Application of antibacterial peptide in in-vitro inhibition of Escherichia coli
A technology of Escherichia coli and antibacterial peptides, applied in the field of cell biology, can solve the problems of inability to meet in vitro sterilization, general sterilization effect, poor sterilization effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
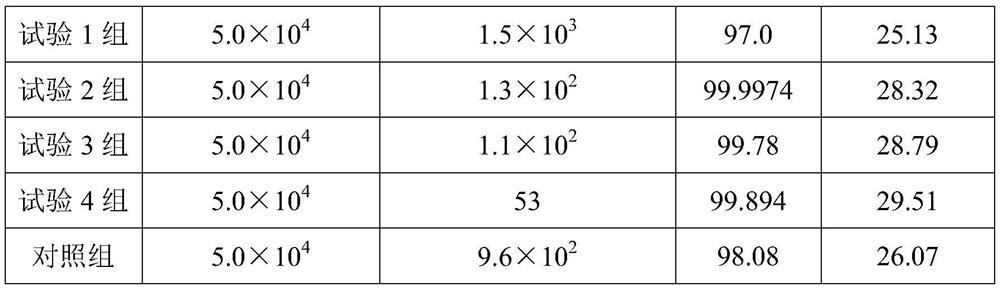
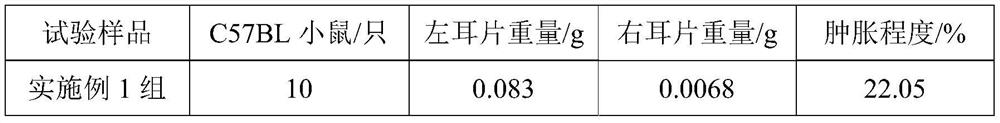
[0021] A kind of pharmaceutical composition of embodiment 1
[0022] The pharmaceutical composition comprises 0.15 g of a mixture of antimicrobial peptide Esculentin-1A and citric acid in a mass ratio of 7:2, 600 mL of normal saline, 0.8 mL of lubricant hydrogenated vegetable oil, 1.3 g of cross-linking agent sodium alginate, apple salt 5g, preservative potassium sorbate 1.3g.
[0023] The preparation process of the pharmaceutical composition is as follows: heating the water for injection to 65° C., and then respectively adding a mixture composed of antibacterial peptide Esculentin-1A and citric acid, a lubricant hydrogenated vegetable oil, a cross-linking agent sodium alginate, malate, Preservative potassium sorbate, stirred at 400rpm for 30min, until mixed evenly and completely dissolved, cooled to 25°C to obtain.
Embodiment 2
[0024] A kind of pharmaceutical composition of embodiment 2
[0025] The ingredients and preparation method of the pharmaceutical composition are similar to those in Example 1, the difference from Example 1 is that the mixture in Example 2 is composed of antibacterial peptide Esculentin-1A and citric acid in a mass ratio of 7-10:2-5 proportional composition.
Embodiment 3
[0026] A kind of pharmaceutical composition of embodiment 3
[0027] The ingredients and preparation method of the pharmaceutical composition are similar to those of Example 1, the difference from Example 1 is that the mixture in Example 3 is composed of antibacterial peptide Esculentin-1A and citric acid in a mass ratio of 9:4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com