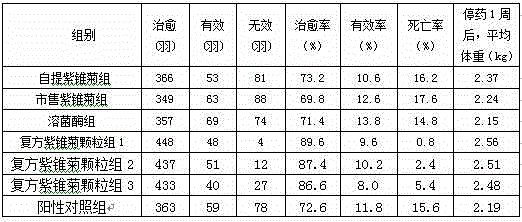
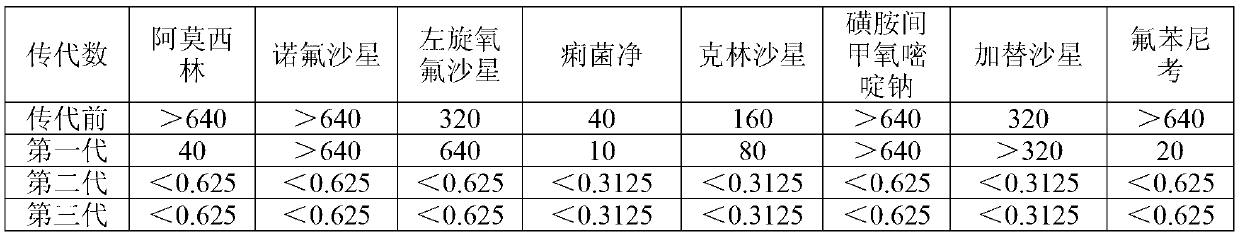
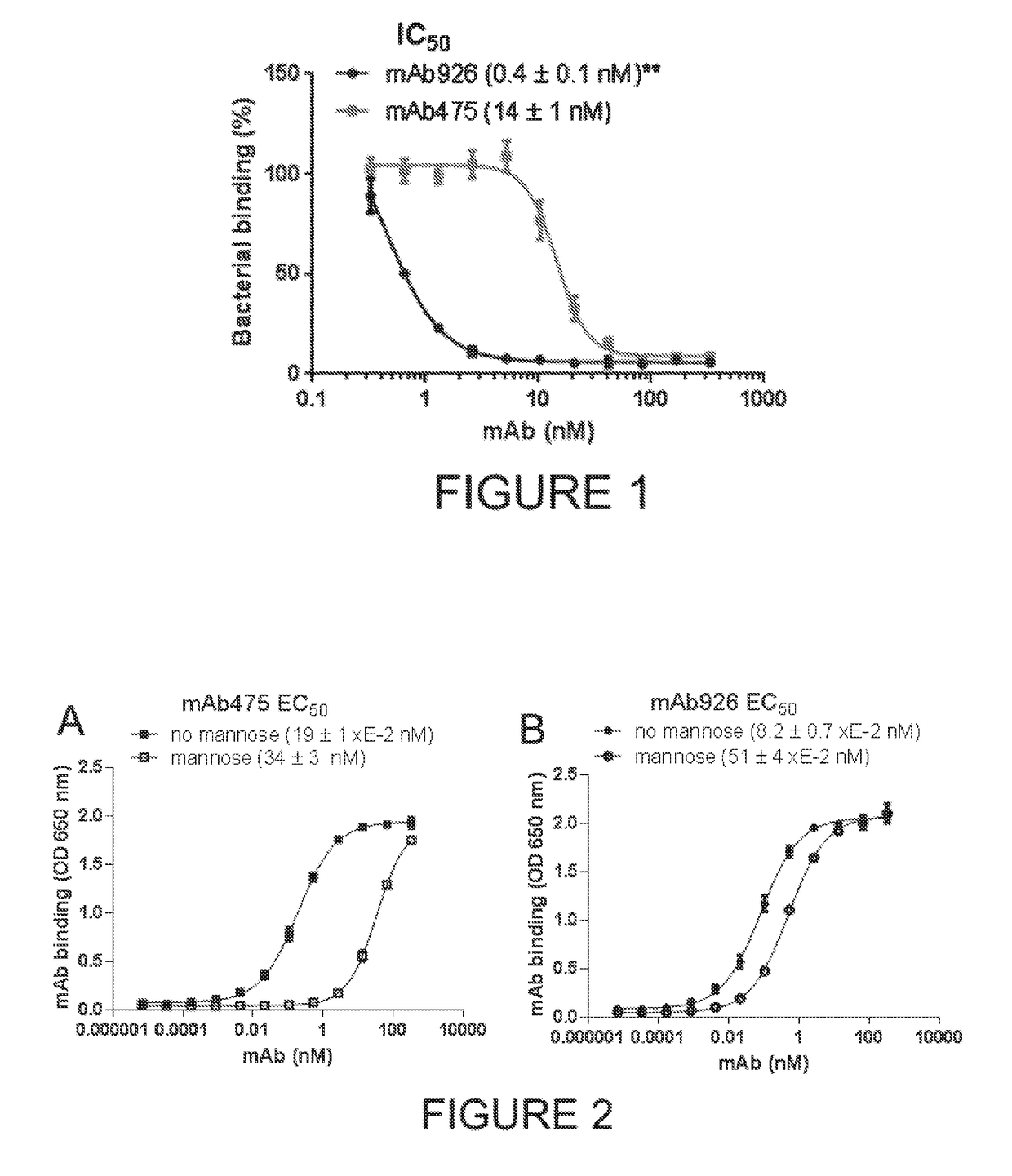
Patents
Literature
47 results about "Escherichia infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
E. coli infection: Symptoms, causes, and treatment E. coli refers to a wide range of bacteria that can cause various diseases, including pneumonia, urinary tract infections, and diarrhea. Most strains of E. coli are harmless to humans. Some strains of E. coli infection can include nausea, vomiting, and fever.
Echinacea extract and preparation method of echinacea extract
ActiveCN102716164AHigh extraction rateSynergisticAntibacterial agentsPeptide/protein ingredientsBiotechnologyMedicinal herbs
The invention relates to an echinacea extract and a preparation method of the echinacea extract. The echinacea extract provided by the invention is obtained through carrying out twice extraction on echinacea medicinal materials by alkaline ethanol solution and lysozyme; and in the product, the polysaccharide content is greater than or equal to 21.50 percent, the polyphenol content is greater than or equal to 8.10 percent, and the chicoric acid content is greater than equal to 5.00 percent. Because the polyphenol content and the chicoric acid content of the echinacea extract are obviously increased, the echinacea extract is prepared into ordinary preparations including powder or oral liquid and the like, and the curative effect is greatly improved. In a compound preparation processed by the echinacea extract and bacteriolysant according to a consumption ratio being 3:1, active ingredients of echinacea and lysozyme totally account for 20 percent, wherein the consumption ratio of the echinacea extract to the lysozyme is (4-1):1, auxiliary materials of cane sugar account for 64 percent, and dextrin accounts for 16 percent. The compound preparation has the functions of improving the immunity of live stock and realizing the sterilization and antivirus functions, and can be used as medicine for preventing and treating animal diseases, particularly chicken diseases caused by escherichia coli infection.
Owner:QILU ANIMAL HEALTH PROD
Holly bark containing compound composition for treating escherichia coli infected diseases of livestock and poultry
ActiveCN103127507AReverse drug resistanceGood treatment effectAntibacterial agentsPlant ingredientsEscherichia coliDisease
The invention relates to a holly bark containing compound composition for treating escherichia coli infected diseases of livestock and poultry. The compound composition is prepared from the following raw materials in parts by weight: 3 to 30 parts of antibacterial drug, and 5 to 200 parts of holly bark. The raw materials are uniformly mixed to prepare powder, tablets, oral liquid or granules which are added into animal feed in a ratio of 0.1 to 5g / kg feed addition amount for feeding, and the drug resistances of bacteria to the antibacterial drug can be reversed, so that the treatment effect of antibacterial drugs can be improved, the administration cost can be greatly reduced, and higher economic benefit can be created for farmers.
Owner:XUZHOU TIANYI ANIMAL PHARMA
Effective antibacterial traditional Chinese medicine composition
The invention relates to an effective antibacterial traditional Chinese medicine composition and in particular relates to a traditional Chinese medicine composition. The effective antibacterial traditional Chinese medicine composition includes radix sophorae flavescentis extract, green tea extract and perilla leaf extract as well as random pharmaceutically acceptable auxiliary materials. The invention further relates to a method for preparing the traditional Chinese medicine composition and application of the traditional Chinese medicine composition. Besides, the traditional Chinese medicine composition can be used for preventing microorganism (for example escherichia coli) infection.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Compositions and methods for treatment and prevention of uropathogenic e. coli infection
ActiveUS20180193457A1Avoid infectionPrevent and inhibit infectionAntibacterial agentsBacterial antigen ingredientsBacteroidesKlebsiella oxytoca
Methods and antibody compositions that displace mannose from the binding pocket of fimbrial adhesin FimH of enterobacteria, including uropathogenic E. coli, Klebsiella oxytoca, or Klebsiella pneumoniae, can be used to disrupt or prevent the attachment of a single layer of bacteria to a mannose-coated surface, or to disrupt or prevent the formation of a multilayer biofilm. The antibody compositions of the invention can thus be used in methods to inhibit, prevent, or reverse the colonization of a surface with enterobacteria that express the bacterial Type 1 fimbrial adhesin FimH, to inhibit or prevent infection of a cell by enterobacteria that express the bacterial Type 1 fimbrial adhesin FimH, such as, for example, uropathogenic E. coli, to treat a bacterial infection in subject in need thereof and to treat or prevent inflammatory bowel disease (IBD), among other uses.
Owner:UNIV OF WASHINGTON
Compound coneflower preparation preparation method
The invention provides a compound coneflower preparation, wherein a use ratio of effective components such as a coneflower extract and lysozyme is 3:1, the total content of the coneflower extract and the lysozyme is 25%, and contents of auxiliary materials such as sucrose and dextrin are respectively 60% and 15%. According to the present invention, the selected coneflower extract is obtained by extracting a coneflower herb with an alkaline ethanol solution and lysozyme; the polysaccharide content exceeds 20%, the polyphenol content exceeds 8%, and the chicoric acid content exceeds 5%; and the composite preparation has functions of animal immunity improving, anti-bacterial and anti-virus, and can be used for preparation of drugs for prevention and treatment of animal diseases, especially prevention and treatment of chicken diseases caused by escherichia coli infection.
Owner:QINGDAO KDN BIOTECH
Compound echinacea purpurea preparation and preparation method and application thereof
ActiveCN102772790AImprove immunityAnti-inflammatoryAntibacterial agentsPeptide/protein ingredientsAnti virusSaccharum
The invention discloses a compound echinacea purpurea preparation. The dose ratio of an echinacea purpurea extract as an effective component to lysozyme is (4-1):1, the dosage of the echinacea purpurea extract and the lysozyme is 20% in total, and the ajudvants contains 64% of saccharose and 16% of dextrin. The echinacea purpurea extract selected by the invention is obtained by extracting echinacea purpurea medicinal material by alkaline ethanol solution and lysozyme for two times, wherein the content of polysaccharide is not less than 21.5%, the content of polyphenol is not less than 8.10% and the content of chicoric acid is not less than 5.00%. The compound preparation provided by the invention has the effects of improving animal immunity, antibiosis and anti-virus, thereby being capable of being taken as the medicine for preventing and treating the animal diseases, in particular to the chicken diseases caused by escherichia coli infections.
Owner:QILU ANIMAL HEALTH PROD +1
Bacteriostatic skin-care cream
InactiveCN104055983AGood treatment effectThorough curative effectAntibacterial agentsHeavy metal active ingredientsBiotechnologyEscherichia coli
Owner:孙占全
Bacteriostatic nourishing liquid for skin
InactiveCN104055896AGood treatment effectThorough curative effectAntibacterial agentsSalicyclic acid active ingredientsCandida albicansSalicylic acid
The invention discloses a bacteriostatic nourishing liquid for skin, which relates to a medicine, especially to an external medicinal liquid. The bacteriostatic nourishing liquid comprises the following raw materials by weight: 4 to 6% of phellodendron, 4 to 6% of coptis, 0.8 to 1.2% of salicylic acid and 86.8 to 91.2% of ethanol with a concentration of 70 to 80%. The bacteriostatic nourishing liquid is prepared by percolating phellodendron and coptis powder with ethanol, collecting percolate, dissolving salicylic acid into the percolate, carrying out uniform mixing with stirring and then successively carrying out filling, sealing and packaging. The bacteriostatic nourishing liquid provided by the invention overcomes the problem that traditional drugs used to treat infection by Staphylococcus aureus, Candida albicans and Escherichia coli caused by moth bites do not have ideal treatment effects and cannot thoroughly treat skin infection after long-term usage.
Owner:孙占全
Polypeptide used for preventing and treating enteropathogenic Escherichia coli infection
ActiveCN105294840AAvoid stickingReduce adsorptionAntibacterial agentsPeptide/protein ingredientsEscherichia coliEnteropathogenic Escherichia coli infection
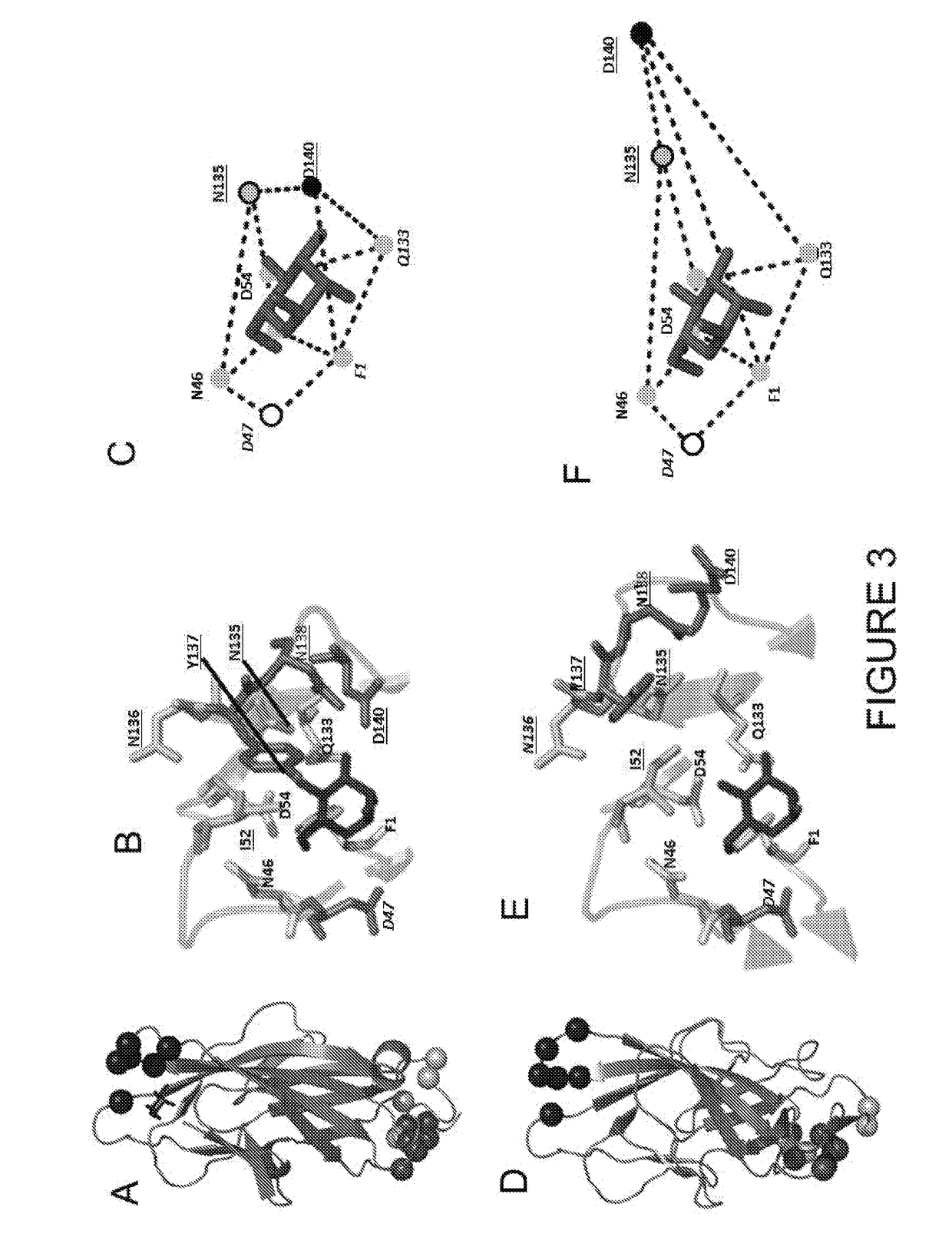
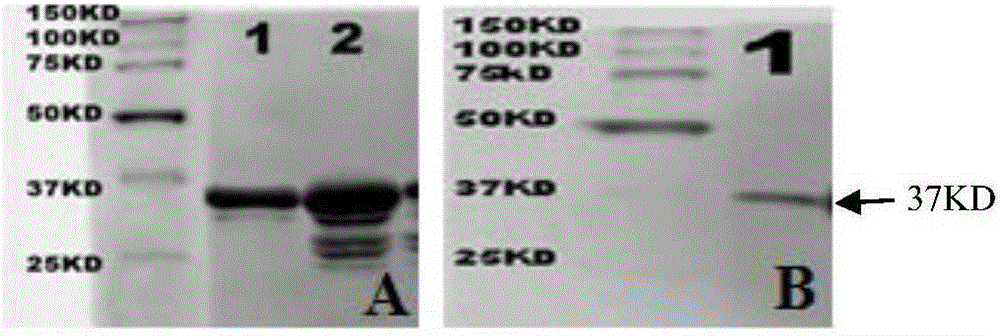
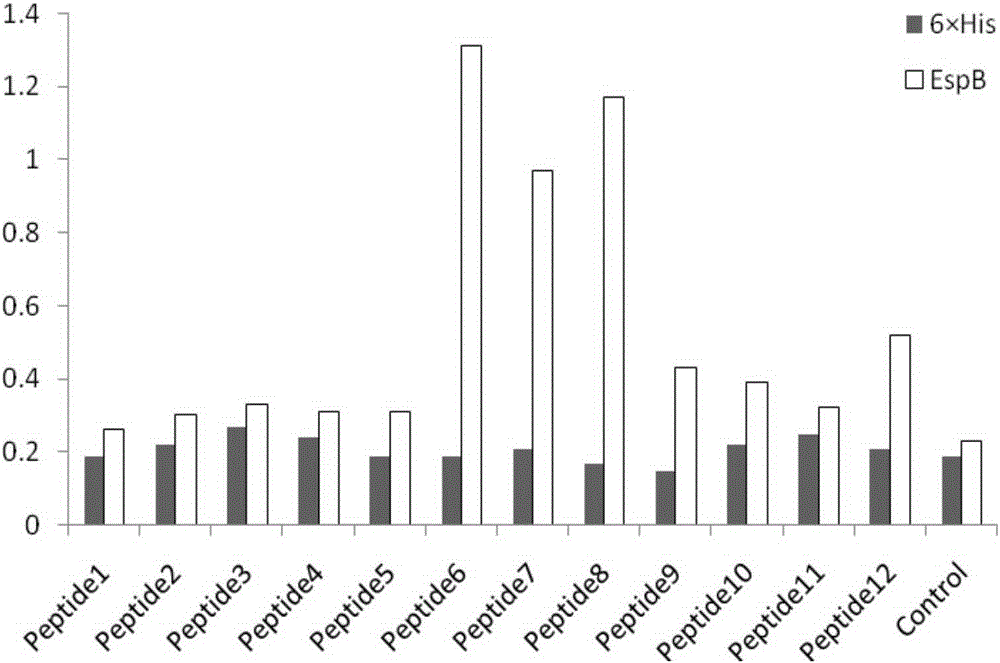
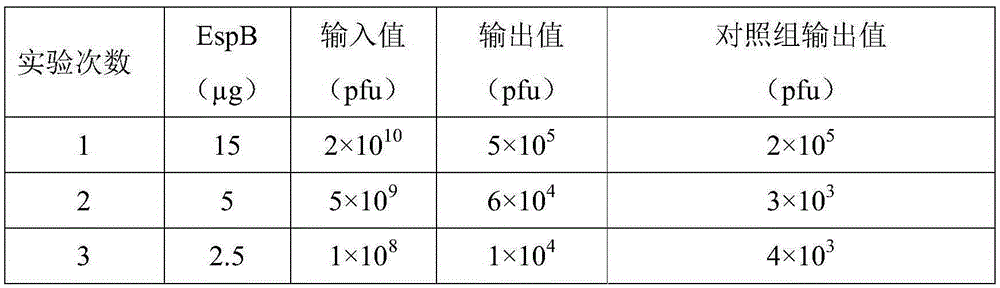
The present invention provides a polypeptide for preventing and treating enteropathogenic Escherichia coli infection. The polypeptide comprises an amino acid sequence of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO:3 or SEQ ID NO: 4. According to the technical scheme of the present invention, the affinity of the polypeptide for preventing and treating enteropathogenic Escherichia coli infection and EspB protein is higher than that of control protein, the polypeptide for preventing and treating enteropathogenic Escherichia coli infection can inhibit adhesion of EPEC an HEp 2 cells and interrupt functions of EspB, thereby reducing epithelial cell adsorption by the EPEC, providing a new direction for EPEC treatment and laying a foundation for researching and developing drugs for preventing and treating the EPEC.
Owner:SHENZHEN NANSHAN DISTRICT PEOPLES HOSPITAL
Pig circRNA sequence, application thereof and cyclization identification method
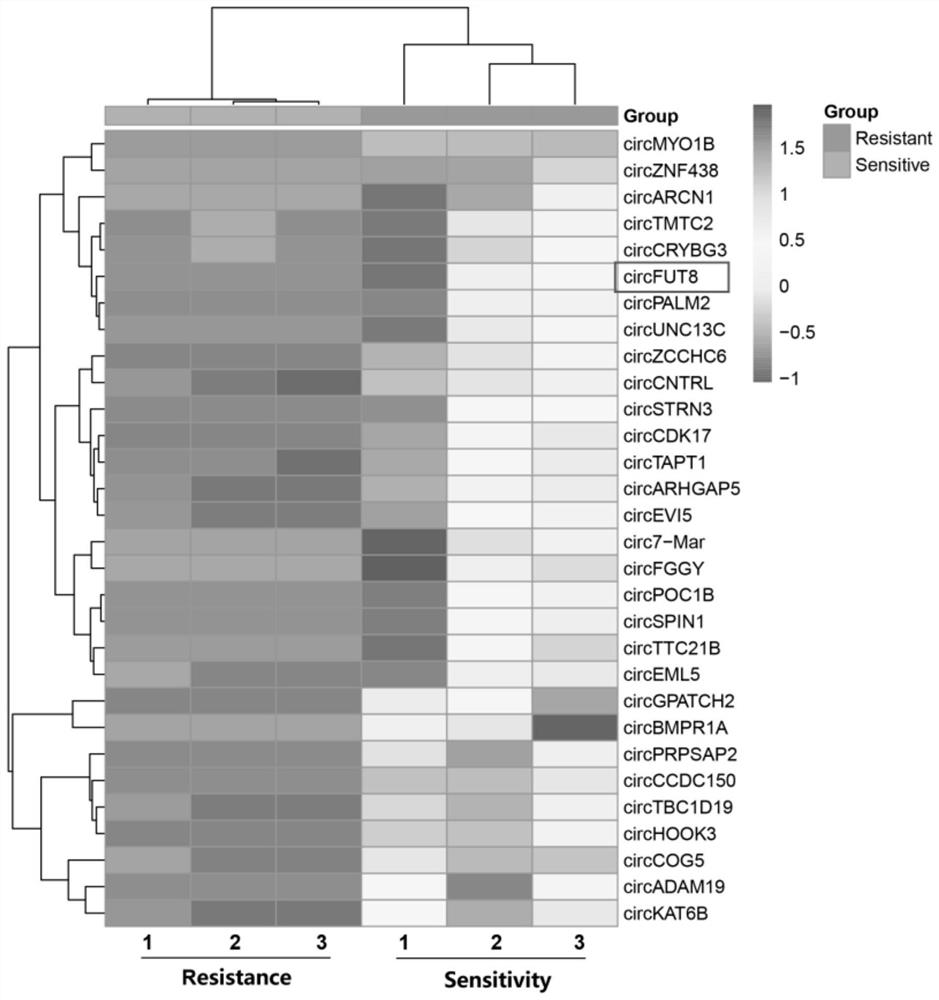
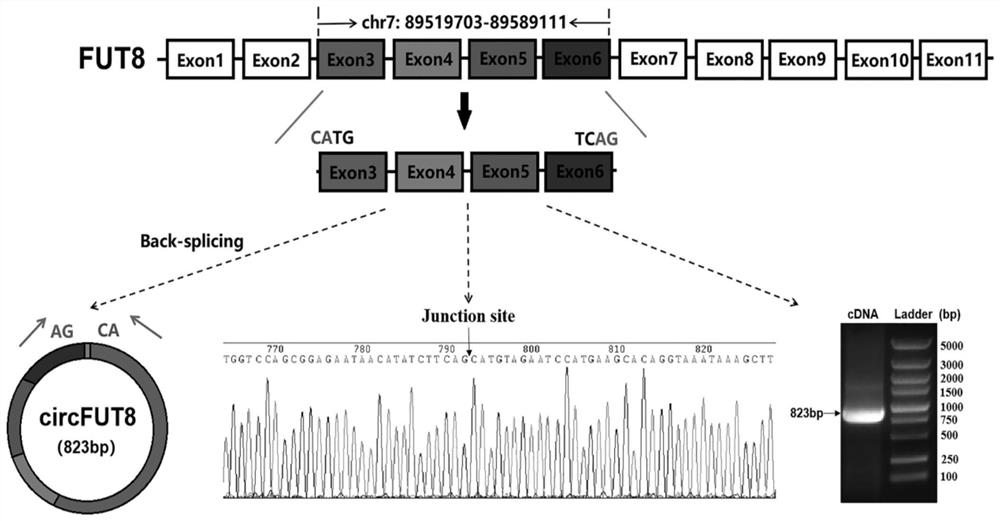
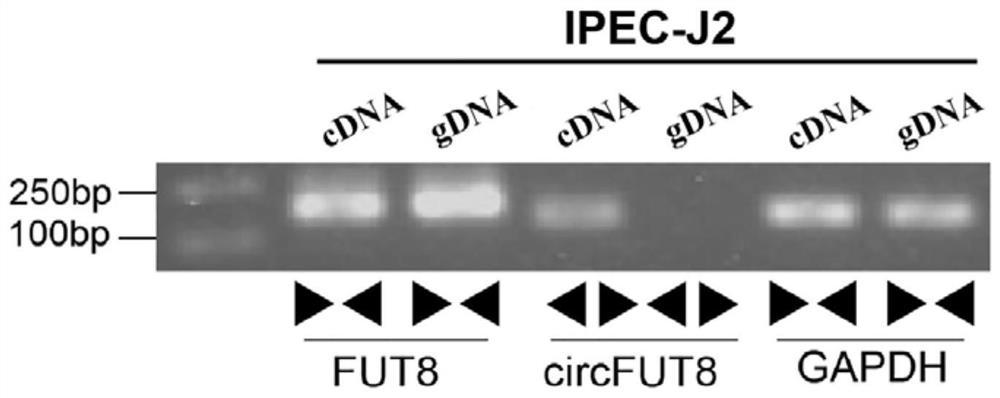
The invention is based on the most common intestinal inflammatory disease, namely piglet bacterial diarrhea (PDW), wherein the F18 Escherichia coli (E. coli F18) is a main pathogen causing weaned piglet bacterial diarrhea, and regulation mechanisms of local variety pigs to the resistance of the E. coli F18 are to be systematically clarified. In the early stage, a key candidate RNA-circRNA-27388 for escherichia coli F18 infection regulation is screened out through omics sequencing, as a circRNA-27388 body gene is FUT8, the circRNA-27388 body gene is named as circ-FUT8, on the basis, the circ-FUT8 sequence is subjected to cyclization identification, and a foundation is laid for follow-up verification of circRNA functions and the regulation mechanisms.
Owner:YANGZHOU UNIV
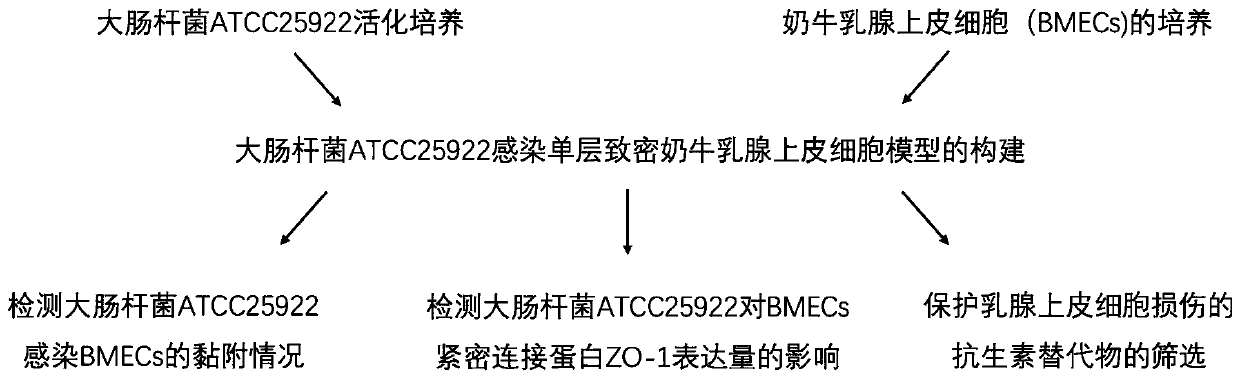
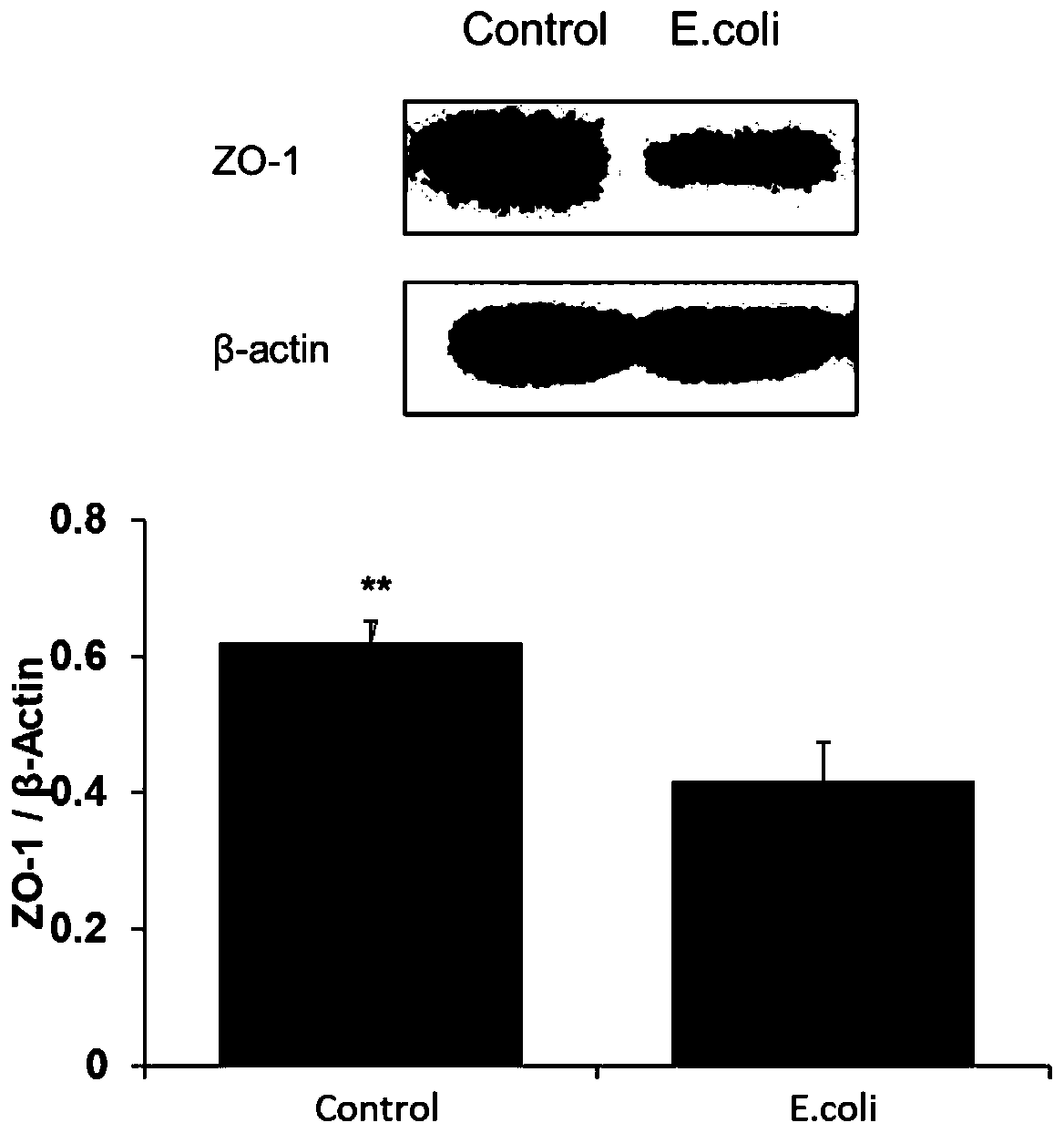
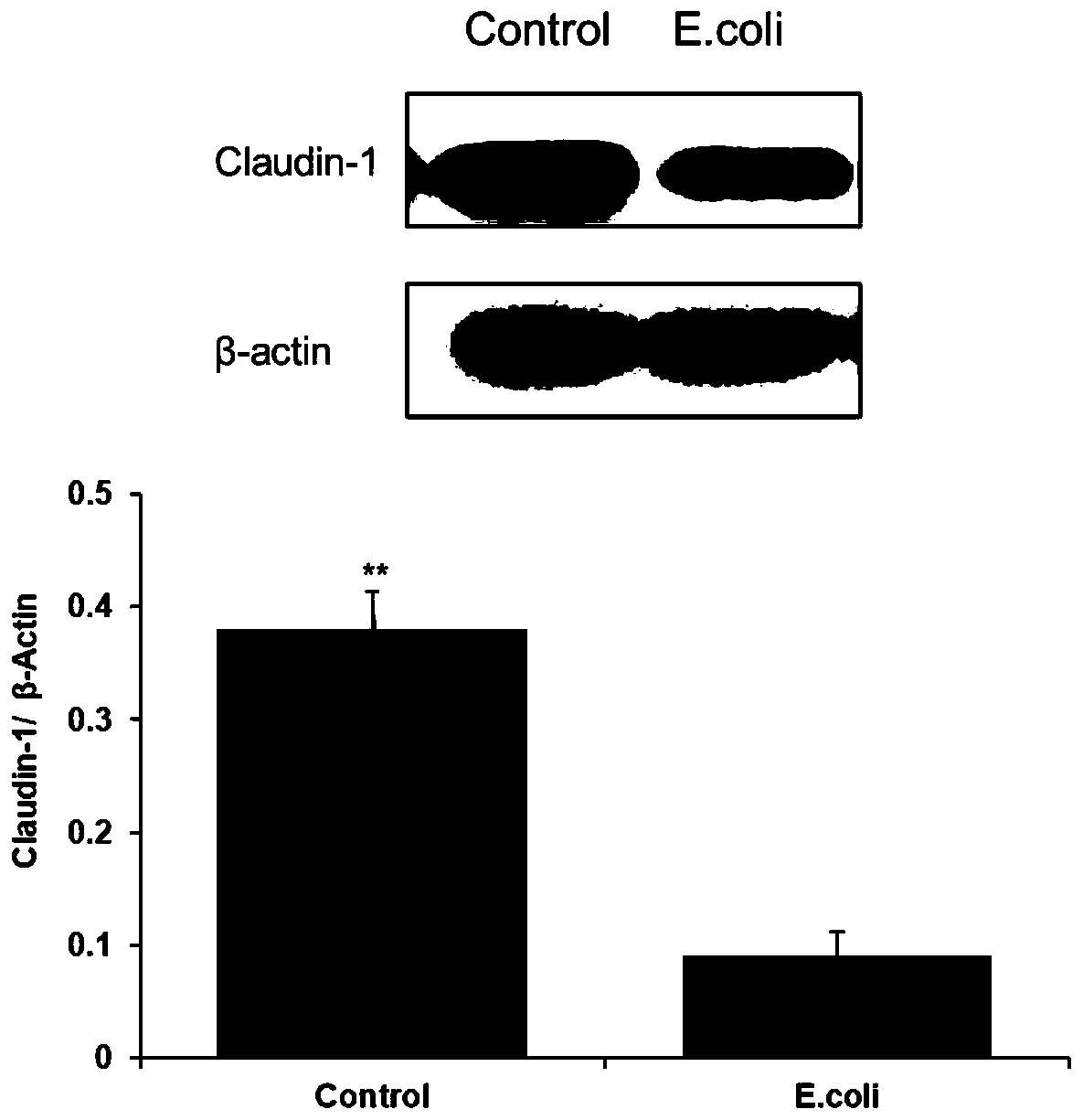
Construction method for escherichia coli infected monolayer compact bovine mammary epithelial cell model
The invention provides a construction method for an escherichia coli infected monolayer compact bovine mammary epithelial cell model. The cell model is a monolayer compact bovine mammary epithelial cell model infected with escherichia coli is ATCC 25922, and during infection, the transepithelial electrical resistance value of the monolayer compact bovine mammary epithelial cell is 400 [omega]. cm<2>. The escherichia coli infected monolayer compact bovine mammary epithelial cell model provided by the invention can be used for detecting the adhesion rate of the escherichia coli infected monolayer compact bovine mammary epithelial cell and an influence of the escherichia coli infected monolayer compact bovine mammary epithelial cell for the expression of bovine mammary epithelial cell tight junction protein, and can be widely applied to related fields, including the screening of an injury mechanism of the escherichia coli induced mastitis for mammary tissues and mammary epithelial cells,and an antibiotic substitute.
Owner:CHINA AGRI UNIV
Herbal veterinary medicine for preventing and treating chicken heat-toxin symptoms, and preparation method and application thereof
InactiveCN103893523AReduce wasteImprove efficacyAntibacterial agentsGranular deliveryMedicinal herbsBaical Skullcap Root
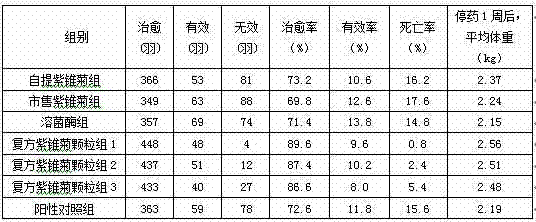
The invention discloses a herbal veterinary medicine for preventing and treating chicken heat-toxin symptoms caused by Escherichia coli infection. The herbal veterinary medicine comprises the following raw materials: on the basis of the amount of crude drugs, 30 to 90 g of gypsum, 3 to 20 g of honeysuckle flower, 2 to 30 g of figwort root, 2 to 15 parts of baical skullcap root, 2 to 15 g of raw rehmannia root, 2 to 15 g of weeping forsythia capsule, 2 to 15 g of cape jasmine, 2 to 15 g of Chinese gentian, 2 to 30 g of Gueldenstaedtia multiflora Bunge., 2 to 40 g of isatis root, 2 to 15 g of common anemarrhena rhizome and 2 to 15 g of dwarf lilyturf tuber. The herbal veterinary medicine is a granule which further comprises an excipient. A preparation method for the summer-heat-removing granule comprises the following steps: (1) extracting the above-mentioned raw materials so as to prepare extract powder; (2) adding the excipient and carrying out uniform mixing; and (3) successively carrying out granulation, drying and split charging so as to obtain the finished granule. The granule provided by the invention is named as summer-heat-removing granule, is capable of clearing heat, removing toxins, nourishing yin and checking dysentery and has the advantages of a substantial effect, convenience in administration and no toxic and side effects.
Owner:QINGDAO VLAND BIOTECH INC
Preparation method of shiga-like toxin Stx1B oral vaccine and product of shiga-like toxin Stx1B oral vaccine
InactiveCN102580118AIncrease productionReduce outputAntibacterial agentsGenetic material ingredientsBiotechnologyOedema disease
The invention discloses a preparation method of a shiga-like toxin Stx1B oral vaccine, which concretely comprises the steps of: cloning a shiga-like toxin Stx1B gene, building a recombinant expression vector containing the Stx1B gene, then preparing a transgenic plant, and finally preparing the shiga-like toxin Stx1B oral vaccine. The prepared shiga-like toxin Stx1B oral vaccine can be directly and orally taken to immunize and can effectively prevent the escherichia coli O157:H7 infection, moreover, the orbidity of edema disease of a pig is reduced, and the death efficiency is also reduced at the same time.
Owner:CHONGQING UNIV
Application of antibacterial peptide in in-vitro inhibition of Escherichia coli
ActiveCN111658759AAvoid survivalPowerful killAntibacterial agentsOrganic active ingredientsPharmaceutical SubstancesAmino acid
The invention belongs to the field of cell biology, and particularly relates to an application of an antibacterial peptide in the in-vitro inhibition of Escherichia coli. The antibacterial peptide selected by the invention is a natural antibacterial peptide Esculentin-1A, and the amino acid sequence information of the antibacterial peptide Esculentin-1A is shown as SEQ ID NO.1. In the research process, it is found that the antibacterial peptide Esculentin-1A and citric acid are mixed according to a mass ratio of (7-10):(2-5), and in-vitro tests prove that the inhibition of the mixture on Escherichia coli reaches 99% or more. The antibacterial peptide and citric acid are mixed for use for the first time, and a new research and development direction is provided for the development of drugs related to the treatment of symptoms such as cholecystitis and ovaritis caused by Escherichia coli infection.
Owner:广州颜如玉生物科技有限公司
Chinese and western drugs for curing bird bacillus coli disease and method of preparing the same
InactiveCN101249153AEffective in treating infectionsTreat infectionAntibacterial agentsOrganic active ingredientsDiseaseEscherichia coli
The invention relates to a Chinese and western drug for treating avian escherichia coli diseases. The invention is characterized in that the Chinese and western drug comprises the components by weight parts as follows: rhubarb is 10 to 30 parts, radix scutellariae is 5 to 15 parts, coptis roots are 1 to 5 parts, radix isatidis is 1 to 5 parts, and enrofloxacin is 0.5 to 5 parts. The invention has a preparation method as follows: (1) the components are got by weight parts as follows: the rhubarb is 10 to 30 parts, the radix scutellariae is 5 to 15 parts, the radix isatidis is 1 to 5 parts, and the radix isatidis is 1 to 5 parts; (2) purified water which is 2 to 3 times the weight of the total quantity of all components is added, and concentrated solution is obtained after diacolation, evaporation and concentration, wherein, the relative density of the concentrated solution is 1.05 to 1.2; (3) the enrofloxacin which is 0.1 to 1 time the weight of the concentrated solution is added in the concentrated solution, and the purified water which is 2 to 5 the weight of the concentrated solution is added, so that the Chinese and western drug is made. The invention has strengthening efficacy after extraction and separation, effectively treats chicken escherichia infection, and reduces the economic loss of raising households. The Chinese and western drug realizes the reasonable collocation and combination by selecting various active ingredients, the cost is low, the curative effect is good, the cure rate is high, and the safety and the reliability are realized.
Owner:TIANJIN SHENGJI GRP CO LTD
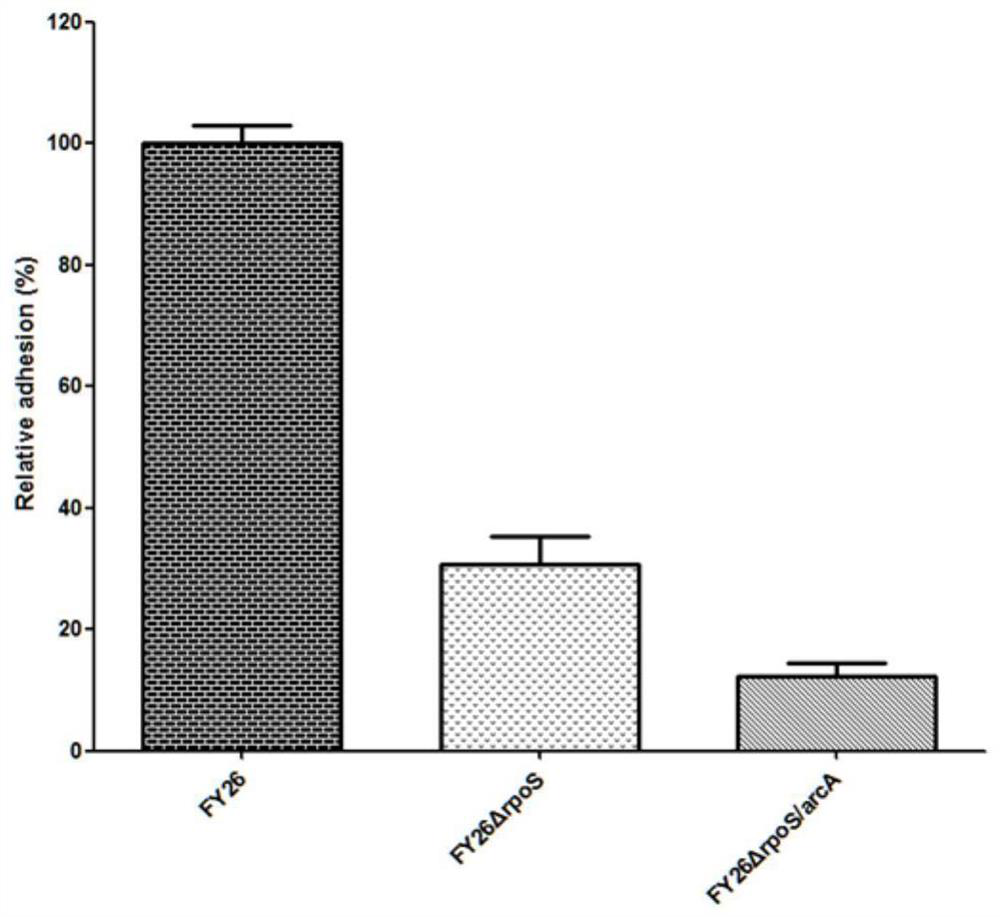
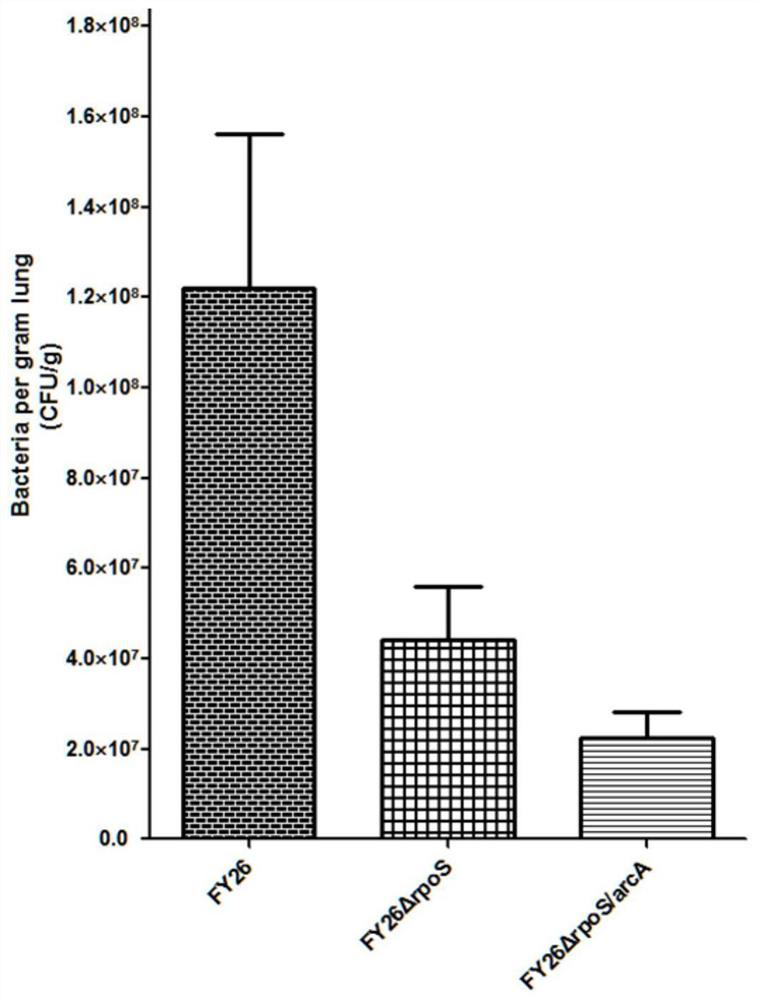
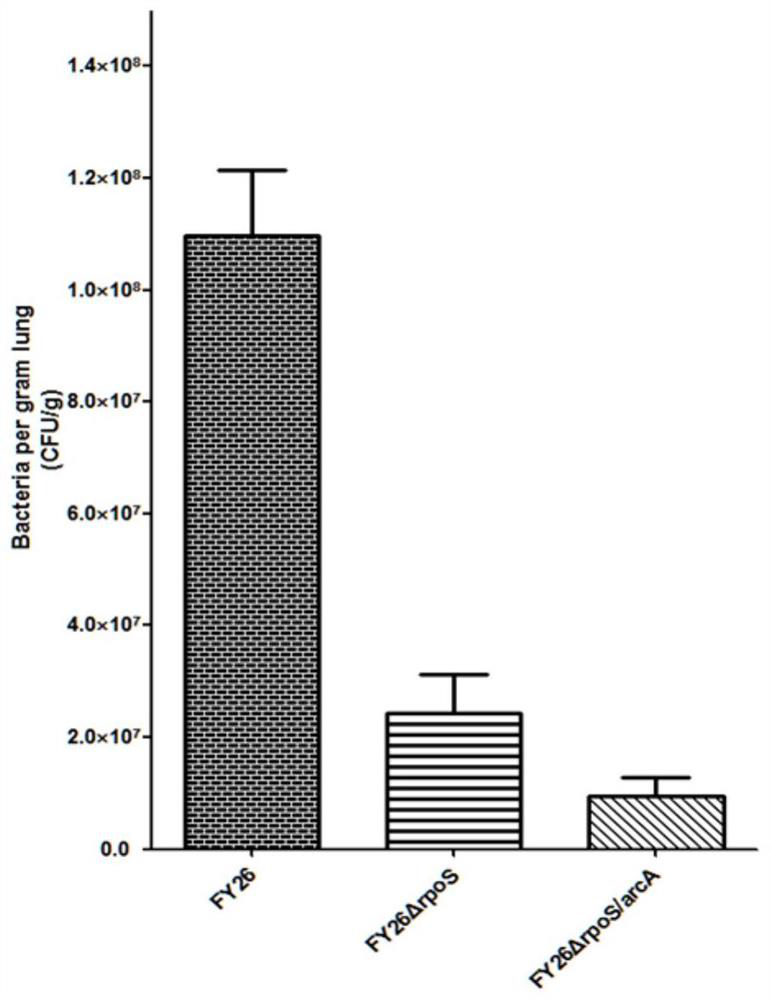
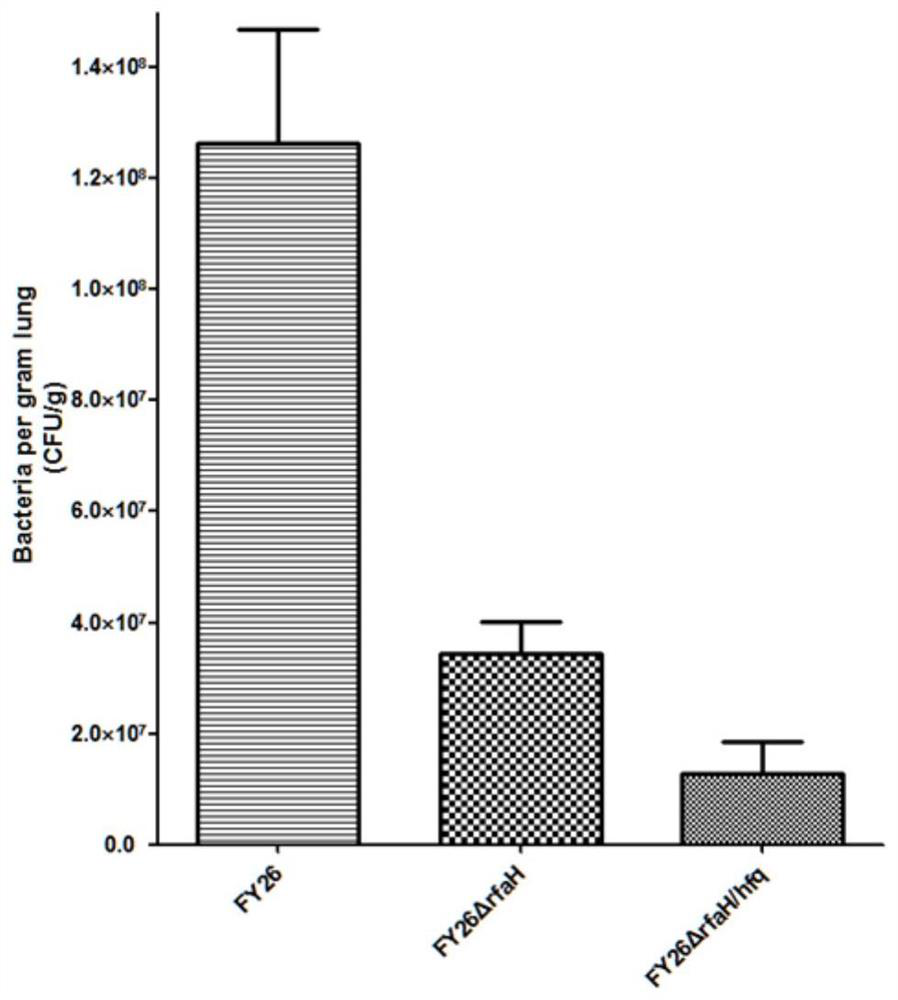
APEC double-gene rpoS and arcA deletion strain and attenuated vaccine
InactiveCN112695004AReduce pathogenicityImprove protectionAntibacterial agentsBacteriaESCHERICHIA COLI ANTIGENImmunogenicity
The invention belongs to the technical field of microorganisms, and discloses an APEC double-gene rpoS and arcA deletion strain and an attenuated vaccine. A construction method of the APEC double-gene rpoS and arcA deletion strain is to knock out rpoS and arcA double genes by a Red homologous recombination method to obtain avian pathogenic Escherichia coli FY26 delta rpoS / arcA which is preserved in China Center for Type Culture Collection with the preservation number of CCTCC M 2020613. The deletion strain has good biological safety and immunogenicity, is low in toxicity, and can be used for preparing avian pathogenic escherichia coli attenuated vaccines to prevent avian pathogenic escherichia coli infection. The attenuated vaccine prepared from the avian pathogenic escherichia coli FY26 delta rpoS / arcA disclosed by the invention has immune protection on avian pathogenic escherichia coli infection and is good in toxin attacking immune protection effect.
Owner:NANTONG UNIVERSITY
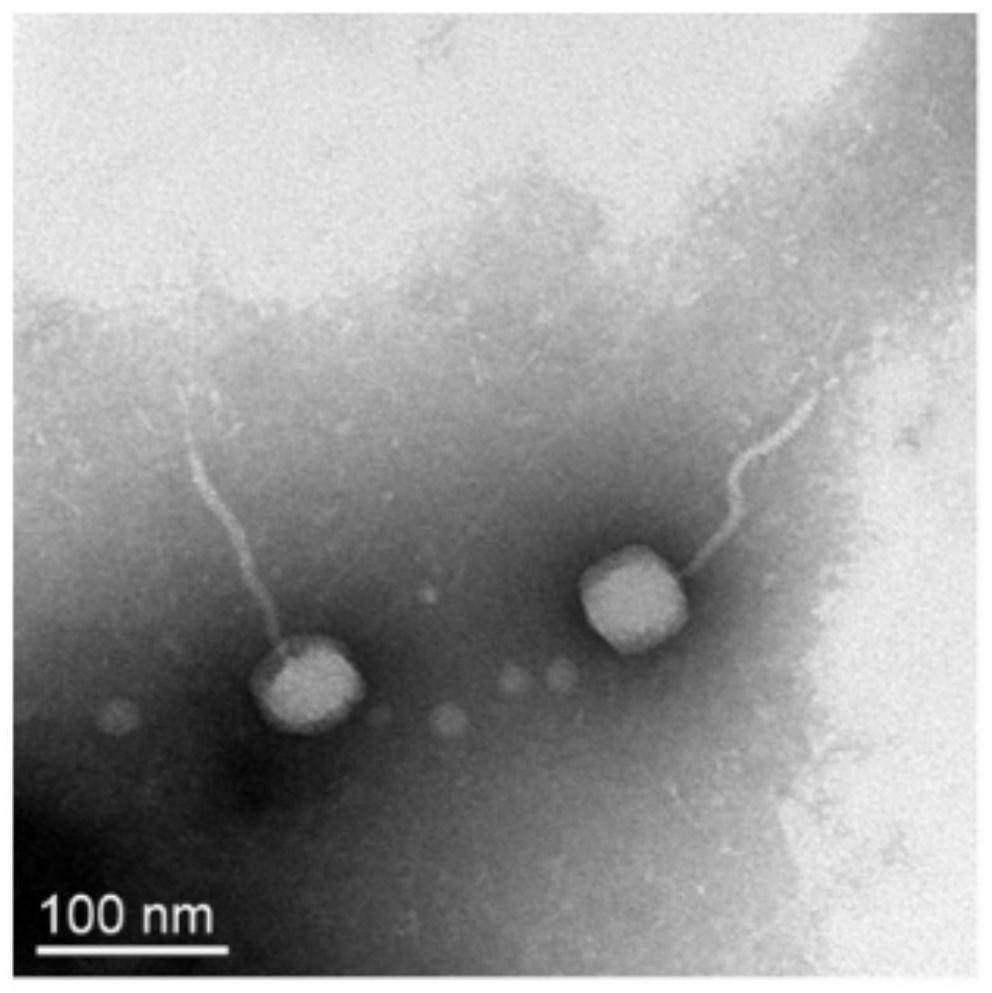
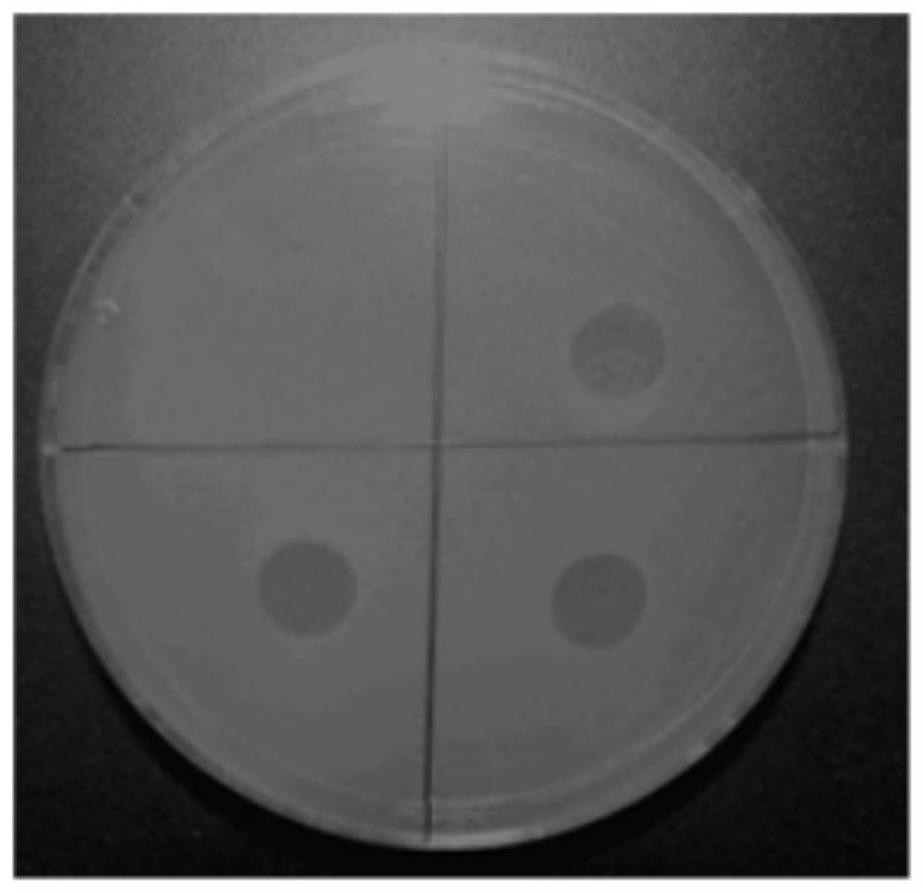
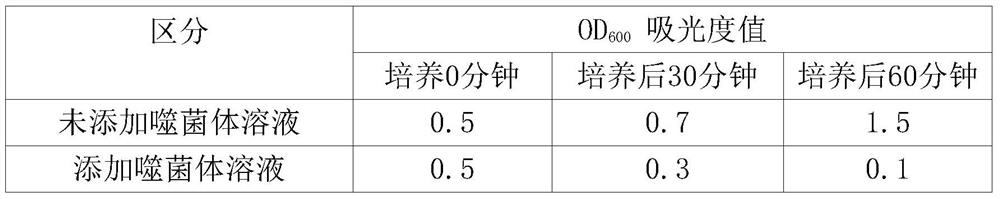
Escherichia coli bacteriophage esc-cop-9 and its use in inhibiting proliferation of pathogenic Escherichia coli
ActiveCN108179136BWeakened immunityStrong specificityAntibacterial agentsViral/bacteriophage medical ingredientsEscherichia coliPathogenicity
The present invention relates to a long-tailed virus family phage Esc-COP-9 (entrusted number: KCTC 13131BP) isolated from nature, characterized in that it carries a genome with the ability to specifically kill Escherichia coli and is marked with sequence number 1, And the present invention also relates to a method for preventing and treating pathogenic Escherichia coli infection by using the composition containing the phage as an active ingredient.
Owner:INTRON BIOTECHNOLOGY INC
Granule for treatment of heat toxin symptom caused by chicken escherichia coli infection and preparation method thereof
ActiveCN102895503BReduce wasteImprove efficacyAntibacterial agentsGranular deliveryBiotechnologyMedicinal herbs
The present invention discloses a granule for treatment of heat toxin symptom caused by chicken escherichia coli infection. The raw material ratio by crude medicines includes: 30-90g of gypsum, 3-20g of honeysuckle, 2-30g of figwort root, 2-15g of baical skullcap root, 2-15g of dried radix rehmannia, 2-15g of fructus forsythiae, 2-15g of fruit of cape jasmine, 2-15g of gentiana scabra bunge, 2-30g of bunge corydalis herb, 2-40g of isatis root, 2-15g of rhizoma anemarrhena, and 2-15g of radix ophiopogonis. The granule also includes an excipient. The preparation method of the granule includes: (1) extracting the prescription medicine to make extract powder; (2) adding the excipient and mixing; and (3) granulating, drying and packaging to get finished products. The granule of the present invention has a trade name of clearing and releasing granule, has the effect of clearing heat and detoxifying, is significant in effects and easy to take, and has no toxic side effects.
Owner:QINGDAO VLAND BIOTECH INC +1
IncRNA marker related to bacterial diarrhea resistance of piglets and application of IncRNA marker
InactiveCN113832235AAntibacterial agentsMicrobiological testing/measurementBiotechnologyBacteroides suis
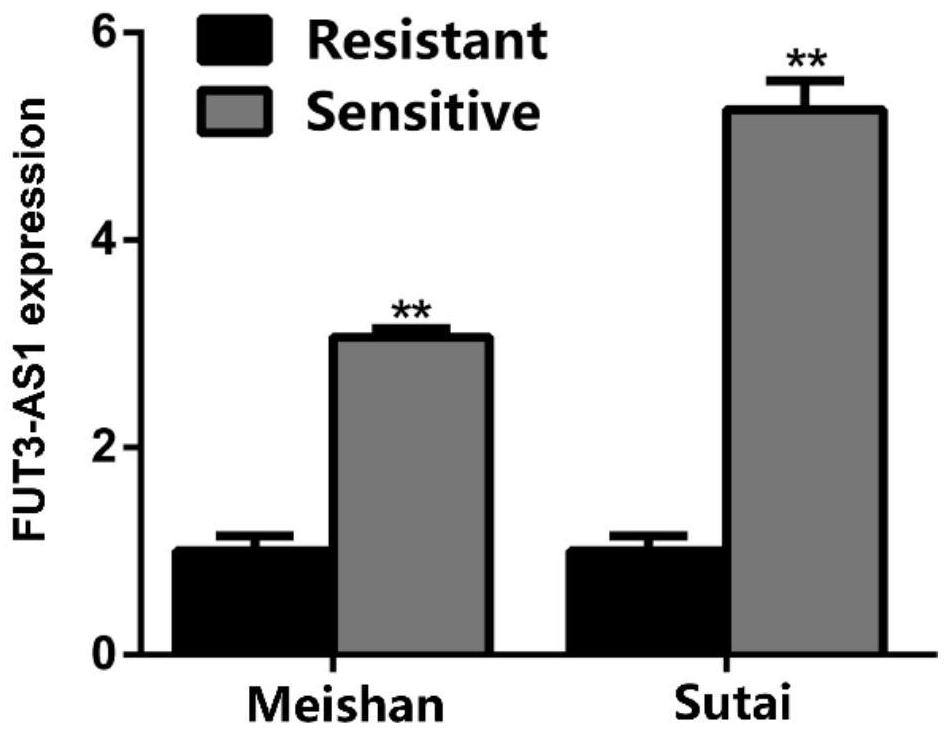
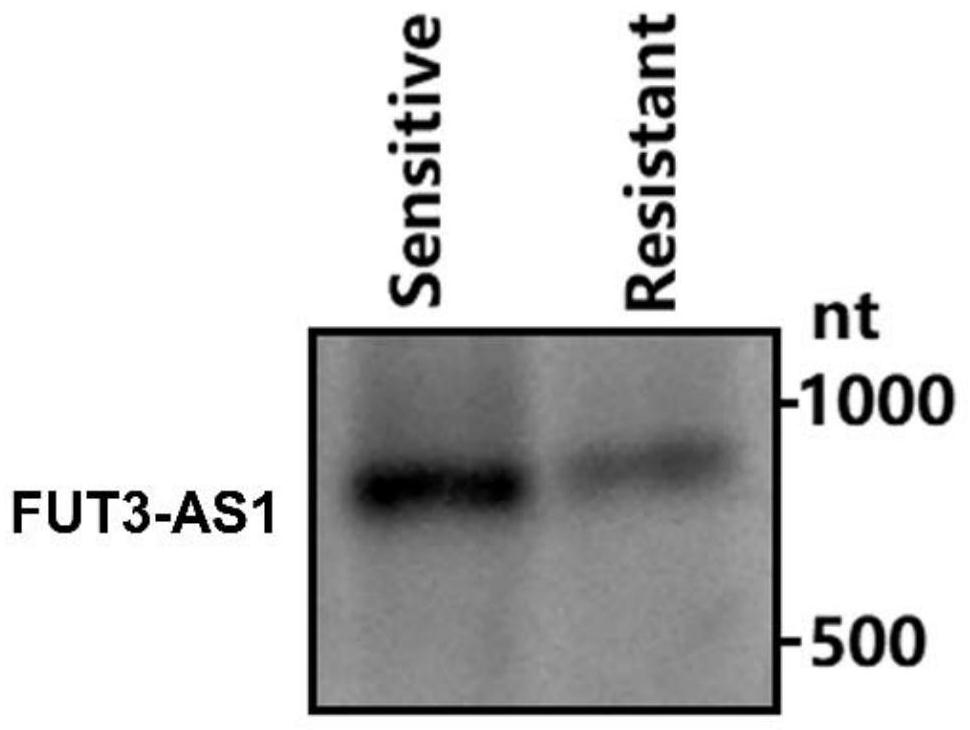
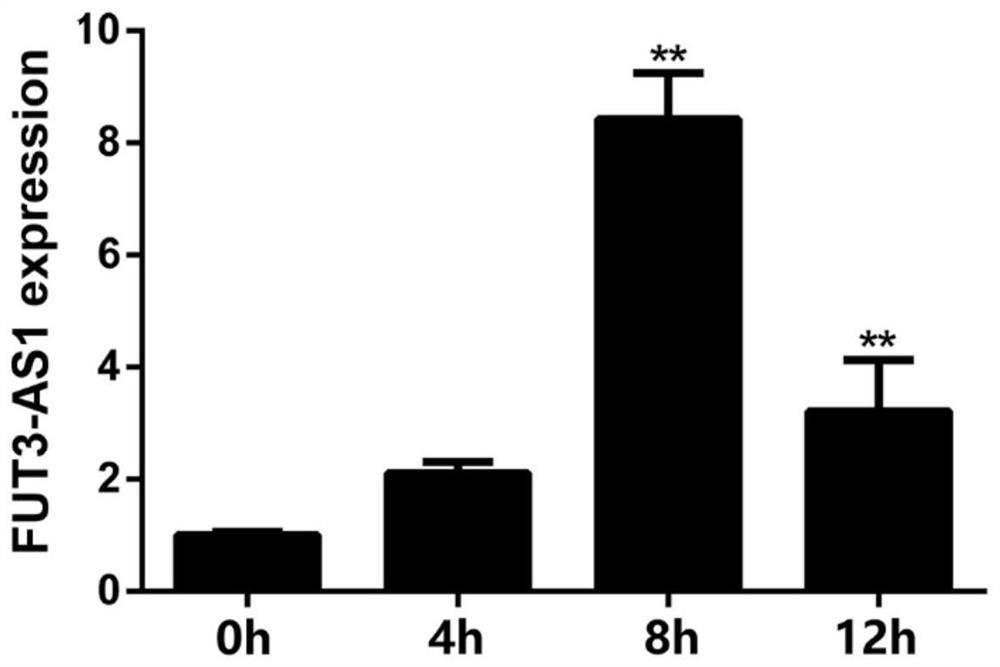
The invention relates to an IncRNA marker related to bacterial diarrhea resistance of piglets and application of the IncRNA marker. According to the IncRNA marker and the application thereof, a key candidate lncRNA-FUT3-AS1 for Escherichia coli F18 infection regulation is screened through omics sequencing, the sequence is SEQ ID NO: 1, and on the basis, deep analysis is carried out around the function and regulation mechanism of the FUT3-AS1 in the research; firstly, qPCR and Northern blot technologies are used for detecting the expression difference condition of the FUT3-AS1 in duodenums of F18 Escherichia coli sensitive and resistant weaned piglets of Meishan pigs and Sutai pigs, lncRNA-FUT3-AS1-silenced pig small intestine epithelial cells are successfully established, and the interference efficiency reaches 68%; and a series of detection means such as bacterial counting, Gram's staining, scanning electron microscopy and indirect immunofluorescence find that the adhesion capability of F18 Escherichia coli to IPEC-J2 cells can be remarkably lowered by silencing lncRNA-FUT3-AS1. Therefore, the lncRNA-FUT3-AS1 plays an important resistance regulation function in a process of resisting F18 Escherichia coli infection of the weaned piglets, the adhesion ability of the small intestine epithelial cells to the F18 Escherichia coli is lowered by down-regulating an expression level of the lncRNA-FUT3-AS1, and the resistance ability of the piglets to the F18 Escherichia coli infection is favorably improved.
Owner:YANGZHOU UNIV
Nourishing bacteriostatic cream for skin
InactiveCN104055836AGood treatment effectThorough curative effectAntibacterial agentsAntimycoticsBiotechnologyBenzoic acid
The invention discloses a nourishing bacteriostatic cream for skin, which relates to a medicine, especially to a bacteriostatic external medicine. The nourishing bacteriostatic cream comprises the following raw materials by weight: 0.9 to 1.1% of menthol, 1.8 to 2.2% of sophora flavescens, 0.9 to 1.1% of borneol, 1.8 to 2.2% of lemonfragrant angelica root, 1.5 to 1.8% of polyoxyethylene dehydrated sorbitol monooleate, 7 to 8% of glycerin, 0.14 to 0.16% of triethanolamine, 8 to 9% of stearic acid, 5 to 6% of Vaseline, 0.9 to 1% of liquid paraffin and 0.16 to 0.19% of benzoic acid, with the balance being purified water. The nourishing bacteriostatic cream for skin provided by the invention overcomes the problem that traditional drugs used to treat skin infection by Staphylococcus aureus, Candida albicans and Escherichia coli do not have ideal treatment effects and cannot thoroughly treat skin infection after long-term usage.
Owner:孙占全
Echinacea extract and preparation method of echinacea extract
The invention relates to an echinacea extract and a preparation method of the echinacea extract. The echinacea extract provided by the invention is obtained through carrying out twice extraction on echinacea medicinal materials by alkaline ethanol solution and lysozyme; and in the product, the polysaccharide content is greater than or equal to 21.50 percent, the polyphenol content is greater than or equal to 8.10 percent, and the chicoric acid content is greater than equal to 5.00 percent. Because the polyphenol content and the chicoric acid content of the echinacea extract are obviously increased, the echinacea extract is prepared into ordinary preparations including powder or oral liquid and the like, and the curative effect is greatly improved. In a compound preparation processed by the echinacea extract and bacteriolysant according to a consumption ratio being 3:1, active ingredients of echinacea and lysozyme totally account for 20 percent, wherein the consumption ratio of the echinacea extract to the lysozyme is (4-1):1, auxiliary materials of cane sugar account for 64 percent, and dextrin accounts for 16 percent. The compound preparation has the functions of improving the immunity of live stock and realizing the sterilization and antivirus functions, and can be used as medicine for preventing and treating animal diseases, particularly chicken diseases caused by escherichia coli infection.
Owner:QILU ANIMAL HEALTH PROD
Inhibitors of f18+ e coli binding
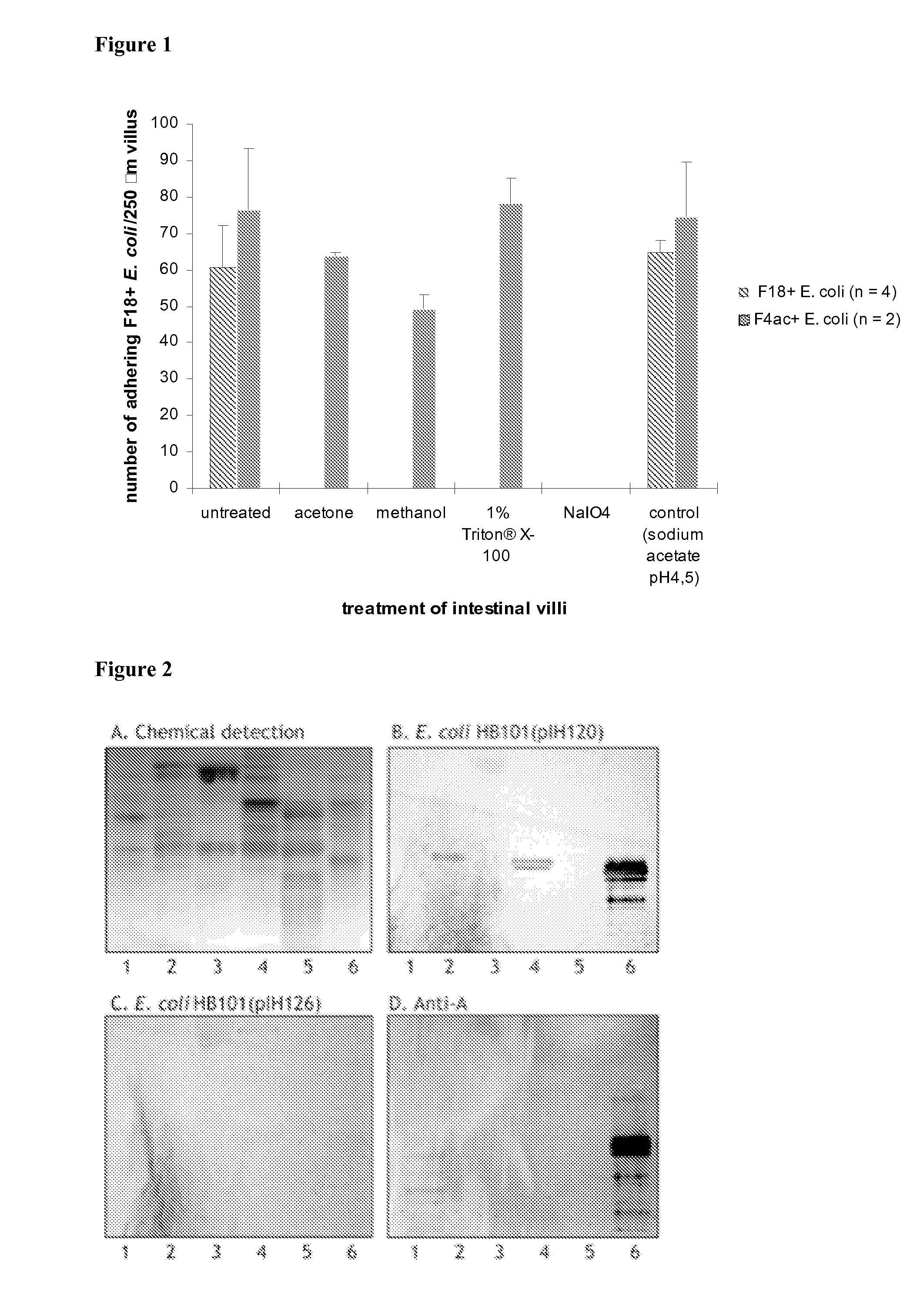
The present invention relates to blood group A / B / H determinant on Type 1 Core glycosphingolipids chains as recognition point for the FedF protein of F18-fimbriated Enterotoxigenic and verotoxinogenic Escherichia coli and the use of compounds comprising such determinants for the treatment of F18′ E. coli infections in pigs and in screening methods.
Owner:UNIV GENT
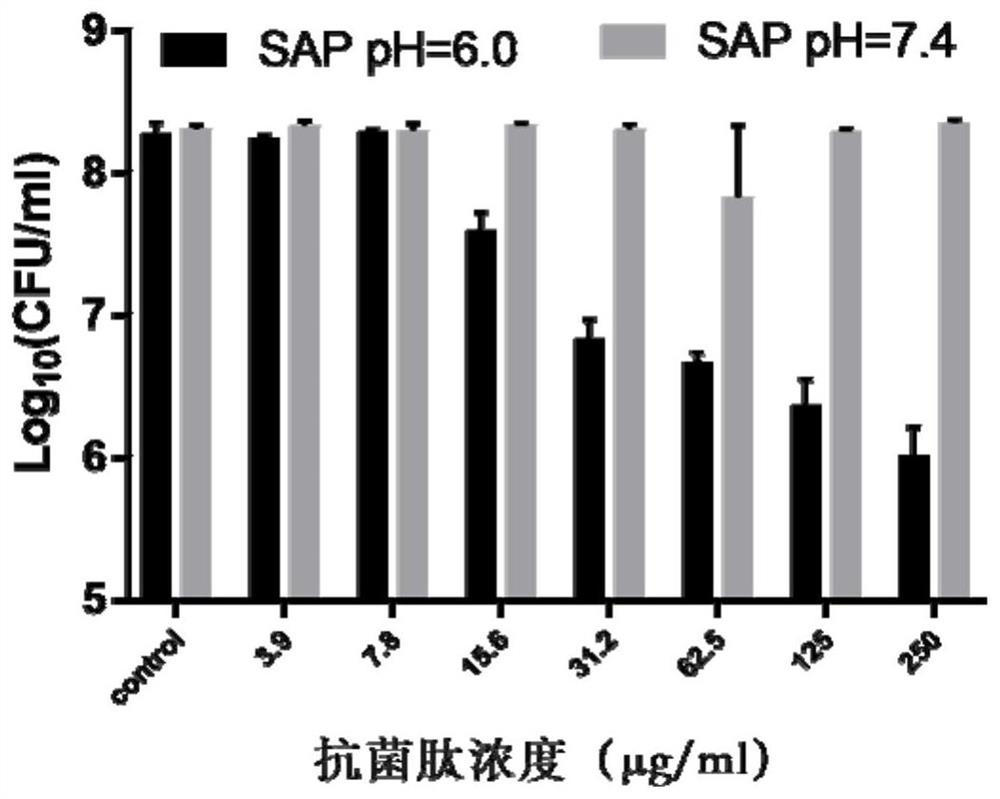
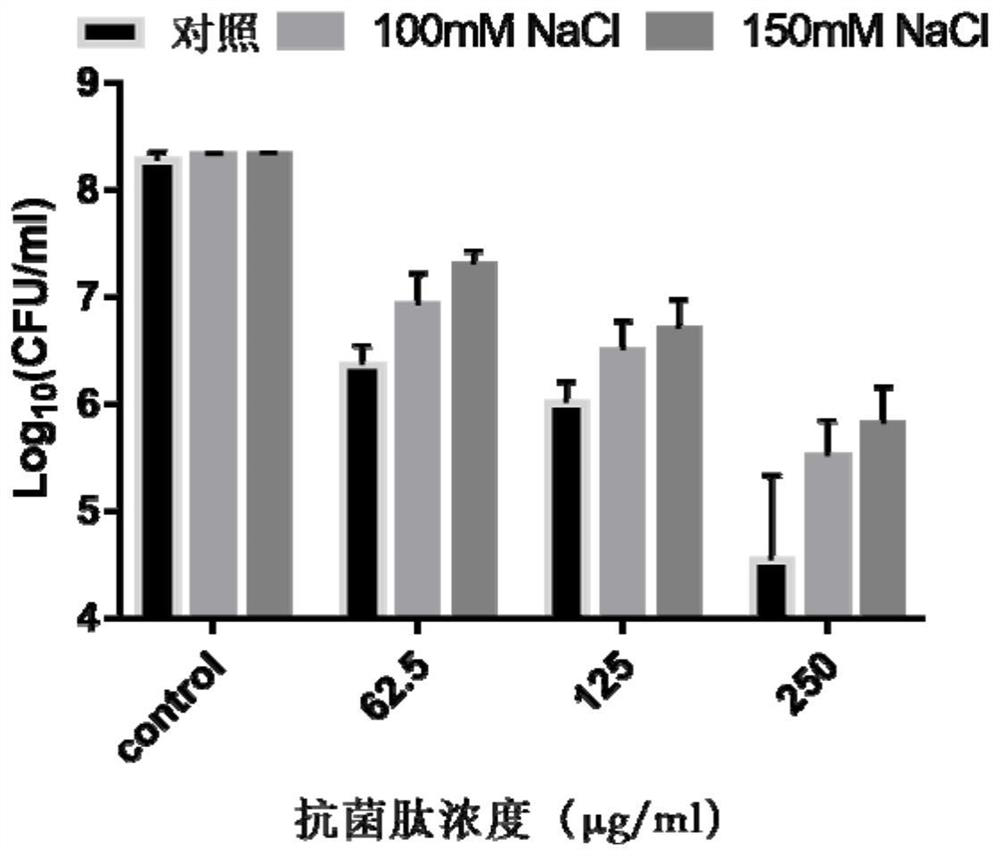
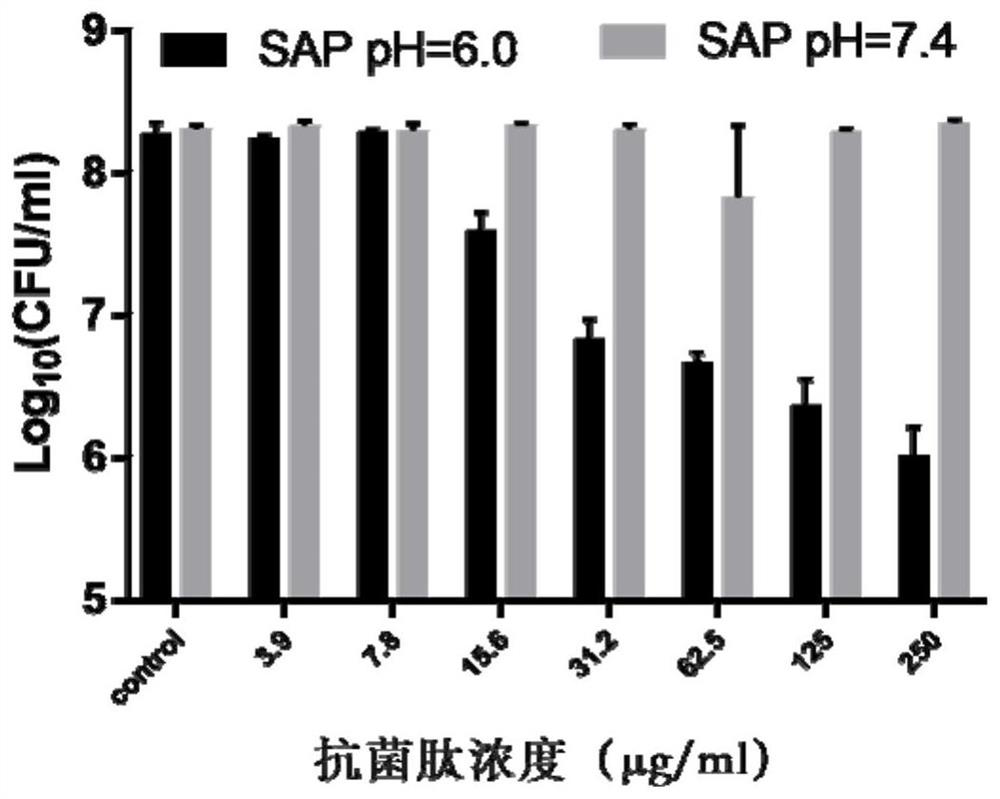
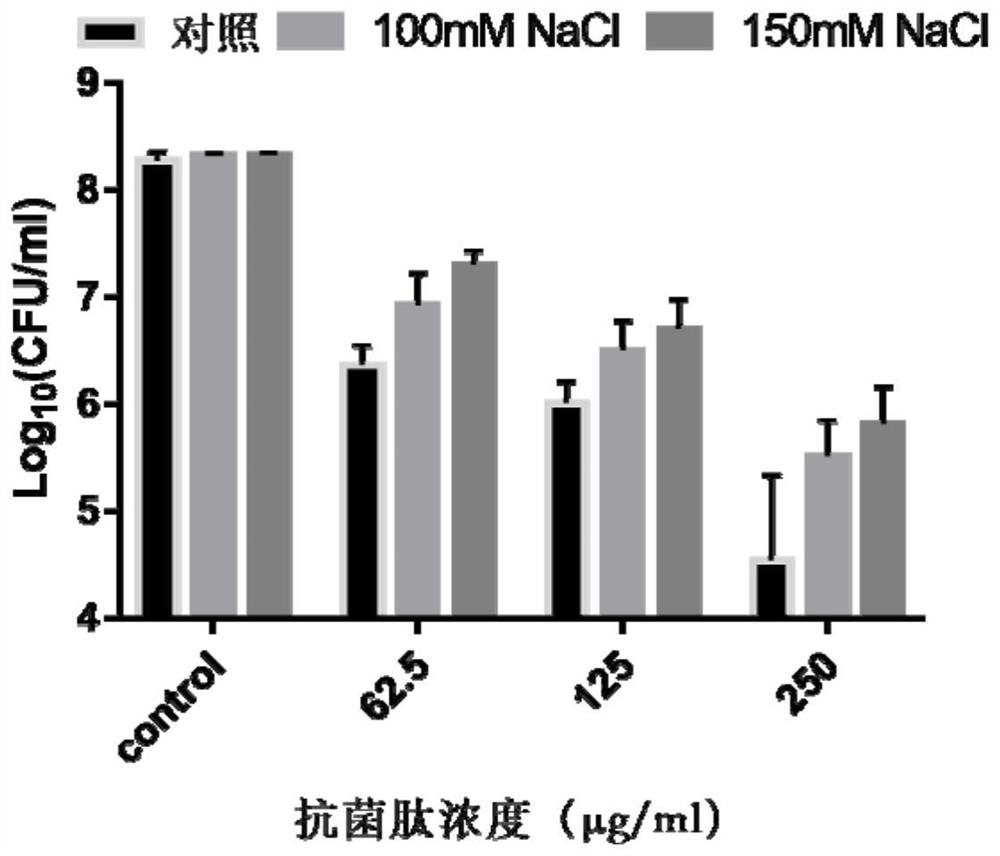
pH regulation self-assembled antibacterial peptide and preparation method and application
ActiveCN111777670ALow toxicityImprove antibacterial propertiesAntibacterial agentsPeptide-nucleic acidsAntimicrobial actionEscherichia coli
The invention discloses a pH regulation self-assembled antibacterial peptide and a preparation method and application. The sequence of an antibacterial peptide SAP is shown as SEQID No.1. A histidineHis functionalization beta folding peptide containing hydrophobic amino acids Trp and Val is designed, the His and the Trp are respectively placed in a non-hydrogen key point adjacent to a beta-folding structure, and are distributed in a diagonal line, the His and the Val which are equivalent are respectively distributed at other hydrogen key points, and DPro-Gly is used as a two-end-peptide chainallowing a beta-corner to be connected with the beta-folding structure. The freeze-dried powder of the antibacterial peptide dissolves in ultra pure water of which the pH is 6.0, and antibacterial peptide monomers are sufficiently self-assembled to obtain nanometer granules in an aqueous solution. The antibacterial peptide is applied to preparation of medicines for treating coli-infection diseases under acid environment. The antibacterial peptide exerts effective antibiotic action under the acid environment, the hemolytic poison is low, the salt ion stability is good, and the defect that a natural antibacterial peptide is low in stability can be overcome.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
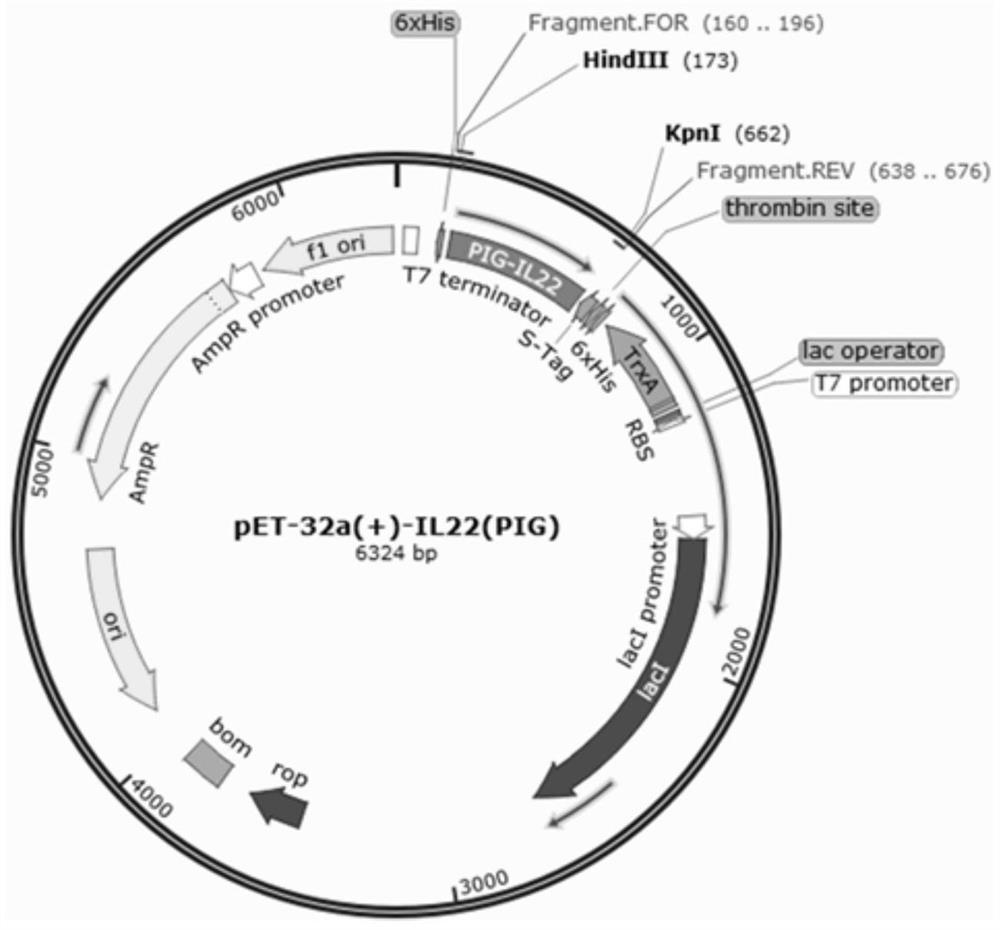
High expression and application of recombinant porcine interleukin 22 in Escherichia coli
ActiveCN108070599BPlasmid high expressionProcess stabilityAntibacterial agentsAntimycoticsTransgenesisCell system
The invention discloses a gene encoding recombinant porcine interleukin-22. The invention also discloses a recombinant expression vector containing the recombinant gene, a transgenic cell system, a transgenic recombinant bacterium and a host cell. The invention also discloses recombinant porcine interleukin 22 and its extraction and purification method. The invention also discloses the application of the recombinant plasmid pET-32a(+)-pIL-22, the recombinant strain and the recombinant protein in anti-apoptosis and anti-Escherichia coli infection. The vector pET‑32a (+) for expressing recombinant porcine interleukin 22 constructed by the present invention contains a His tag, and the recombinant porcine interleukin 22 protein extracted from Escherichia coli is connected with the His tag, and highly purified recombinant porcine interleukin can be obtained through the His column 22 protein; the expression yield of recombinant porcine interleukin 22 protein was counted by Quantity One software, and the purity was as high as 95.7%.
Owner:NANJING AGRICULTURAL UNIVERSITY
A kind of ph-regulated self-assembled antimicrobial peptide and its preparation method and application
ActiveCN111777670BLow toxicityImprove antibacterial propertiesAntibacterial agentsPeptide-nucleic acidsEscherichia coliAntimicrobial action
The invention discloses a pH-regulating self-assembled antibacterial peptide, its preparation method and application. The sequence of the antibacterial peptide SAP is shown in SEQ ID No.1. Design a histidine His-functionalized β-sheet peptide containing hydrophobic amino acids Trp and Val. His and Trp are respectively placed at the non-hydrogen bond sites adjacent to the β-sheet structure, distributed in a diagonal form, and other hydrogen bond sites distribute equal amounts of His and Val, respectively, D Pro-Gly acts as a β-turn to connect the two peptide chains of the β-sheet structure. The freeze-dried powder of the antimicrobial peptide is dissolved in ultrapure water with pH=6.0, and the monomers of the antimicrobial peptide fully self-assemble into nanoparticles in the aqueous solution. The antimicrobial peptide is used in the preparation of medicines for treating Escherichia coli infectious diseases in acidic environment. Antimicrobial peptides exert effective antibacterial effects in acidic environments, have low hemolytic toxicity, and have good salt ion stability, which overcomes the weakness of low stability of natural antimicrobial peptides.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
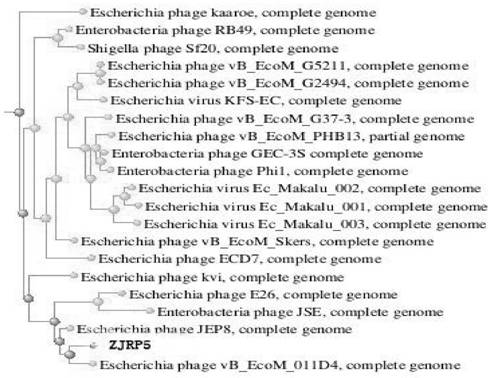
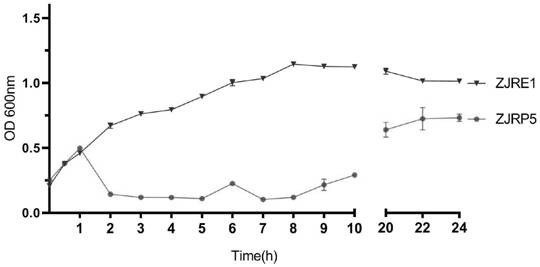
Escherichia coli bacteriophage ZJRP5, application thereof, bactericide and medicine
ActiveCN114292822AAntibacterial agentsViral/bacteriophage medical ingredientsEnterobacterialesBacteriocidal Agents
The invention relates to the field of disease control, in particular to an escherichia coli bacteriophage ZJRP5 and application thereof, a bactericide and a medicine. The invention relates to an escherichia coli bacteriophage ZJRP5 with a bactericidal effect. The preservation number of the escherichia coli bacteriophage ZJRP5 is CCTCC (China Center For Type Culture Collection) NO.M202183. The escherichia coli bacteriophage ZJRP5 has a bactericidal effect. The escherichia coli bacteriophage ZJRP5 disclosed by the invention has a relatively wide host range and can be used for effectively preventing and treating escherichia coli infection.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
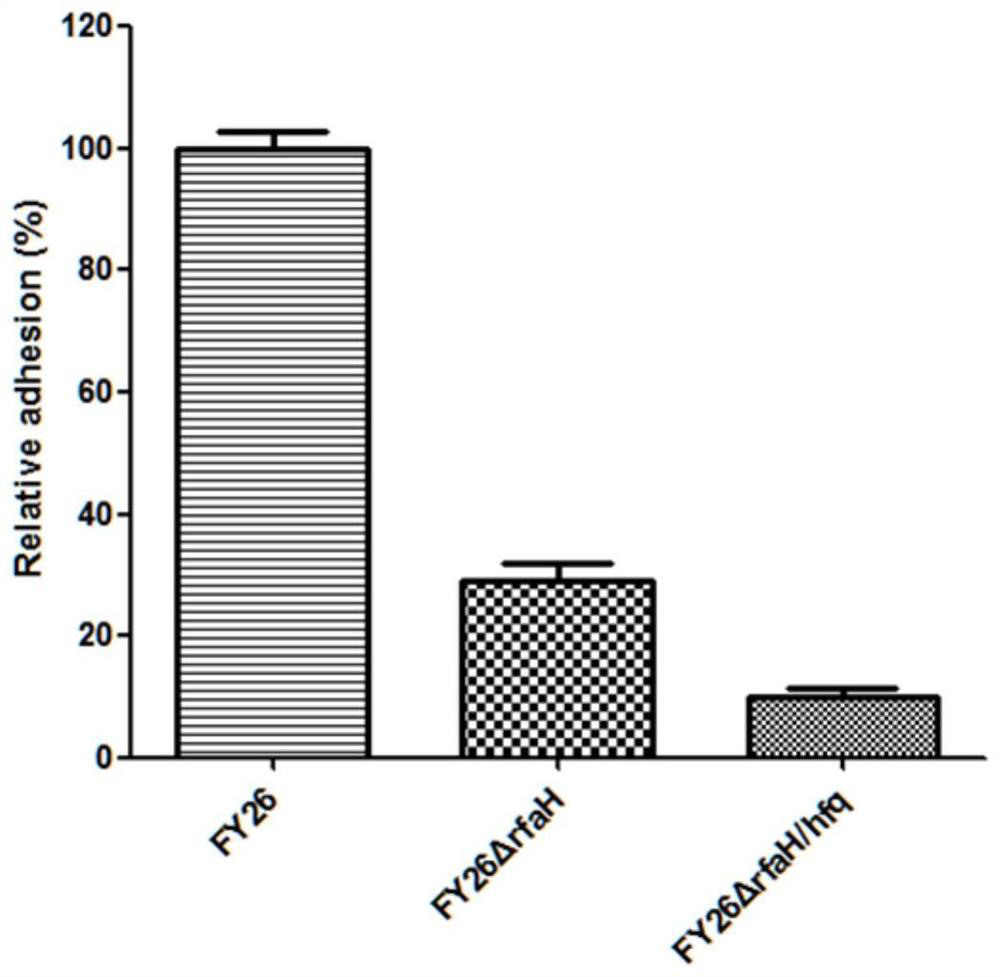
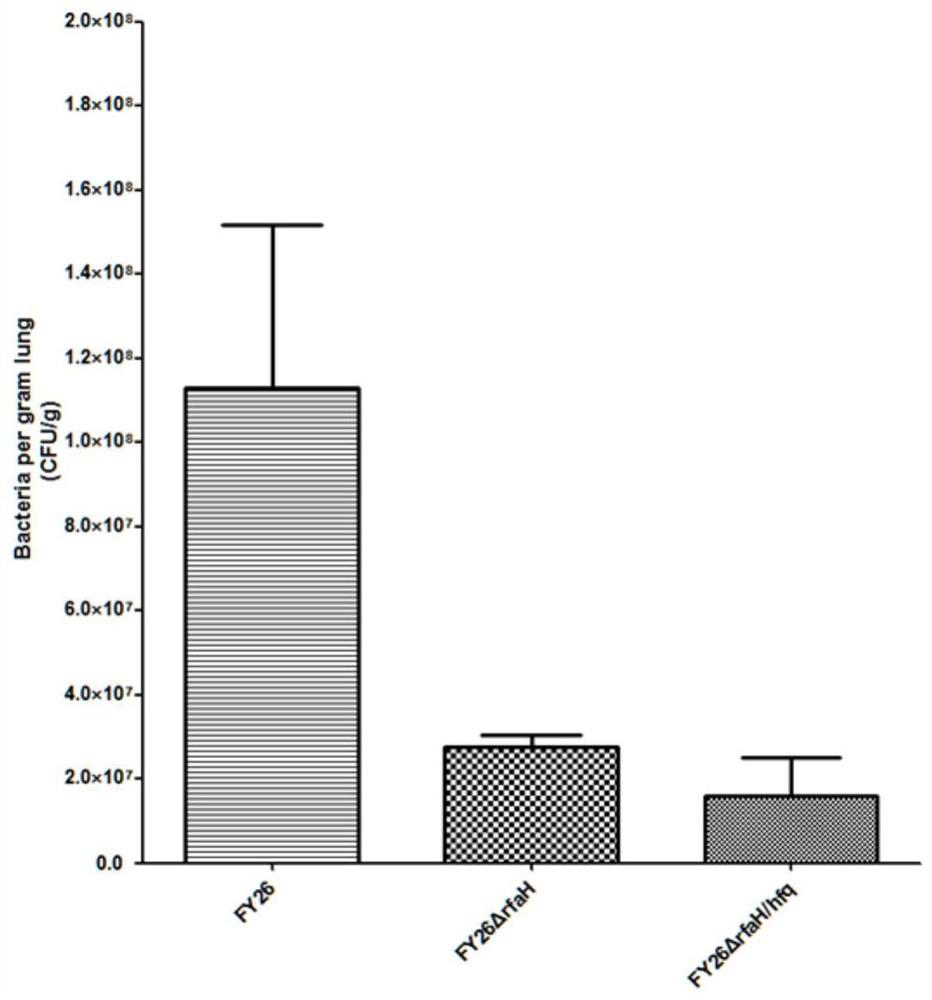
APEC (avian pathogenic E .coli) double-gene rfaH and hfq deleted strain and attenuated vaccine
InactiveCN112680391AReduce pathogenicityImprove protectionAntibacterial agentsBacteriaImmunogenicityTGE VACCINE
The invention belongs to the field of microorganisms, and discloses an APEC (avian pathogenic E .coli) double-gene rfaH and hfq deleted strain and an attenuated vaccine. A construction method of the APEC double-gene deletion strain is a Red homologous recombination method, rfaH and hfq double genes are knocked out to obtain APEC FY26[delta]rfaH / hfq, and the APEC FY26[delta]rfaH / hfq is preserved in China Center for Type Culture Collection, and the preservation number of the strain is CCTCC NO: M 2020612. The deleted strain has good biological safety and immunogenicity, is low in toxicity, and can be used for preparing the APEC attenuated vaccine to prevent APEC infection. The attenuated vaccine prepared from the APEC FY26[delta]rfaH / hfq has immune protection against APEC infection, and has good toxicity attacking immune protection effect.
Owner:NANTONG UNIVERSITY
Breeding method of disease-resistant pigs
ActiveCN113142130AStable disease resistanceIncrease resistanceAnimal husbandryDiseaseAfrican swine fever
The invention relates to the technical field of animal genetic breeding and reproduction, in particular to a breeding method of disease-resistant pigs. The breeding method of the disease-resistant pigs comprises the following steps that (1), one or more factors of noise, driving, transition and temperature serve as a stress source, and anti-stress pigs are bred through a group subculture breeding method; and (2) the anti-stress pigs in the step (1) are taken as a basic group, and anti-stress and anti-escherichia coli infection pigs are bred through the group subculture breeding method. According to the breeding method of the disease-resistant pigs, the variety obtained through a traditional disease-resistant breeding means is stable in disease resistance and has multiple resistances; the bred anti-stress and anti-escherichia coli infection pigs also have good resistance to other diseases such as swine fever, porcine reproductive and respiratory syndrome and the like, especially have extremely high resistance to the African swine fever, and the death rate is almost zero after the pigs are infected with the African swine fever under natural conditions.
Owner:山东融发生物科技开发有限公司
Preparation method of shiga-like toxin Stx1B oral vaccine and product of shiga-like toxin Stx1B oral vaccine
InactiveCN102580118BIncrease productionReduce outputAntibacterial agentsGenetic material ingredientsBiotechnologyOedema disease
Owner:CHONGQING UNIV
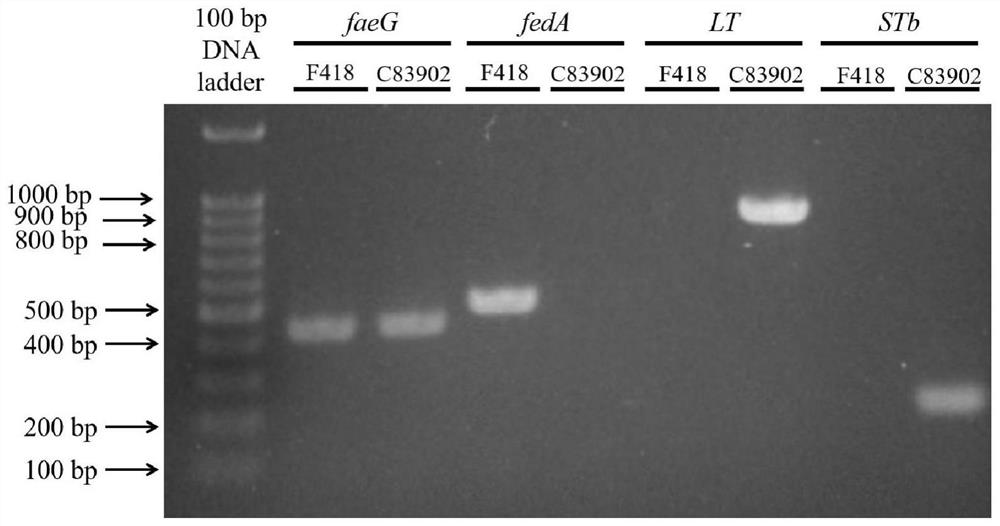
Swine Escherichia coli non-toxic isolate capable of simultaneously expressing F4 and F18 pili and application of swine Escherichia coli non-toxic isolate
ActiveCN114752531AImprove adhesionGood adhesion colonizationAntibacterial agentsBacteriaAntigenPilus
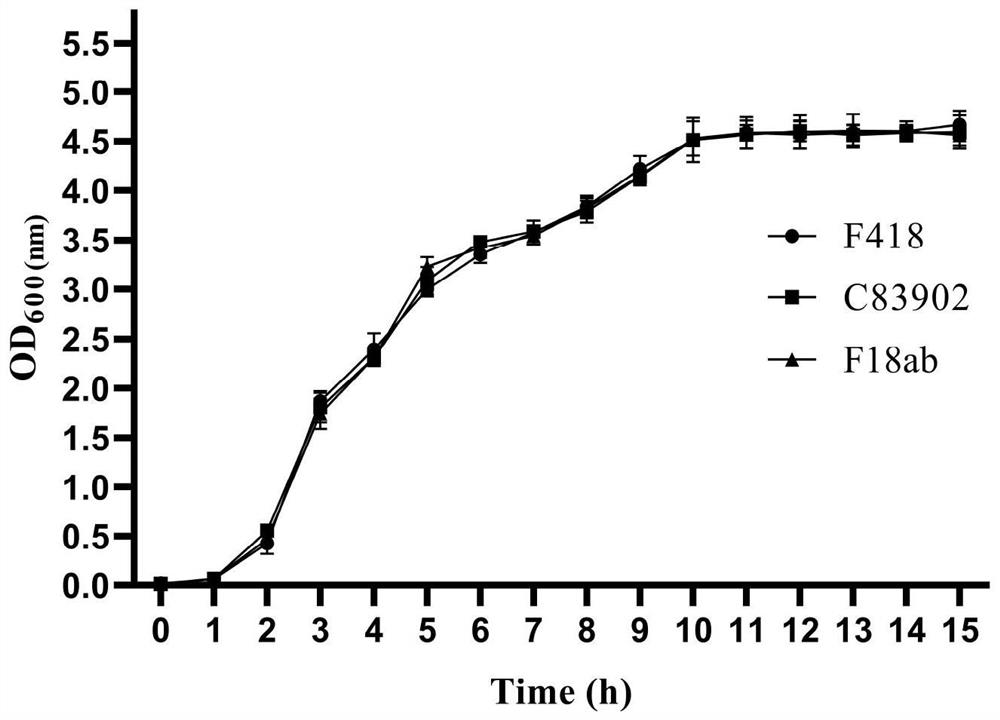
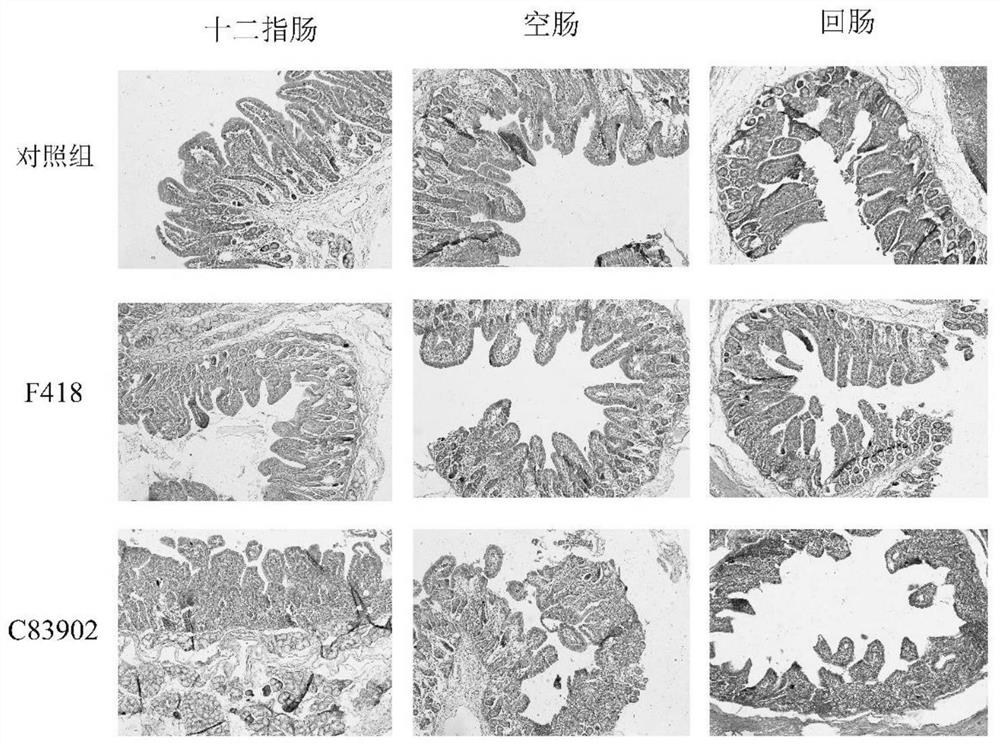
The invention relates to a swine Escherichia coli non-toxic isolated strain capable of simultaneously expressing F4 and F18 pili and application of the swine Escherichia coli non-toxic isolated strain, the preservation number of the swine Escherichia coli non-toxic isolated strain F418 is CCTCC M 2022089, and the preservation date is January 18, 2022. The isolated strain is escherichia coli, can simultaneously express F4 and F18 fimbriae antigens and is a swine-derived natural isolated strain, PCR (Polymerase Chain Reaction) tests show that the strain lacks LT and STb enterotoxin genes, does not generate enterotoxin, can be well adhered and colonized to a host pig, has the potential of being developed into a bacterial non-toxic live vaccine candidate strain, and can be used for preparing a bacterial non-toxic live vaccine candidate strain. The strain can be well adhered and colonized to a host pig by oral administration, a piglet is induced to generate immune response for resisting F4 and F18 pilus antigens, a certain immune efficacy is exerted, the safety is guaranteed, and the strain is expected to become a potential live vaccine candidate strain for preventing and controlling F4 and F18 escherichia coli infection.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com