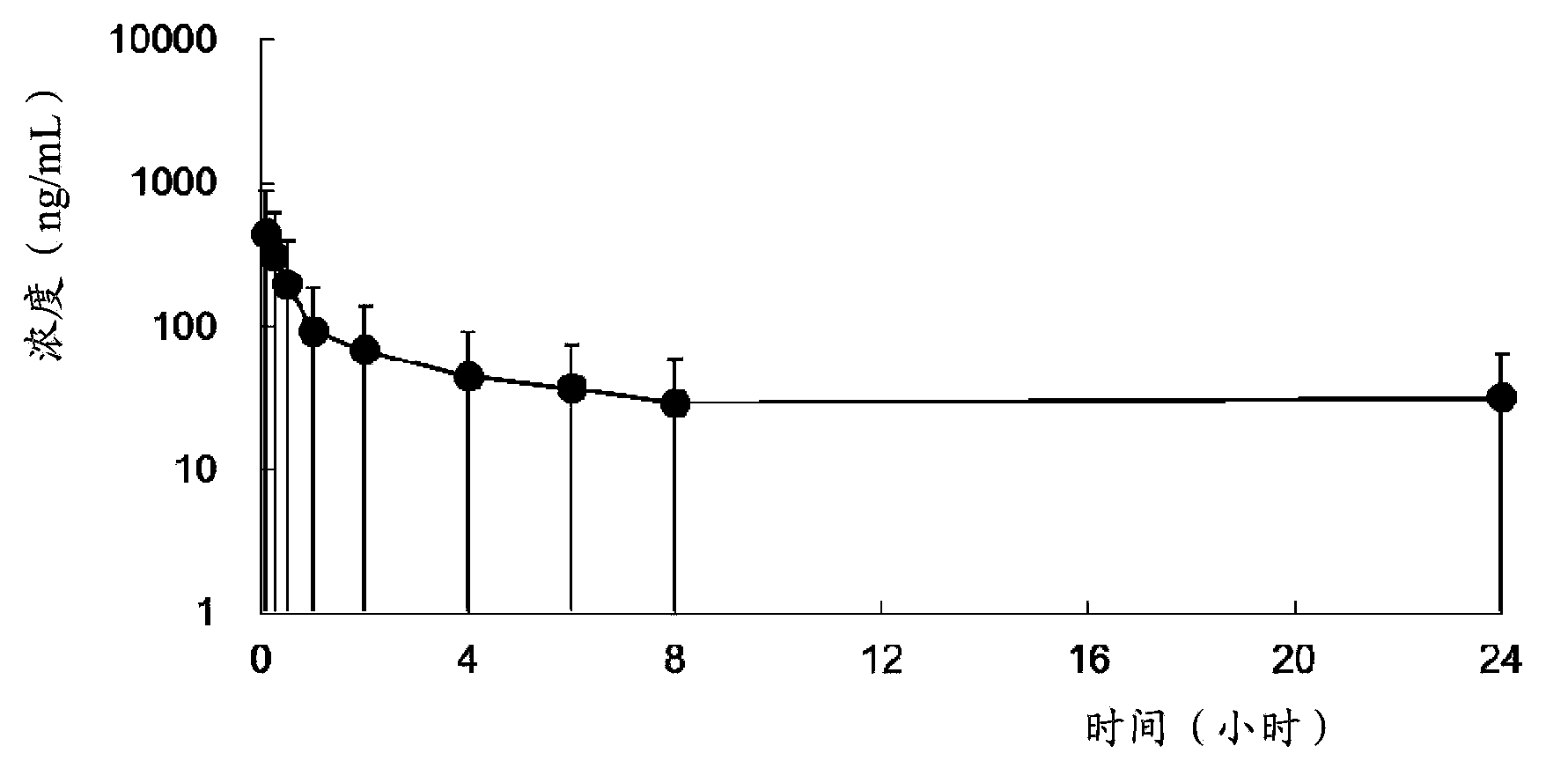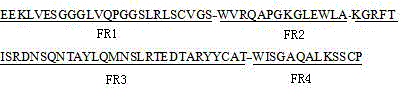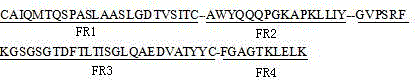Patents
Literature
107 results about "Swine origin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Swine It is believed that the majority of the breeds we now know are descended from the Eurasian Wild Boar (Sus scrofa). Archaeological evidence from the Middle East indicates domestication of the pig occurs as early as 9,000 years ago, with some evidence for domestication even earlier in China.
Use of neuraminidase inhibitor and neuraminidase inhibitor prodrug
ActiveCN103845316AExtended half-lifeOrganic chemistryOrganic compound preparationPatient needViral infection
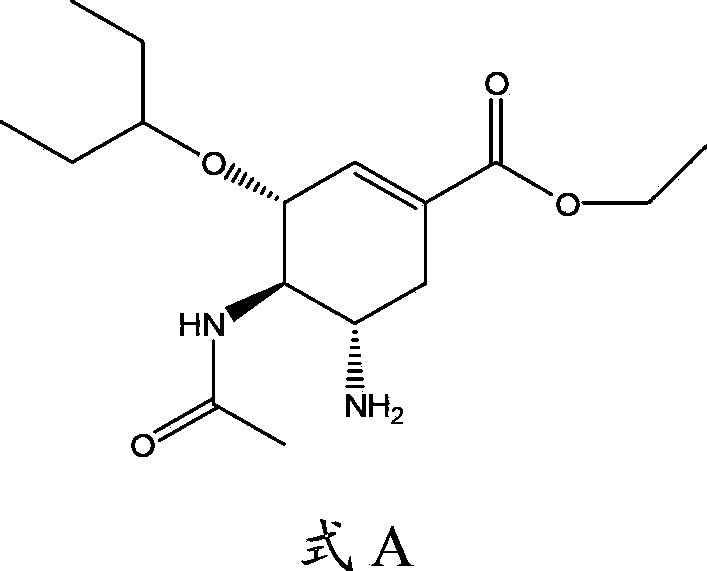
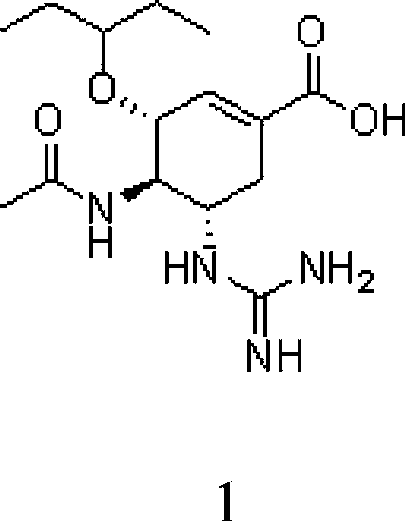
The invention provides use of a compound shown as formula Ia or a prodrug thereof in preparation of drugs for the prevention and / or treatment of Tamiflu resistant influenza viruses strain infection, a Tamiflu resistant influenza viruses strain is preferably H1N1 Swine-origin influenza A virus H274Y. The invention also provides a method for the treatment, prevention or delay of virus infection caused by the Tamiflu resistant influenza viruses strain, and the method comprises administering of a therapeutically effective amount of the compound or the prodrug thereof to patients needing the treatment.
Owner:BEIJING PRELUDE PHARM SCI & TECH
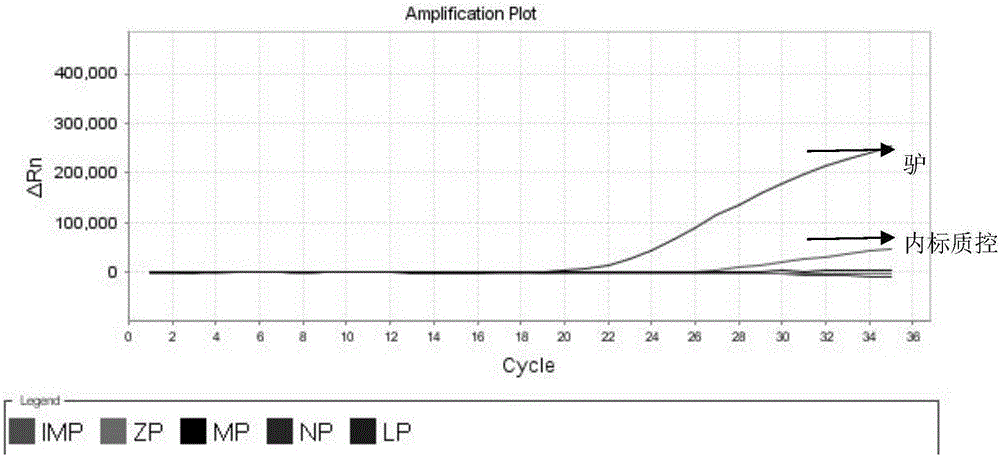
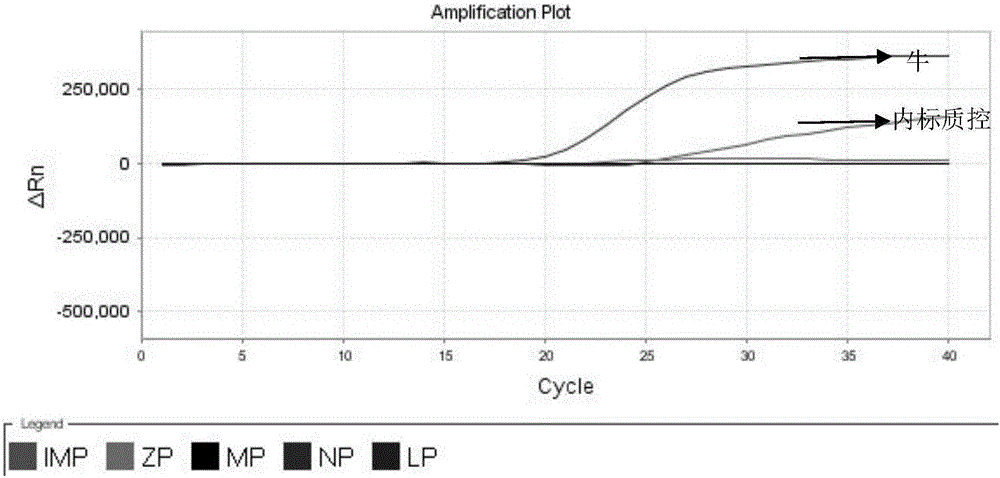
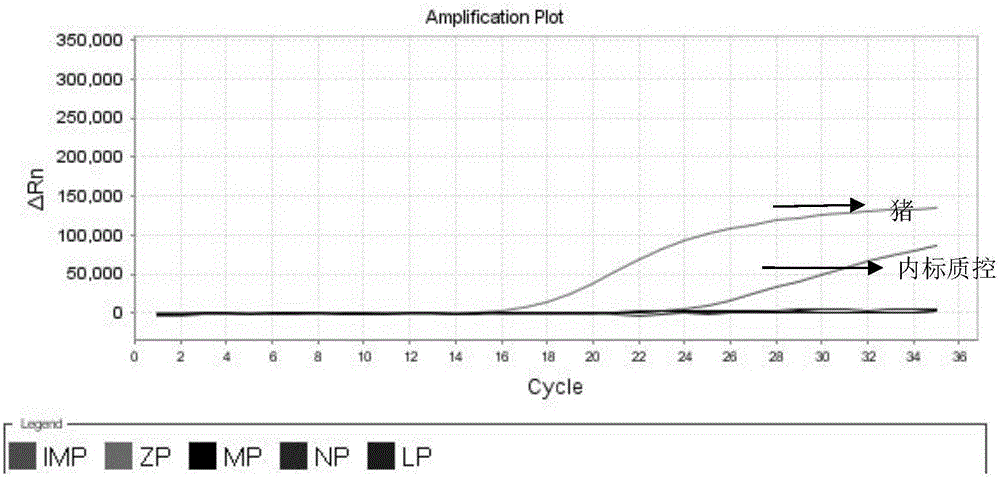
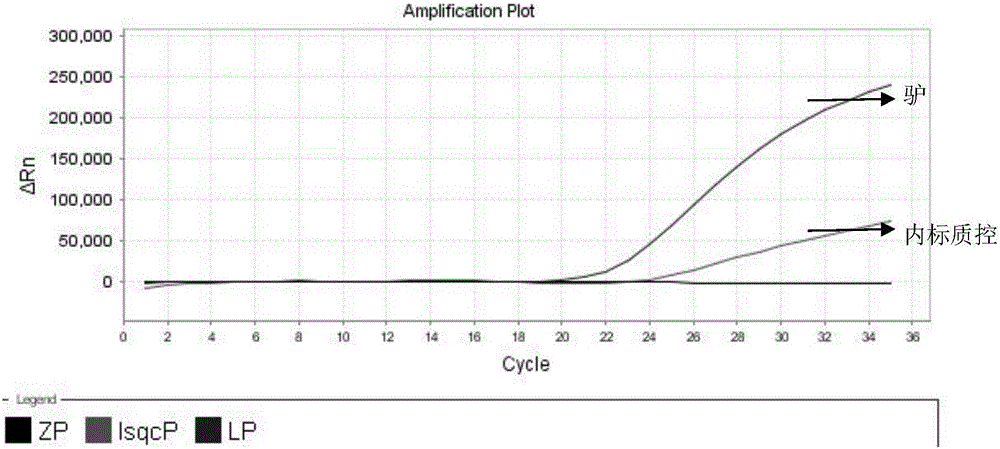
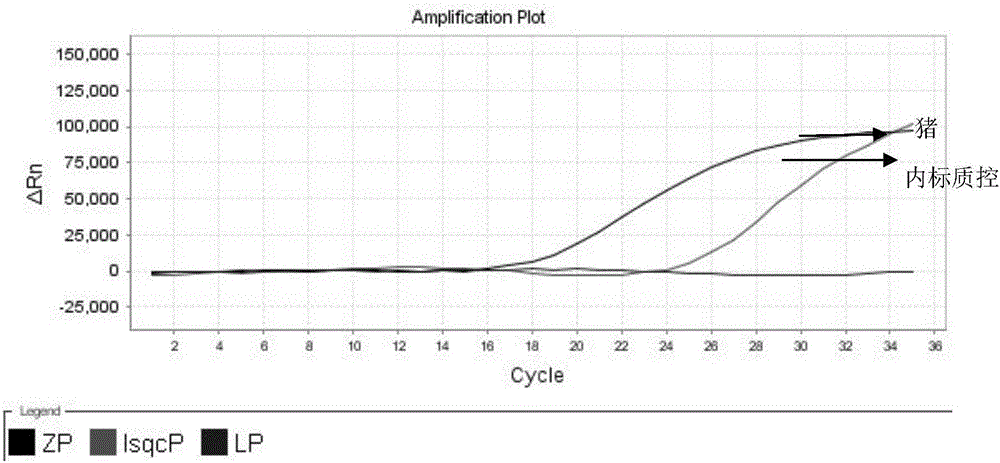
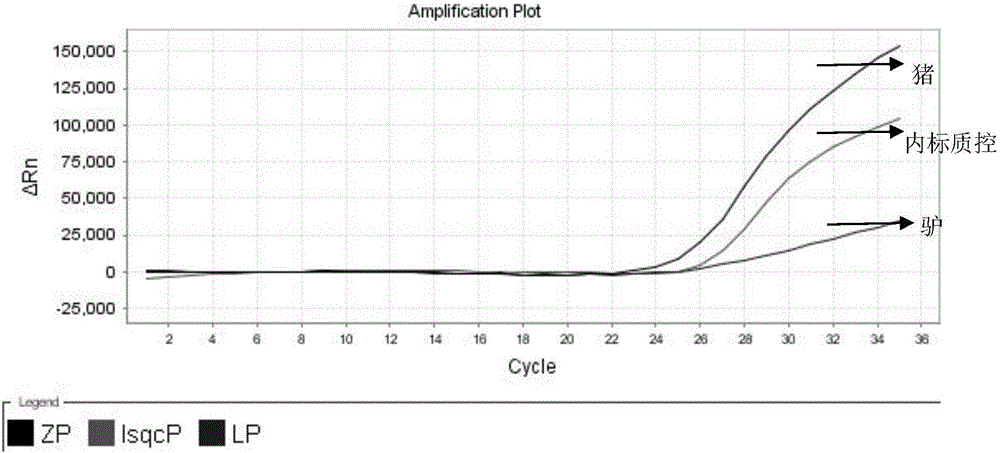
Nested fluorescent PCR detection primer, probe, kit and detection method of donkey-origin, horse-origin, swine-origin and bovine-origin in donkey-hide gelatin, and applications thereof
InactiveCN105907852AImprove real reliabilityImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationFluorescent pcrGelatin
The invention provides a nested fluorescent PCR detection primer, a probe, a kit and a detection method of donkey-origin, horse-origin, swine-origin and bovine-origin in donkey-hide gelatin, and applications thereof, and relates to the technical field of molecular biology. Using combinations of the primer and probe of the invention as well as multiple nested fluorescent PCR detection kits can quickly detection the donkey-origin, horse-origin, swine-origin and bovine-origin components in donkey-hide gelatin. Aiming at microscale DNA or degradation DNA in donkey-hide gelatin, the nested fluorescent PCR detection primer, the probe, the kit and the detection method greatly increase the effective target spot identification rate of the primer and the probe by nested PCR and short PCR fragment technologies, thereby greatly increasing the detection accuracy and sensitivity; two steps of PCR amplifications are conducted within one system, thereby providing advantages such as closed pipe operation, low contamination possibility, high detection sensitivity, good specificity, high accuracy, great flux, etc.
Owner:BIOTECH RES CENT SHANDONG ACADEMY OF AGRI SCI
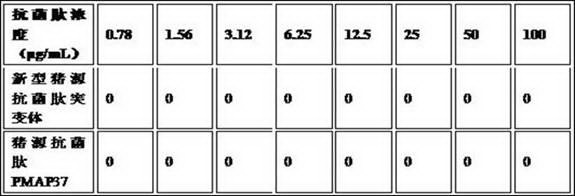
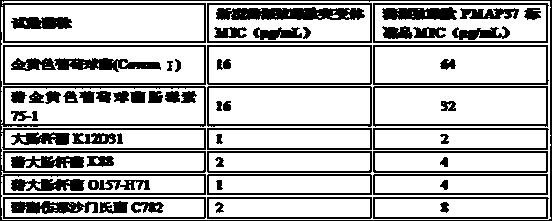
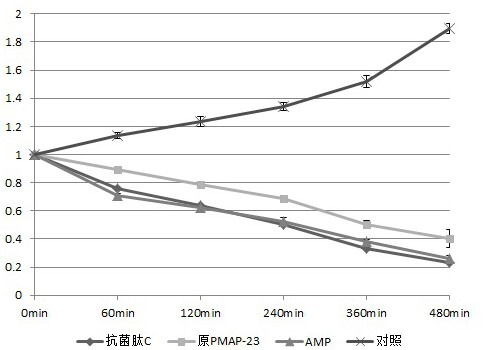
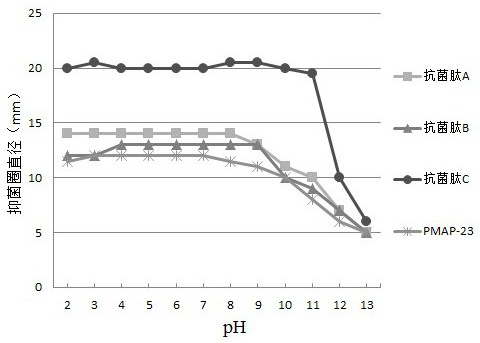
Novel pig-source antibacterial peptide mutant, preparation method and application thereof
InactiveCN104341497AHigh antibacterial potencyBroad antibacterial spectrumMicroorganism based processesFermentationPichia pastorisAntimicrobial peptides
The invention provides a novel pig-source antibacterial peptide mutant, a preparation method and an application thereof. According to the invention, the spatial structure analysis is preformed on the amino acid sequence of pig-source antibacterial peptide PMAP37 through biology software and three amino acids (L6K, K20L and L31K) of the thirty-seven amino acids are mutated, so that the novel pig-source antibacterial peptide mutant is acquired and the antibacterial effect is obviously increased. On the basis, a pichia pastoris biased codon is selected and a novel pig-source antibacterial peptide mutant gene is artificially synthesized and is cloned in expression of the pichia pastoris, so that a novel antimicrobial peptide recombined yeast strain is acquired, the fermentation scale is amplified to the fermentation tank level and the high-density fermentation and high-efficient expression of the antibacterial peptide products are realized. After the fermentation liquor is further purified, the fermentation liquor can be prepared into an antibacterial peptide preparation, such as powder and liquid, used for preventing and treating livestock diseases.
Owner:QINGDAO AGRI UNIV
Recipient beta-blocker of prevention weaning piggy diarrhea and piggy oedematous disease, preparing and application
InactiveCN101199556AProtection from diseaseEffective combinationBacteriaBacteria material medical ingredientsMultiple drug resistanceAntigen
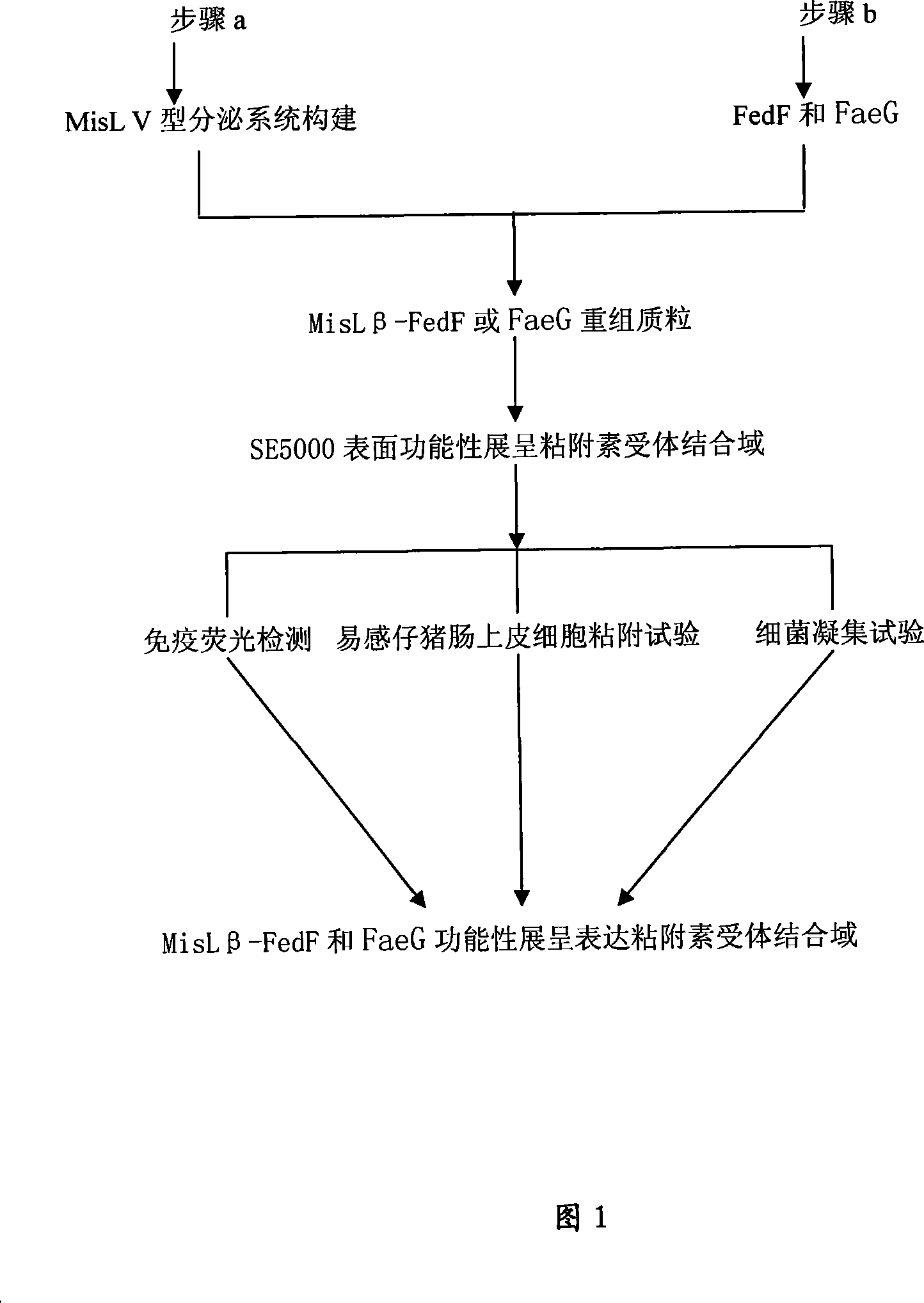
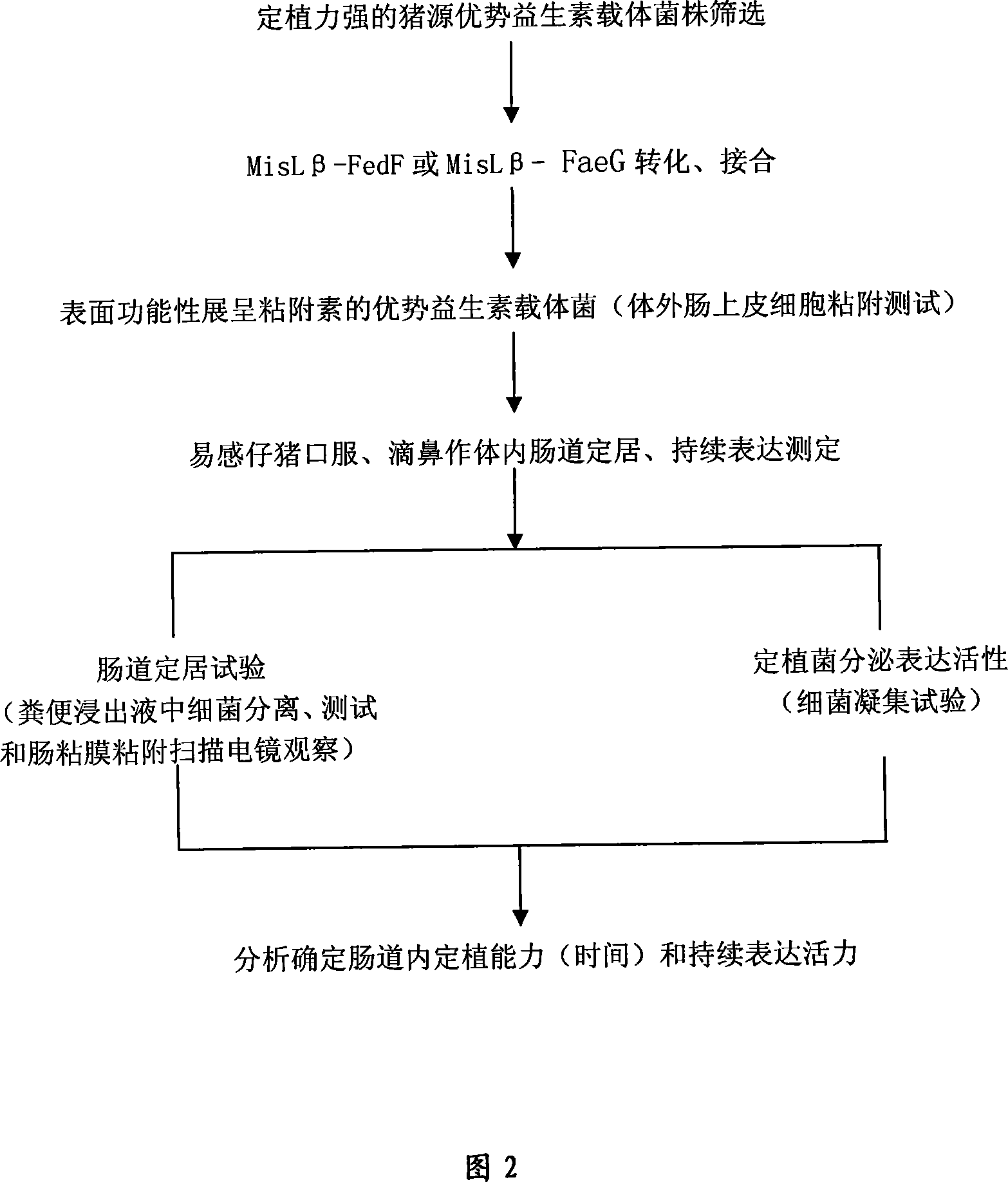
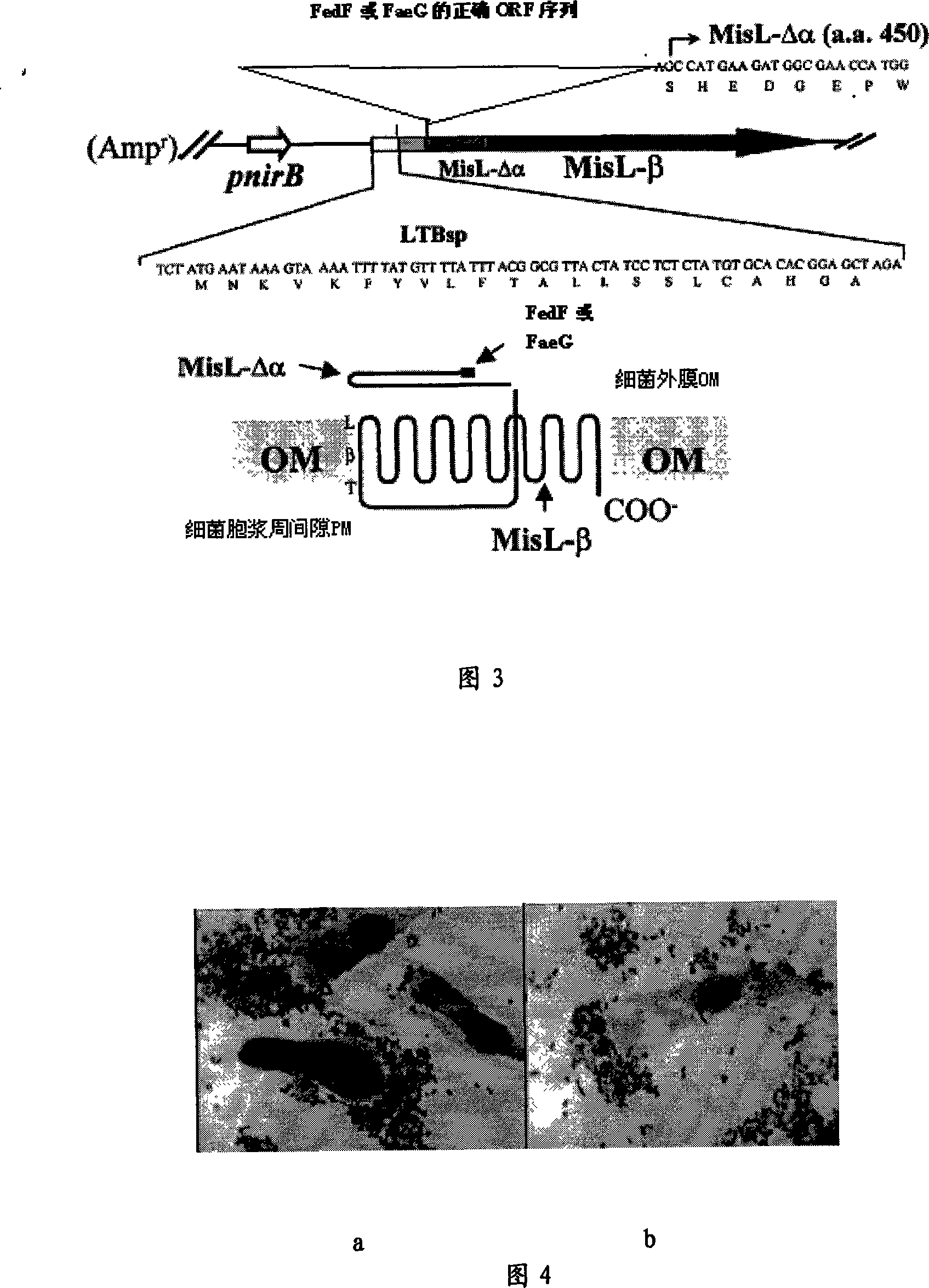
The invention relates to a receptor antagonist for preventing diarrhea occurring on the weaned piglets and edema disease occurring on the piglets, a preparation method and an application. The receptor antagonist of the invention is an assemblage series of recombinant strains of probiotics, and is a MisL-Beta recombinant vector which expresses FedF formed by F18 or Fed adhering to prime subunit gene, and FaeG formed by K88 or F4 or Fae adhering to the prime subunit gene, based on the recombinant strain EP2 of the probiotics of escherichia coli with pig origin advantage and strain LP2 of probiotics of lactobacillus with pig origin advantage. The surface of the cell is good to express antigen technology, so after the piglets orally administrate the cell, the cell can colonize, sedentarily live and proliferate in the intestine. Furthermore, the cell on the surface of the epithelium of the intestine functionally shows secretory expression of highly conservative and cofunctional adhesins FedF and FaeG, that is, the receptor binding domain of F18 and K88 adhesins, which is preferentially bound with the surface of the epithelium of the intestine of the piglets, and thus the diarrhea occurring on the weaned piglets and the edema disease occurring on the piglets are controlled. The invention overcomes the defects that the present antibiotics are almost ineffective in the clinical treatment as most of piglets suffering from the edema disease die though treated by the present antibiotics, and the present antibiotics causes multiple drug resistance.
Owner:YANGZHOU UNIV
Recombinant adenovirus for expression of goat alpha interferon and construction method and application thereof
ActiveCN103981192AHigh titerStrong antiviral activityGenetic material ingredientsViral/bacteriophage medical ingredientsRuminant animalMicroorganism
The invention discloses recombinant adenovirus for expression of goat alpha interferon and a construction method and application thereof. The invention first provides an optimized goat alpha interferon gene with a nucleotide sequence shown as SEQ ID NO:1; the invention also provides the recombinant adenovirus for stable expression of the goat alpha interferon, and the microbial preservation number is: CGMCC (China General Microbiological Culture Collection Center) No.8757. The invention further provides a construction method of the recombinant adenovirus. The recombinant adenovirus constructed by the construction method is capable of stable expression of the goat alpha interferon, high in virus titer, and high in antiviral activity; the recombinant adenovirus and the goat alpha interferon expressed by the recombinant adenovirus both have antiviral activity in swine cells IBRS2, bovine cells MDBK and primary sheep skin cells, and can be used in the prevention or treatment of ruminant animal virus infectious diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antibacterial peptide and application thereof
InactiveCN108314722AAntibacterial agentsPeptide/protein ingredientsSpatial structureCombinatorial chemistry
The invention relates to an antibacterial peptide. The antibacterial peptide is characterized in that the amino acid sequence of the antibacterial peptide is shown in SEQ ID No. 1. The antibacterial peptide enables five amino acids in an amino acid sequence of a swine-origin antibacterial peptide PMAP-36 to mutate based on the spatial structure analysis of the amino acid sequence of the swine-origin antibacterial peptide PMAP-36, and the five amino acids are respectively L7V, T11S, I18E, I25A and I32A. The antibacterial peptide product with higher antibacterial activity and better safety is obtained.
Owner:江西华牧生物科技有限公司
African swine fever prevention and/or treatment neutralizing antibody and preparation method and application thereof
ActiveCN110845604AFold preciselyImproving immunogenicityImmunoglobulins against virusesAntiviralsClassical swine fever virus CSFVAfrican swine fever virus Antigen
The invention provides an African swine fever prevention and / or treatment neutralizing antibody which is an embedded monoclonal antibody and comprises a heavy chain and a light chain. A light chain variable region has a sequence shown as SEQ ID No:6, a heavy chain variable region has a sequence shown as SEQ ID No:2, and both a light chain constant region and a heavy chain constant region come froma swine derived antibody. The invention further provides a gene coding the embedded monoclonal antibody and an expression vector or a transformant comprising the coding gene. The expression carrier can be obtained by inserting the coding gene into pAAV-CAG. The invention further provides a method of utilizing the expression vector to prepare AAV. The antibody sequences or the transformant can beused for preparing detection reagents for African swine fever virus, agents for inducing immunoreaction aiming at African swine fever virus antigen in tested animals or agents for preventing animals from being infected by the African swine fever virus, and rapid and lasting antibody protection such as African swine fever virus vaccines can be provided.
Owner:苏州沃美生物有限公司
Escherichia coli bacteriophage vBE_coM_swi3 and application thereof
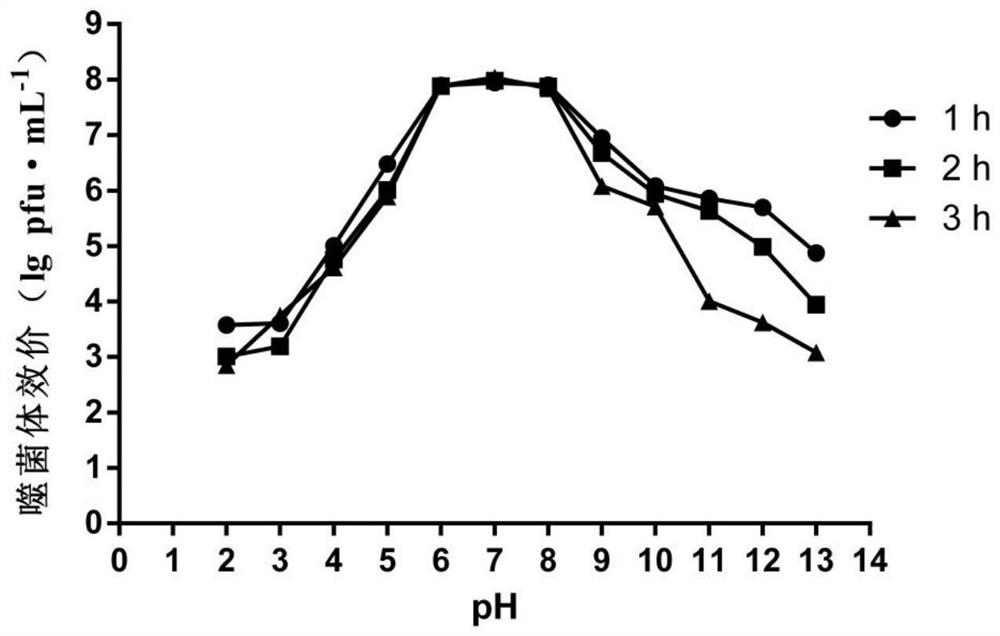
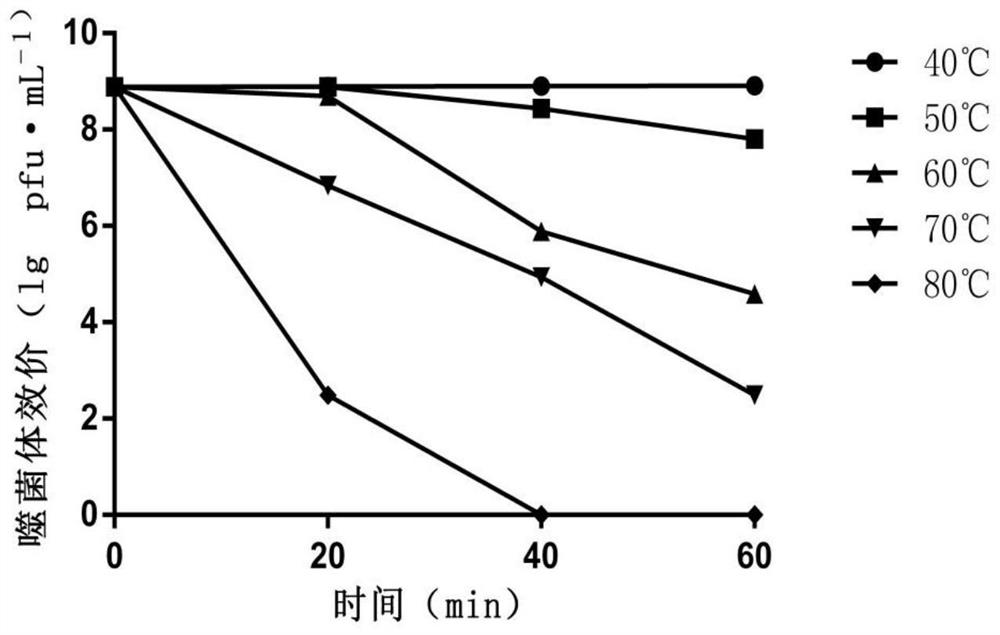
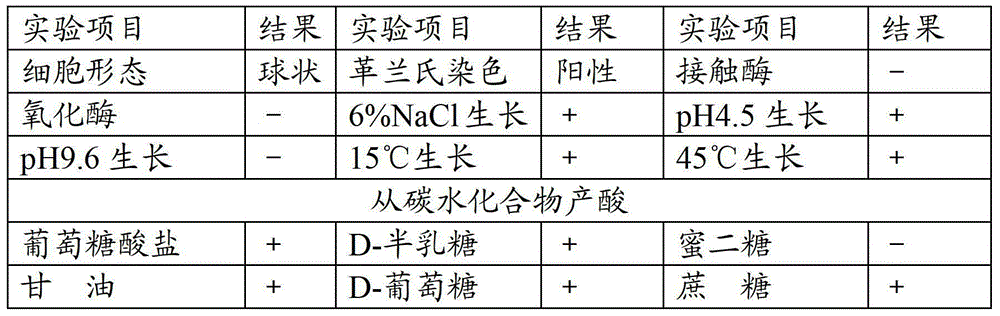
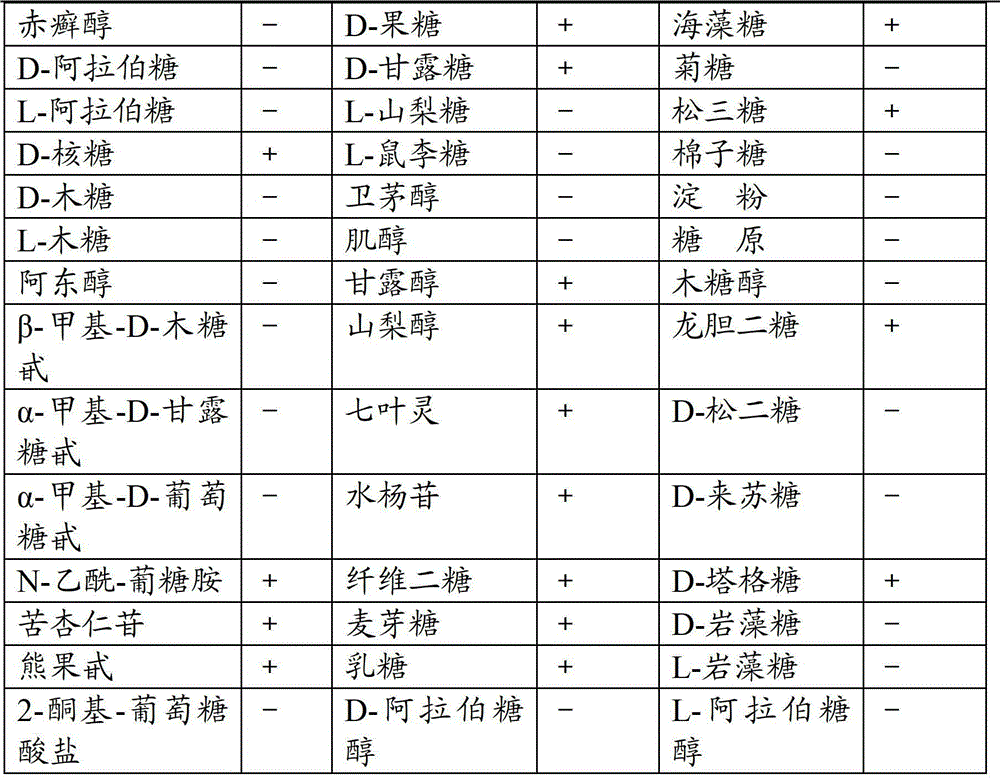
ActiveCN112280749APhysical and chemical factors are well toleratedShort latencyBiocideMicroorganism based processesESCHERICHIA COLI ANTIGENPathogenicity
The invention discloses an escherichia coli bacteriophage vBE_coM_swi3 with a preservation number of CCTCC NO: M2019467. The bacteriophage belongs to myoviridae and has a polyhedral head and a telescopic tail structure, the diameter of the head is about 80 nm, and the length of the tail is about 120 nm; transparent empty spots can be formed on a solid culture medium, no halo ring exists around thesolid culture medium, the edge is clear and regular, and the diameter is about 1-1.5 mm; the bacteriophage has a good cracking effect on swine-derived and chicken-derived escherichia coli in a breeding environment, particularly on swine-derived and chicken-derived pathogenic escherichia coli, and has very wide application prospects in multiple aspects of pharmaceutical preparations, feed additives and the like for preventing and treating swine colibacillosis and chicken colibacillosis.
Owner:QINGDAO AGRI UNIV
Enterococcus faecalis, micro-ecological preparation and application of micro-ecological preparation
ActiveCN103333830AStrong resistanceGenetically stableBacteriaBacteria material medical ingredientsLaboratory cultureSwine origin
The invention discloses enterococcus faecalis, a micro-ecological preparation and an application of the micro-ecological preparation. The 16SrDNA sequence of enterococcus faecalis is shown in SEQ ID NO:1 and the collection number of enterococcus faecalis is CGMCC (China General Microbiological Culture Collection Center) No.6970. The micro-ecological preparation comprises enterococcus faecalis. Enterococcus faecalis is resistant to heat, acid, alkali and dryness, has strong resistance, more stable heredity and no toxicity and can suppress eight common pathogenic bacteria of pigs and cows. The micro-ecological preparation can promote sperm motility of pigs and cows and prolong the lives of pigs and cows, thus purifying the environments of genital tracts of female animals, improving and maintaining micro-ecological balance, ensuring normal physiological functions so that the female animals rut and ovulate on time and improving the fertility rate and litter size. The micro-ecological preparation has great scientific significance and good economic benefits when being applied to preventing various reproductive disturbance diseases of female animals.
Owner:中山市健邦生物科技有限公司
Extraction method of swine-origin antibacterial peptide
InactiveCN106977595AIncrease usageAvoid damagePeptide preparation methodsCationic antimicrobial peptidesWater bathsFiltration
The invention discloses an extraction method of a swine-origin antibacterial peptide. The method comprises the following steps of firstly carrying out heat treatment on pretreated small intestines of a pig in water of 80-100 DEG C for 15-25s to achieve the targets of improving the raw material utilization rate and making cell disruption fuller during repeated freezing and thawing; preparing homogenate of the small intestines of the pig, mixing the homogenate with injection water at the mass ratio of 100:(90-99) evenly, repeatedly freezing and thawing for 5-6 times, mixing the homogenate with acetic acid (analytically pure) in a mass ratio of 100:(1-10), carrying out a boiling water bath, cooling and centrifuging, collecting sediments and primary supernate; mixing the sediment with 2.87% acetic acid solution in a mass ratio of 1:(0.8-1) evenly, freezing, centrifuging and collecting secondary supernate; and merging the supernate and then carrying out tangential flow filtration and ultrafiltration in sequence, adjusting a pH value and filtering again and finally obtaining the swine-origin antibacterial peptide. According to the method, the utilization rate of the raw materials can be improved, the loss of an instrument in the tissue mashing process is reduced, meanwhile, cell disruption is more sufficient during repeated freezing and thawing, and the extraction rate of the swine-origin antibacterial peptide is improved.
Owner:派生特(福州)生物科技有限公司
Detection kit for pig origin component identification and detection of multi-species origin components in products
ActiveCN105296646AStrong specificityLow costMicrobiological testing/measurementDNA/RNA fragmentationPositive controlBiology
Belonging to the technical field of animal origin component molecular detection, the invention provides a detection kit for pig origin component identification and detection of multi-species origin components in products. The kit includes primers shown as SEQ ID No.2&3, a reaction reagent, positive control and blank control. In addition, the kit also includes specific primers of fox, mink, rat, chicken, duck and equus, and the nucleotide sequences thereof are shown as SEQ ID No.4-25. The primers involved in the invention are employed for amplification of the sample DNA and analysis of the PCR amplification product to determine whether the sample contains pig origin components and is mixed with equus, fox, mink, rat, chicken, duck and other animal components. The kit provided by the invention provides an effective, accurate and reliable means for animal origin component molecular identification and detection of adulterated components therein, and has the characteristics of simplicity, fastness, comprehensiveness, strong specificity, low cost, and wide applicability.
Owner:HUAZHONG AGRI UNIV
Recombinant bordetella bronchiseptica strain, vaccine and use
ActiveCN103421728AGood immune protectionImmunization is easy to operateAntibacterial agentsBacterial antigen ingredientsBiotechnologyBordetella bronchiseptica
The invention belongs to the technical field of animal bacteriology and vaccine genetic engineering preparation, and relates to a resistance mark-free recombinant bordetella bronchiseptica strain for expression of a pig toxaigenic pasteurella multocida toxA gene fragment, and its vaccine containing the resistance mark-free recombinant bordetella bronchiseptica strain, a preparation method and a use. The resistance mark-free recombinant bordetella bronchiseptica strain QH0814 delta aroA / toxA-N for expression of the pig toxaigenic pasteurella multocida toxA gene fragment does not contain a 5-enolpyrul-shikimate-3-phosphate synthase aroA gene of bordetella bronchiseptica, and contains the pig toxaigenic pasteurella multocida toxA gene fragment. The invention also discloses the preparation method of the resistance mark-free recombinant bordetella bronchiseptica strain and the vaccine use. The genetic engineering vaccine can stimulate a pig to produce a protective immune response for resisting pig toxaigenic pasteurella multocida and a wild strain of pig bordetella bronchiseptica, and can effectively prevent the double infection caused by pig toxaigenic pasteurella multocida and pig bordetella bronchiseptica.
Owner:HUAZHONG AGRI UNIV
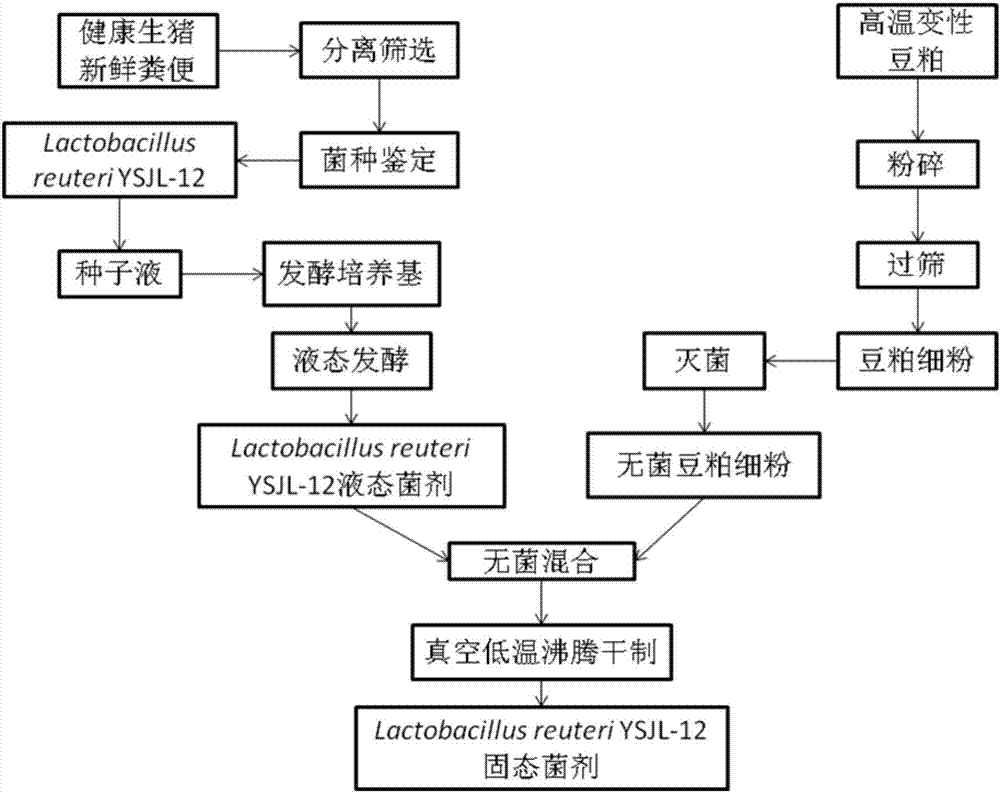
Swine-origin lactobacillus reuteri and prepared solid probiotics
The invention relates to swine-origin lactobacillus reuteri and prepared solid probiotics. The bacterial strain is separated from fresh night soil of healthy live pig, and is indentified as Lactobacillus reuteri YSJL-12 through a morphological biochemistry and molecular biology method, and a preservation number is CGMCC No. 9062. The bacterial strain inhibits Listeria monocytogenes CICC21633 to positive. The prepared solid probiotics contain soybean meal and active Lactobacillus reuteri YSJL-12, can substitute aureomycin for preventing and treating diarrhoea while added in a live pig basic feed, and the feed-gain ratio of the breeding pork pig at age in days of 28-168 is 2.61. The bacterial strain has the characteristics of strong host specificity, large live bacteria quantity, low cost and high cost performance, added value of high temperature soybean meal is increased, and the green live pig culture cost is reduced.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Anti-African swine fever virus single-chain antibody as well as preparation method and application thereof
ActiveCN111560069AControl the spread of the diseaseReactivity hasMicroorganism based processesImmunoglobulins against virusesSingle-Chain AntibodiesAfrican swine fever
The invention discloses an anti-African swine fever virus single-chain antibody as well as a preparation method and application thereof. According to the invention, lymphocytes are separated from peripheral blood of naturally infected African swine fever immune tolerant pigs; extracting is carried out to obtain the total mRNA of the separated lymphocytes; the total cDNA fragments are obtained through an RT-PCR method; cDNA is taken as a template, under the action of a corresponding primer with a Linker joint, swine ScFv antibody gene sequence is obtained through an SOE-PCR method; constructingof an ScFv antibody gene sequence to a pET-30a vector is carried out, and BL21 competent cell transformation is carried out; the ScFv antibody (VH-VL kappa 6) aiming at the African swine fever virusis screened from a single colony after transformation, and preliminary activity identification is carried out on the screened ScFv antibody (VH-VL kappa 6) by adopting an ELISA test, it shows that theantibody has African swine fever reaction activity. The invention provides a new material for early diagnosis, prevention and control of African swine fever, and provides a new technical means for controlling epidemic situation propagation of African swine fever as soon as possible.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
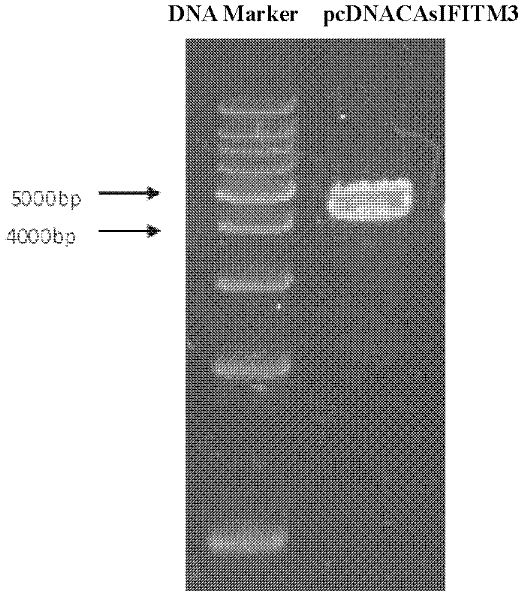
Breeding method for transgenic pigs expressing sIFITM3 genes
InactiveCN102391990ASimple methodSimple and fast operationAnimal reproductionMicrobiological testing/measurementAnimal virusEmbryo transfer
The invention discloses a breeding method for transgenic pigs expressing sIFITM3 (swine interferon induced transmembrane 3) genes. The breeding method comprises the steps as follows: firstly, the construction of swine fibroblast cell lines stably expressing the sIFITM3 genes comprises the following steps: the step a refers to the construction and the linearization of pcDNACAsIITM3 vectors, wherein, primers are designed, lymph node tissue RNA (Ribose Nucleic Acid) of pigs is extracted, the sIFITM3 genes are obtained through RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification, ethanol precipitation is carried out, and sterile water is used for dissolving; the step b refers to liposome transfection, wherein, swine fibroblasts expressing sIFITM3 are constructed; the step c refers to screening and proliferation of nuclear donor cells transfected with the sIFITM3 genes; secondly, recombination blastula acquisition based on a manual nucleus transplantation method comprises the following steps: the step a refers to the preparation of recipient cells; the step b refers to enucleation and injection of the nuclear donor cells; and thirdly, the embryo transplantation and the acquisition of the transgenic pigs comprise the following steps: the step a refers to embryo transplantation, wherein, bred blastulas are transferred to a fallopian tube of a surrogate sow, and then detection is carried out; and the step b refers to transgenic individual identification. The breeding method is feasible, and is simple and convenient to operate; in addition, the pigs are the transgenic pigs expressing sIFITM3, and have the potential capability to resist animal virus such as foot-and-mouth disease virus, Japanese encephalitis virus, swine influenza virus and the like.
Owner:HUAZHONG AGRI UNIV
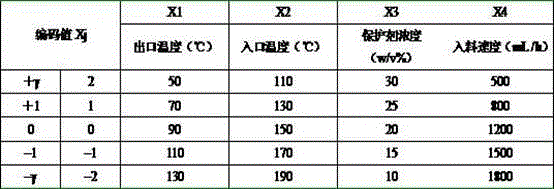
Pig-origin bacillus amyloliquefaciens spray drying technology
The invention discloses a pig-origin bacillus amyloliquefaciens spray drying technology, and relates to the field of pig-origin bacillus amyloliquefaciens preparation technologies. The technology comprises the steps that firstly, pig-origin bacillus amyloliquefaciens fermented liquid is prepared; secondly, a protective agent with the concentration of 20% (W / V) is added into the pig-origin bacillus amyloliquefaciens fermented liquid obtained in the first step; thirdly, spray drying is carried out by a spray drying machine, wherein the feeding speed is 1200 mL / h, the inlet temperature is 150 DEG C, and the outlet temperature is 90 DEG C. The pig-origin bacillus amyloliquefaciens spray drying technology is used for pig-origin bacillus amyloliquefaciens spray drying.
Owner:黑龙江省兽医科学研究所
Pork component identifying molecular marker and application thereof
ActiveCN110373473AQuantitatively detectableAvoid pollutionMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyPork meat
The invention discloses a pork component identifying molecular marker and application thereof. According to a SYBR Green I dye based real-time fluorescence quantitative PCR technology, a high-specificity and high-sensitivity pork component detection method is created by taking a pig single-copy gene in a cell nucleus as the molecular marker. By adoption of the method, pigs can be accurately distinguished from other 13 animals and 5 plants, and by practical detection of 6 meat products on the market, whether porcine-derived components are contained or not can be accurately judged. The porcine specific real-time fluorescence quantitative PCR detection method provides technical references for qualitative and quantitative detection of porcine-derived components in mixed meat products.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
ShRNA (short hairpin ribonucleic acid) suppressing IRS1 (insulin receptor substrate 1) gene expression and application
InactiveCN103589730AIncrease blood sugar concentrationVector-based foreign material introductionAnimal husbandryNucleotideIRS1
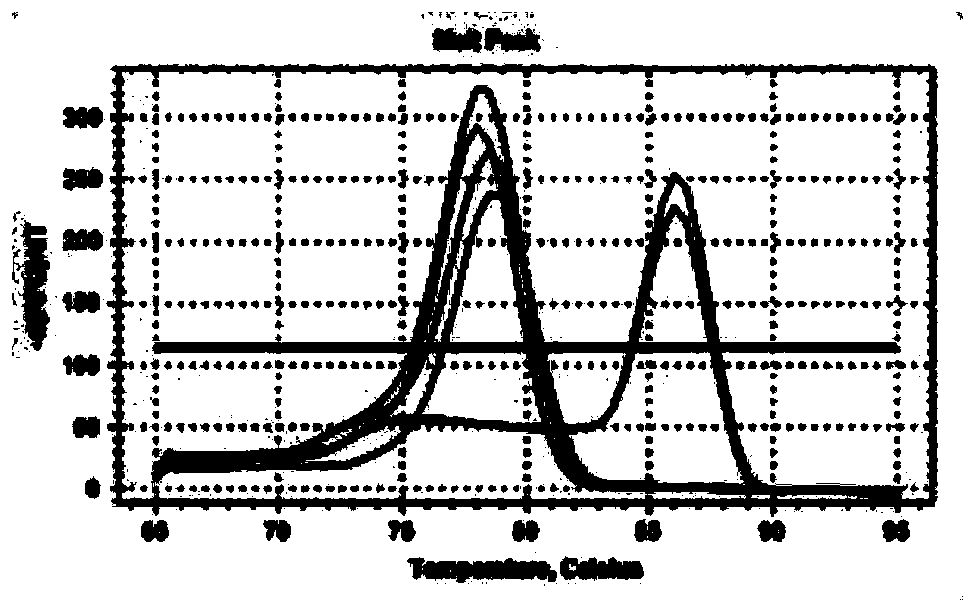
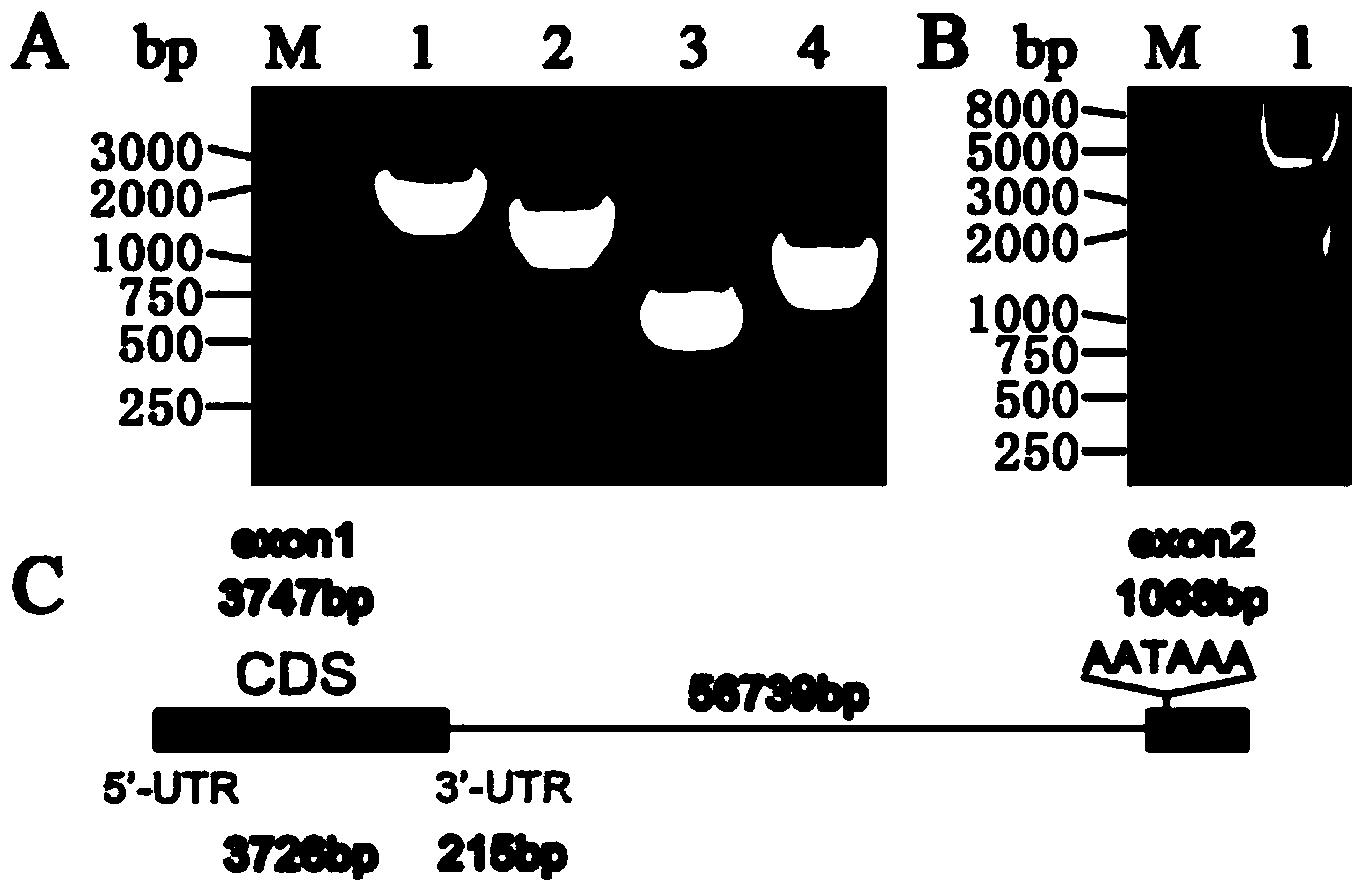
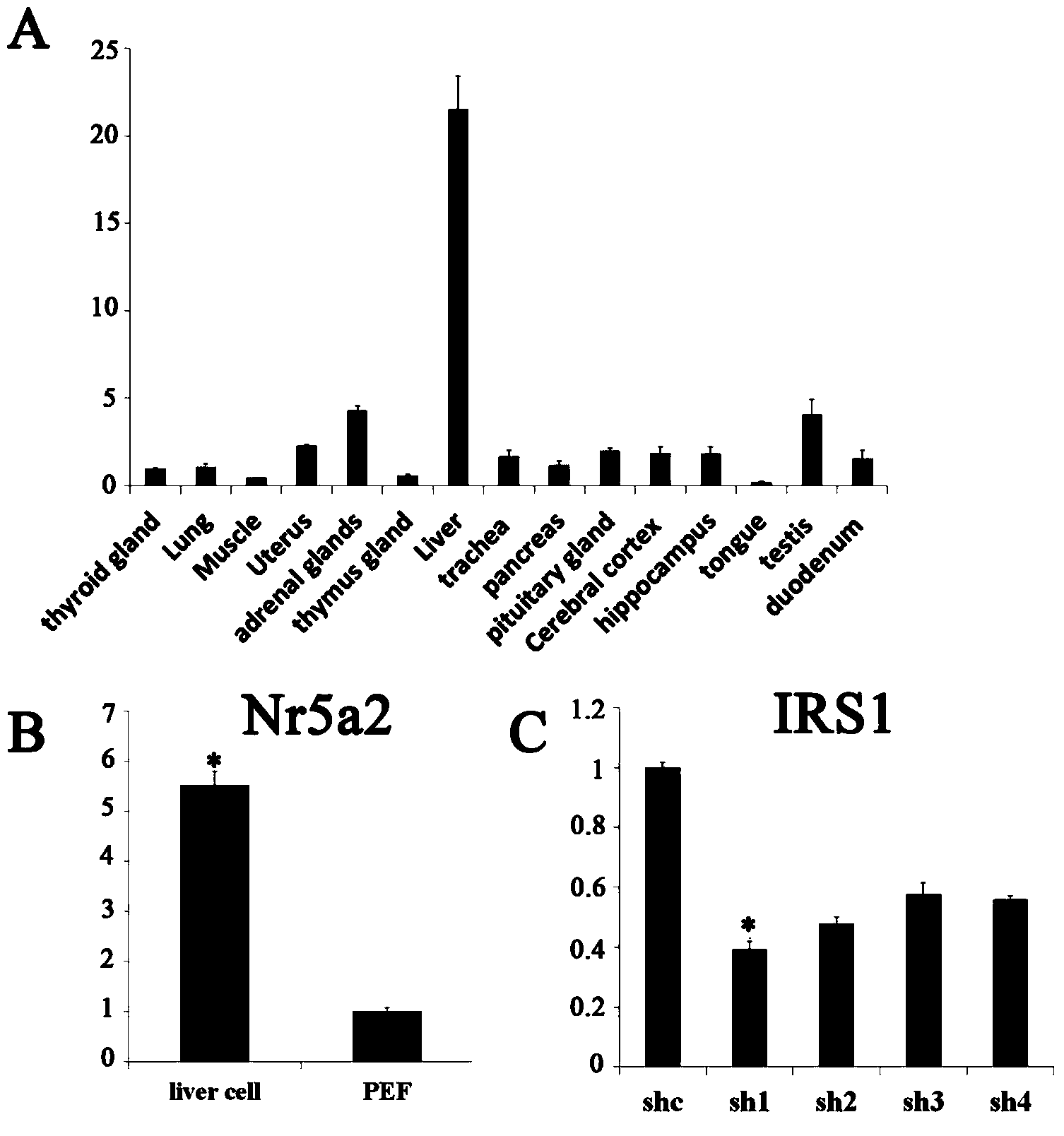
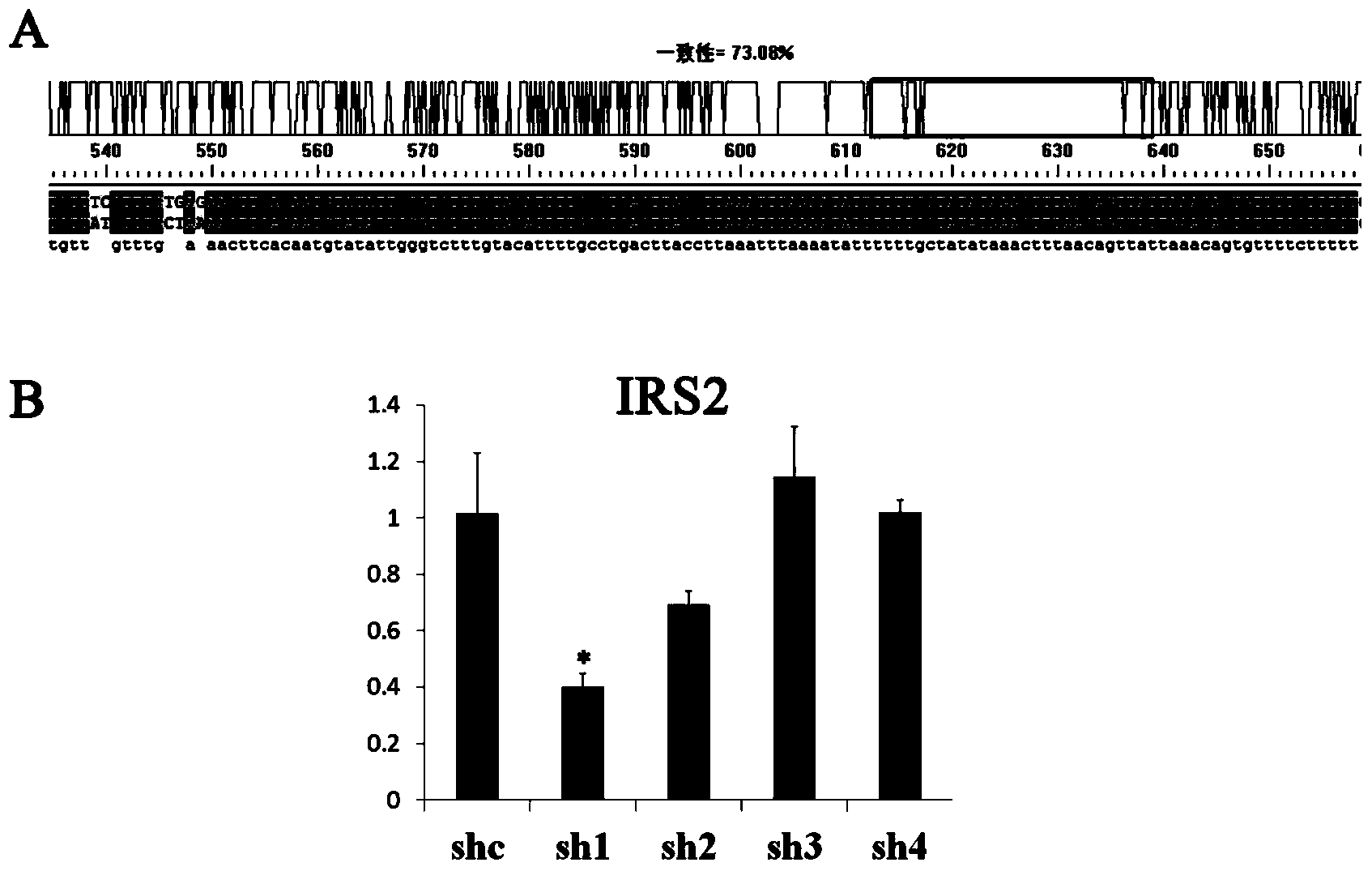
The invention discloses shRNA (short hairpin ribonucleic acid) suppressing IRS1 (insulin receptor substrate 1) gene expression and an application, and belongs to the technical field of gene engineering. A nucleotide sequence of the shRNA is represented by SEQ ID NO.1. The invention further discloses a shRNA interference vector capable of lowering swine-origin IRS1 and IRS2 gene expression simultaneously through connection with a plasmid vector comprising IRS2 genes. Results of swine liver cell IRS gene expression tests indicate that IRS1 genes and the IRS2 genes of swine are lowered by 78% and 64% by the shRNA interference vector respectively. Meanwhile, lowering of the IRS gene expression level affects glucolipid metabolism of swine liver cells, so that the blood sugar concentration of the swine liver cells is increased. According to the shRNA, a foundation is laid for establishment of a type 2 diabetes mellitus swine model, and helpful exploration for increase of the swine blood sugar concentration is conducted.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Swine-derived reshaped antibody with insecticidal activity, and preparation method and application thereof
ActiveCN105085683AEfficiently obtainedImprove securityBiocideHybrid immunoglobulinsBiotechnologySingle-Chain Antibodies
The invention relates to a swine-derived reshaped antibody with insecticidal activity, and a preparation method and application thereof. The preparation method comprises the following steps: predicting 8 FRs of swine-derived antibody heavy and light chain variable regions by using biological information science, implanting 6 CDRs (complementary determining region) of an anti-specific single chain antibody A12 to determine the sequence of the swine-derived reshaped antibody, carrying out artificial synthesis to construct the swine-derived reshaped antibody of A12, and substituting a PIT2 vector capable of easy bacteriophage expression to obtain the swine-derived reshaped antibody. The reshaped antibody has certain combining capacity with intestine BBMV in cabbage moth, and has insecticidal activity. The method of utilizing the biological information science prediction and artificial synthesis can quickly and efficiently obtain the animal-derived reshaped antibody, and uses the gene engineering antibody technique to search and develop the possibility of the novel high-safety insecticidal protein resources, thereby providing a new idea and direction for developing novel insecticides.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Construction method of swine-origin collagen membrane-autologous chondrocyte composite scaffold
InactiveCN106924814AStrong persistenceTear resistantSkeletal/connective tissue cellsCell culture supports/coatingRough surfaceTherapeutic effect
The invention discloses a method for constructing a composite scaffold of pig-derived collagen membrane-autologous chondrocytes. The specific steps are as follows: take the patient's articular cartilage, shake and clean it; cut the cartilage tissue into pieces; obtain cell suspension; precipitate as chondrocytes; Dried the porcine type Ⅰ / Ⅲ collagen membrane, placed it in a sterile plastic plate, slowly dripped the cell suspension on the rough surface of the prepared porcine type Ⅰ / Ⅲ collagen membrane with a plastic sleeve until the collagen membrane reached Wet and saturated state; wait for 10-15 minutes after the collagen membrane adsorbs the cells. The invention has the advantages of good therapeutic effect and the like.
Owner:安徽安龙基因科技有限公司
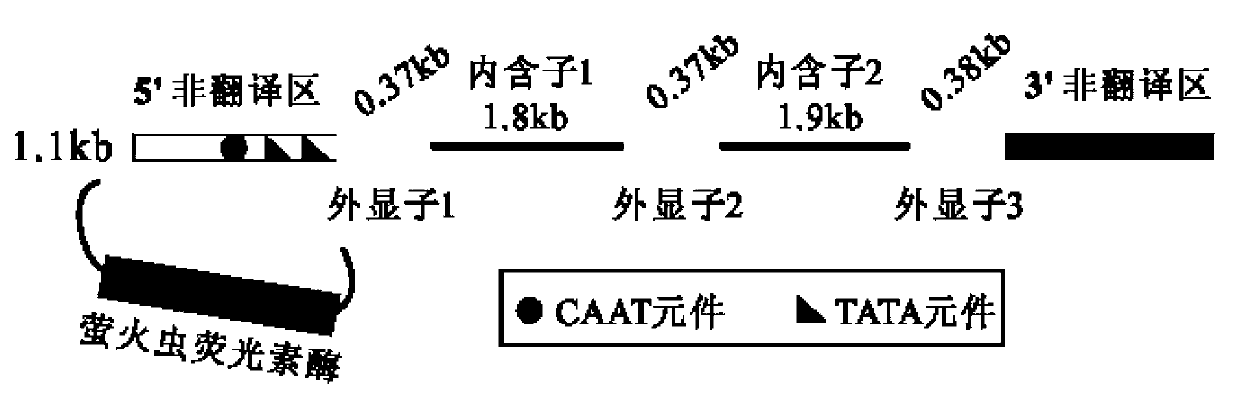
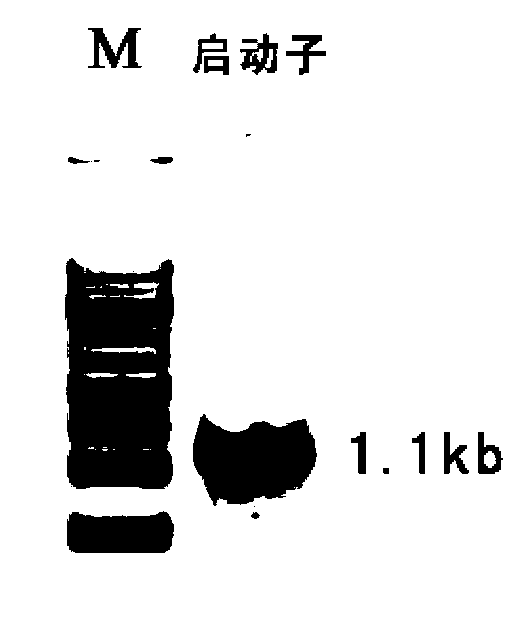
Pig myostatin gene promoter and its applications
ActiveCN102725408AFix stability issuesResolve locationBacteriaGenetic material ingredientsAgricultural scienceHuman cell
A pig myostatin gene promoter and its applications are disclosed in the present invention. The DNA fragment provided in the present invention which is derived from pigs is anyone of the following DNA molecules 1)-4): 1) the DNA molecule of SEQ ID NO:2 shown in the Sequence Listing; 2) the DNA molecule of SEQ ID NO:3 shown in the Sequence Listing; 3) the DNA molecule which hybridizes to the DNA sequence in 1) or 2) under strict conditions and has promoter function; 4) the DNA molecule which has more than 90% homology with the DNA sequence in 1) or 2) and has promoter function. The experiments in the present invention prove that the promoter provided in the present invention can drive the expression of firefly luciferase reporter gene. The promoter can also be built into the reporter vector and then the vector is transfected into cultured swine and human cells, and the activity and efficiency of the promoter are identified by accurate quantitative methods through reporter gene test.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
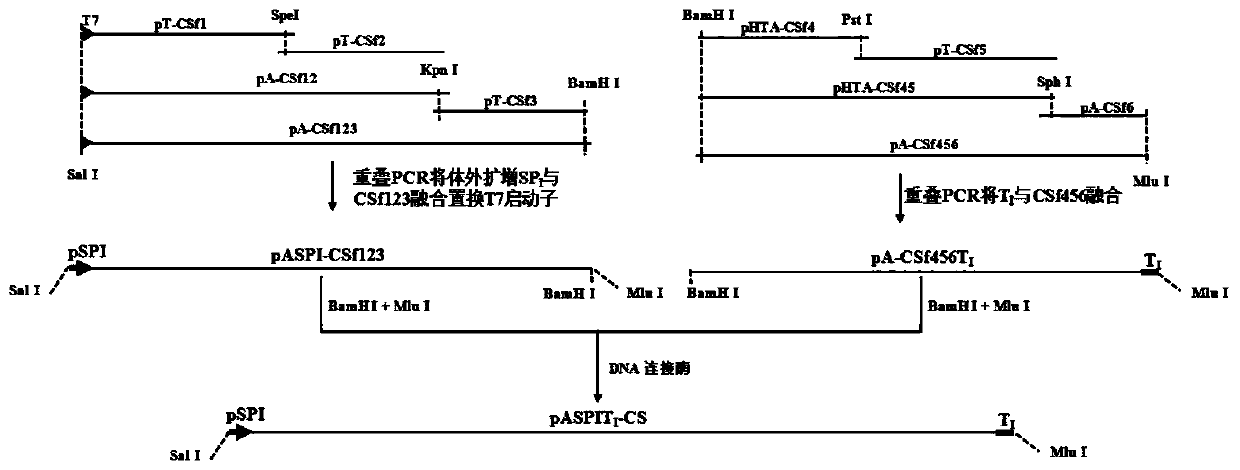
Hog cholera vaccine infectious cDNA (Complementary Deoxyribonucleic Acid) as well as construction method and application thereof
ActiveCN104651378AImprove efficiencyAntiviralsViruses/bacteriophagesEscherichia coliViral transformation
The invention belongs to the technical field of animal biological products, and in particular relates to construction of plasmid pASPITI-CS. The plasmid comprises hog cholera vaccine C strain infectious cDNA clone, fusion of a hog RNA (Ribonucleic Acid) polymerase I promoter sequence at the 5' tail end of the genome cDNA, and insertion of plasmid pASPITI-CS of a mouse RNA polymerase I termination sub-sequence at the 3' tail end. The plasmid is transfected to hog origin cells PK15, so that hog cholera vaccine C strain virus can be directly generated. Escherichia coli DH10B / pASPITI-CS transformed with the plasmid pASPITI-CS is preserved in the China centre for type culture collection; and the preservation number is CCTCC NO.M2015062. The hog cholera vaccine C strain infectious cDNA clone can be used for genetically modifying to prepare high-titre hog cholera vaccines or constructing bivalent vaccines based on a hog cholera vaccine vector.
Owner:WUHAN UNIV
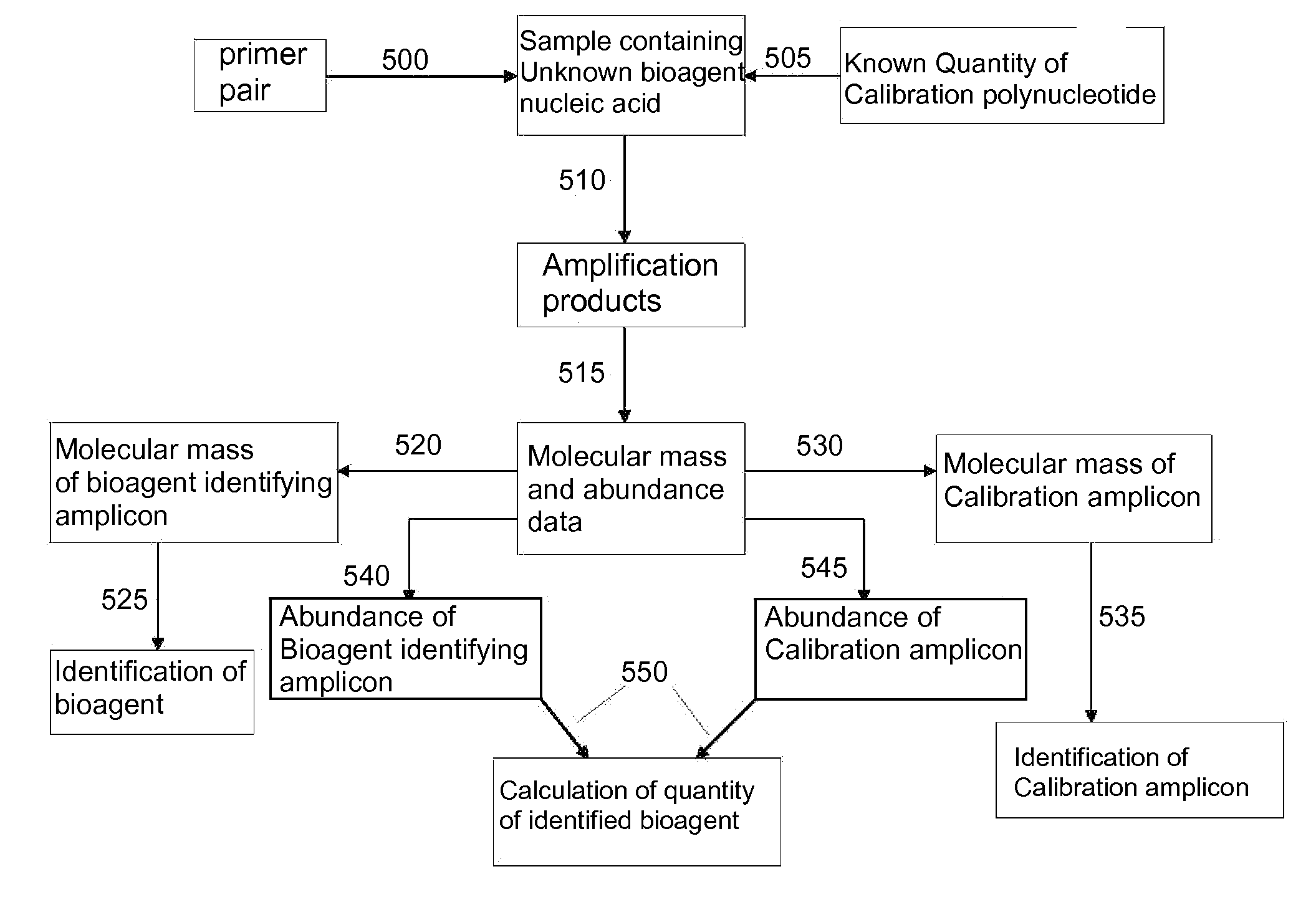
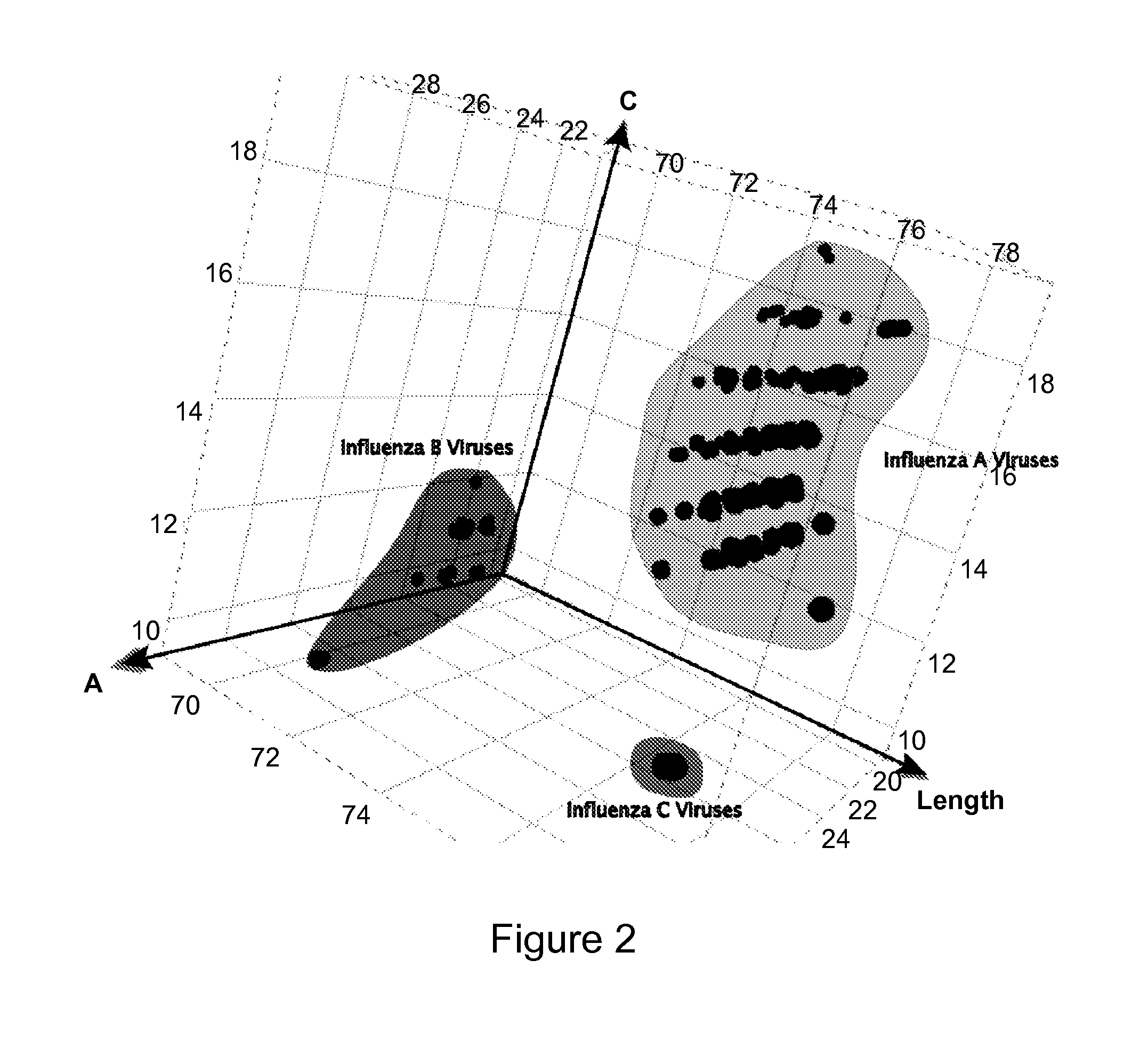
Identification of swine-origin influenza a (H1N1) virus
InactiveUS20120094274A1Quick identificationMicrobiological testing/measurementRapid identificationViral nucleic acid
The present invention provides oligonucleotide primers, compositions, and kits containing the same for rapid identification of viruses (e.g., swine-origin influenza A (H1N1) virus) which are members of the influenza virus family by amplification of a segment of viral nucleic acid followed by molecular mass analysis.
Owner:IBIS BIOSCI
Pig-source lactobacillus mucosae and application thereof
ActiveCN113512516AGood in vitro probiotic potentialGrowth inhibitionAntibacterial agentsBacteriaBiotechnologyFeces
The invention discloses a pig-source lactobacillus mucosae and an application thereof. The strain is named as lactobacillus mucosae LM410, and the preservation number is CGMCC No.22828. The strain is separated from manure of healthy pigs, and has good acid production, acid resistance, cholate resistance and pathogenic bacterium antagonism. The lactobacillus mucosae LM410 provided by the invention can inhibit the growth of C-type clostridium perfringens, has an obvious protection effect on pig intestine epithelial cell injury caused by the C-type clostridium perfringens, and has a wide development prospect and application value in the aspect of preventing and treating bacterial infectious intestinal diseases of livestock and poultry.
Owner:GANSU AGRI UNIV +1
Wormwood pill containing 95% of wormwood ethyl alcohol extract that has inactivation efficacy of the h1n1 virus and the h9n2 avian influenza virus
A mugwort pill containing a 95% ethanol extract of wormwood having an inactivation power with respect to a novel swine-origin influenza virus and an avian influenza virus is disclosed. In more details, the conventional mugwort pill is prepared by a hot water extraction method and has 0.8% of crude fat component; however the ethanol extract mugwort crude fat of the present invention is 11.6%, in particular the crude fat component is 14.5 times higher, and the novel swine-origin influenza virus (H1N1) can be inactivated 99.99%, and it has a good disinfection effect to an avian influenza virus (H9N2). When a mugwort pill containing a 95% ethanol extract of wormwood having an inactivation power with respect to a novel swine-origin influenza virus and an avian influenza virus is administrated to people or fowls, the people or fowls can have immunity with respect to a novel swine-origin influenza virus and an avian influenza virus.
Owner:JO BONGKWAN +1
Generic inert carrier salmonella and potential application thereof
ActiveCN111500504AImprove and refine featuresImprove and perfect the technical bottlenecks where the sensitivity needs to be improvedBacteriaMicroorganism based processesBiotechnologyZoology
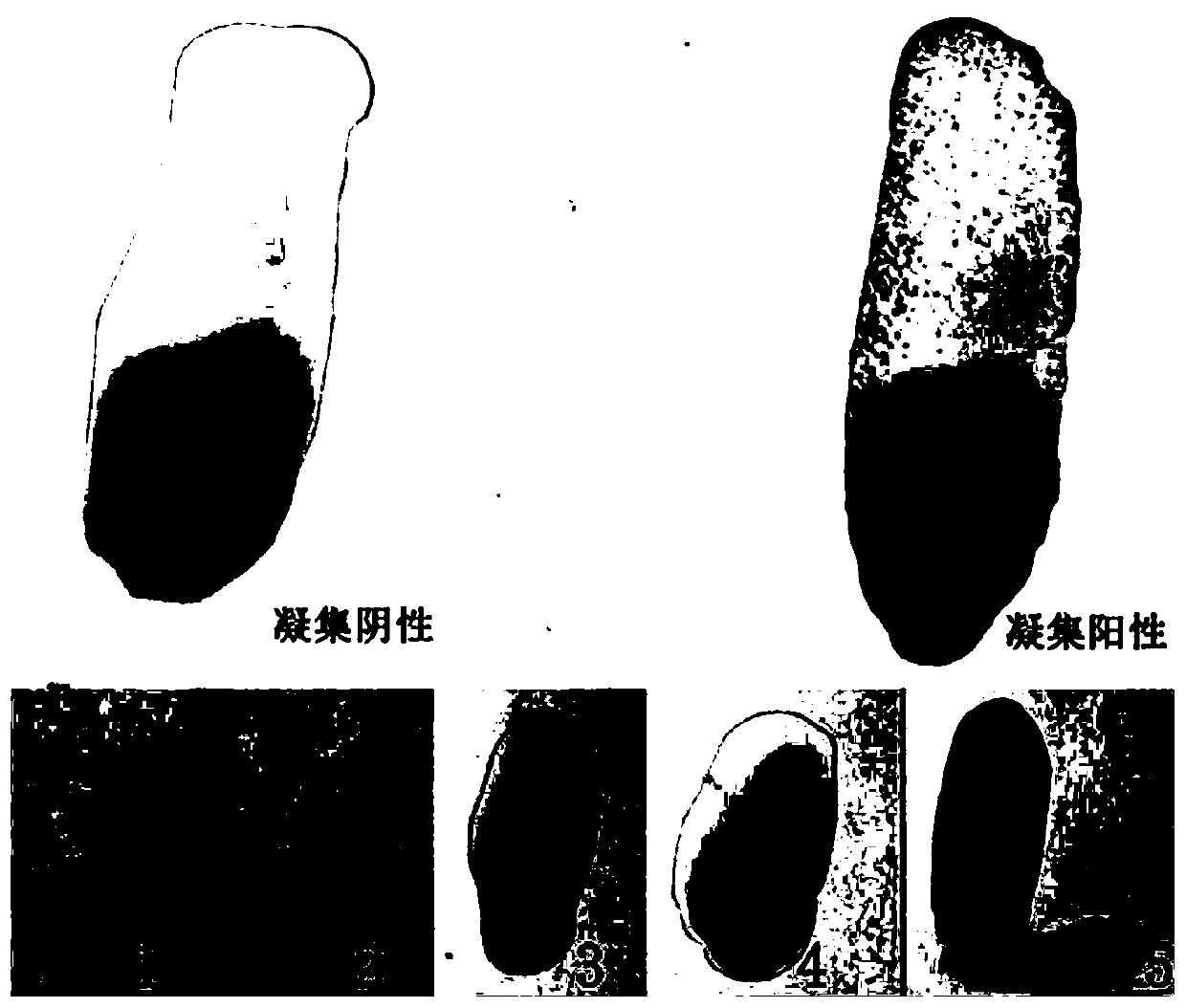
The invention discloses generic inert carrier salmonella S9H and a potential application thereof. The generic inert carrier salmonella S9H is obtained by continuously culturing inert carrier bacteriaS9 in vitro by using an LB solid and liquid culture medium for passage to the fortieth generation, under the condition of the quantity of bacteria with working concentration, the non-specific agglutination reaction with serum and whole blood of human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys, pigeons and quails) can be avoided, the characteristics of carrying and surface expression displaying human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys, pigeonsand quails) for different resistance factors are realized, the generic inert carrier salmonella S9H can be applied to development of an indirect agglutination test detection method for simply, conveniently and quickly detecting human and various animal antigens or infected antibodies, improves and perfects the technical bottleneck of poor specificity and sensitivity of an existing agglutination test for agglutination antigen and antibody detection, and has a wide application value and market prospect.
Owner:YANGZHOU UNIV
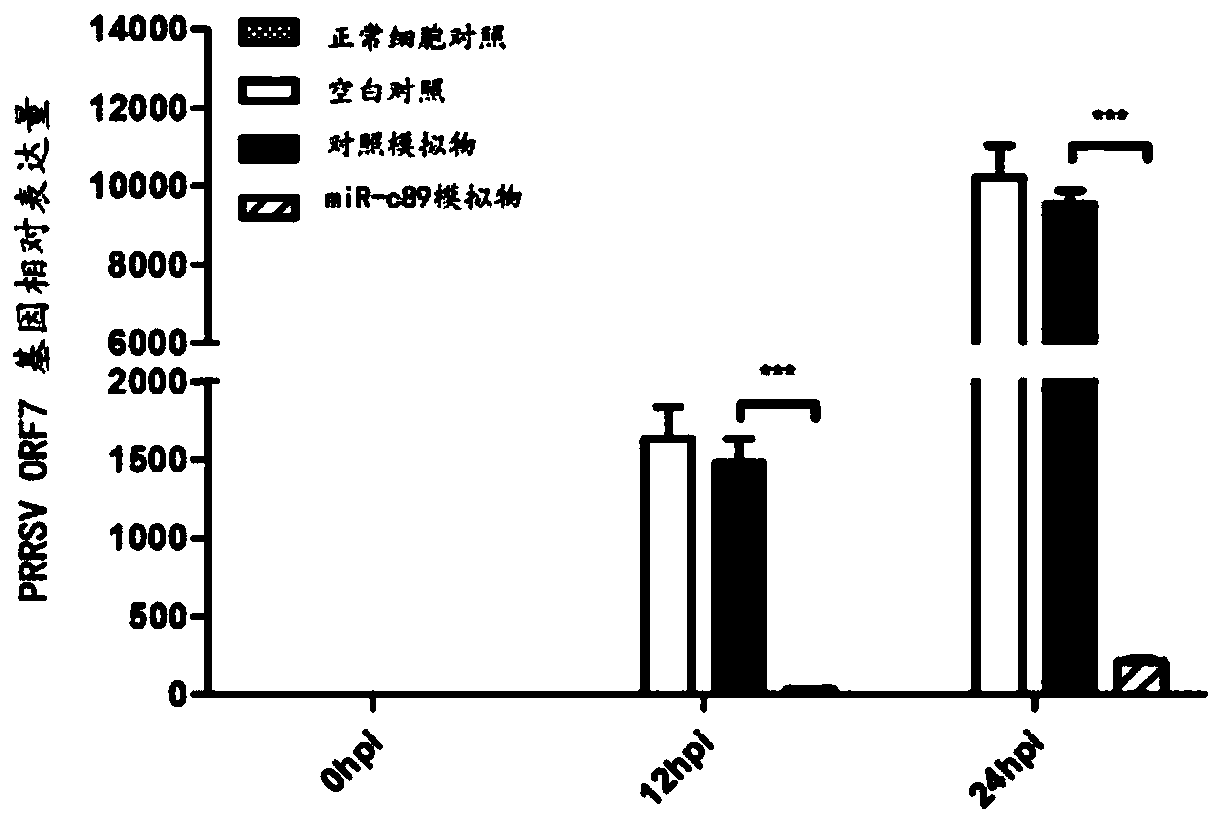
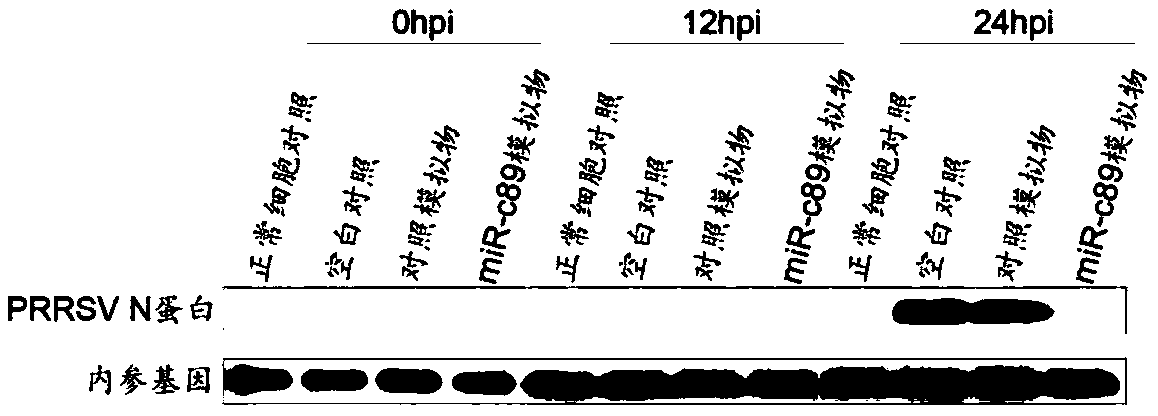
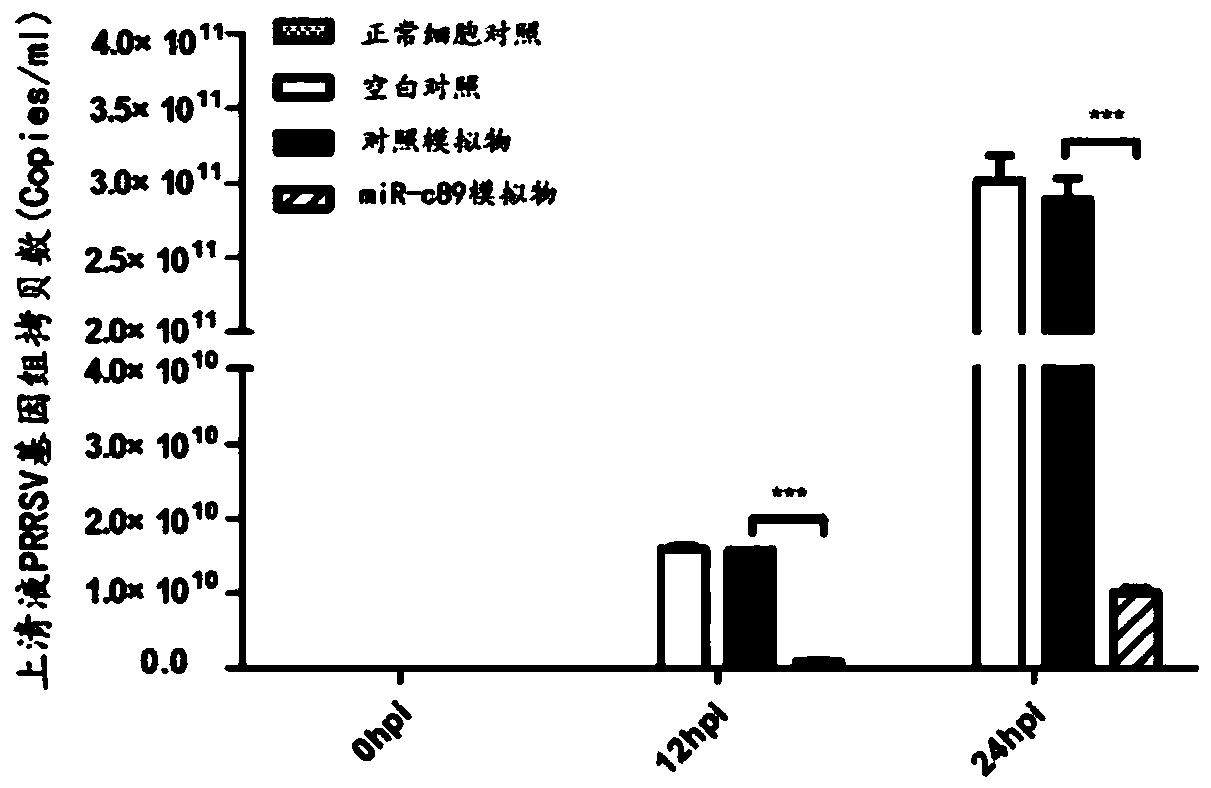
Swine-origin miR-c89 capable of resisting PRRSV (Porcine reproductive and respiratory syndrome virus) infection, and application thereof
ActiveCN109679952AInhibition of replicationPrevent proliferationOrganic active ingredientsAntiviralsWestern blotPorcine reproductive and respiratory syndrome virus
The invention discloses a swine-origin miR-c89 capable of resisting PRRSV (Porcine reproductive and respiratory syndrome virus) infection. Through RT-qPCR (Real Time-quantitative Polymerase Chain Reaction), TCID50 (Tissue Culture Infectious Dose 50) and western blot methods, miR-c89 derived from swine coding is proved to obviously inhibit the replication and the proliferation of high pathogenicityand low pathogenicity PRRSV for the first time, the micoRNA is hopefully developed into a novel medicine capable of preventing and curing porcine reproductive and respiratory syndrome and used for researching the gene-modified pig capable of resisting the porcine reproductive and respiratory syndrome, and a foundation is laid for the prevention and control of the PRRSV.
Owner:NORTHWEST A & F UNIV
Nest-type fluorescence PCR detection primers, probe composition and kit for donkey and pig-sourced ingredients in colla corii asini and detection method and application
InactiveCN105734156AImprove efficiencyHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAdditive ingredientSmall fragment
The invention provides nest-type fluorescence PCR detection primers, a probe composition and a kit for donkey and pig-sourced ingredients in colla corii asini and a detection method and application, and relates to the technical field of molecular biology. By means of the primers, the probe composition and the multiple nest-type fluorescence PCR detection kit, the donkey and pig-sourced ingredients in colla corii asini can be rapidly identified. For trace DNA or degraded DNA in colla corii asini, a nest-type PCR and small fragment amplification technology is adopted, the probability for effectively identifying target points by means of the primers and a probe is greatly increased, and therefore the detection accuracy and sensitivity are greatly improved; two steps of PCR amplification are conducted in a same system, and the advantages that closed tube operation is achieved, the contamination rate is low, the detection sensitivity is high, the specificity is good, the accuracy is high, and the flux is large are achieved.
Owner:BIOTECH RES CENT SHANDONG ACADEMY OF AGRI SCI
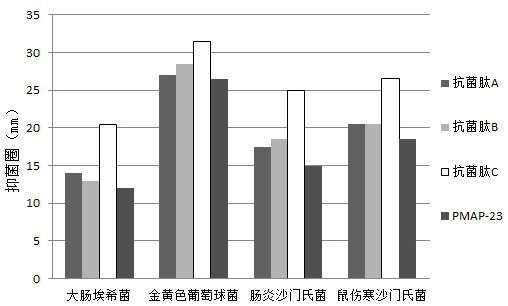
Porcine antibacterial peptide PMAP-23 variant and application thereof in preparation of feed
ActiveCN113024653AHigh activityImprove biological activityAntibacterial agentsPeptide/protein ingredientsBiotechnologyAnimal science
The invention relates to a porcine antibacterial peptide PMAP-23 variant and application thereof in preparation of feed. A peptide fragment of a porcine antibacterial peptide PMAP-23 is modified, so that the antibacterial activity and safety of the antibacterial peptide are improved, the antibacterial peptide variant has improved thermal stability, the porcine antibacterial peptide PMAP-23 variant is further prepared into a feed additive which can be used for feeding piglets and other animals, and is used for improving the growth performance of the animals, reducing the feed conversion ratio and improving the economic benefit of breeding; and as the variant of the natural antibacterial peptide, the antibacterial peptide variant does not bring drug resistance to fed animals, and has positive social significance.
Owner:山东中科康源生物科技有限公司 +1
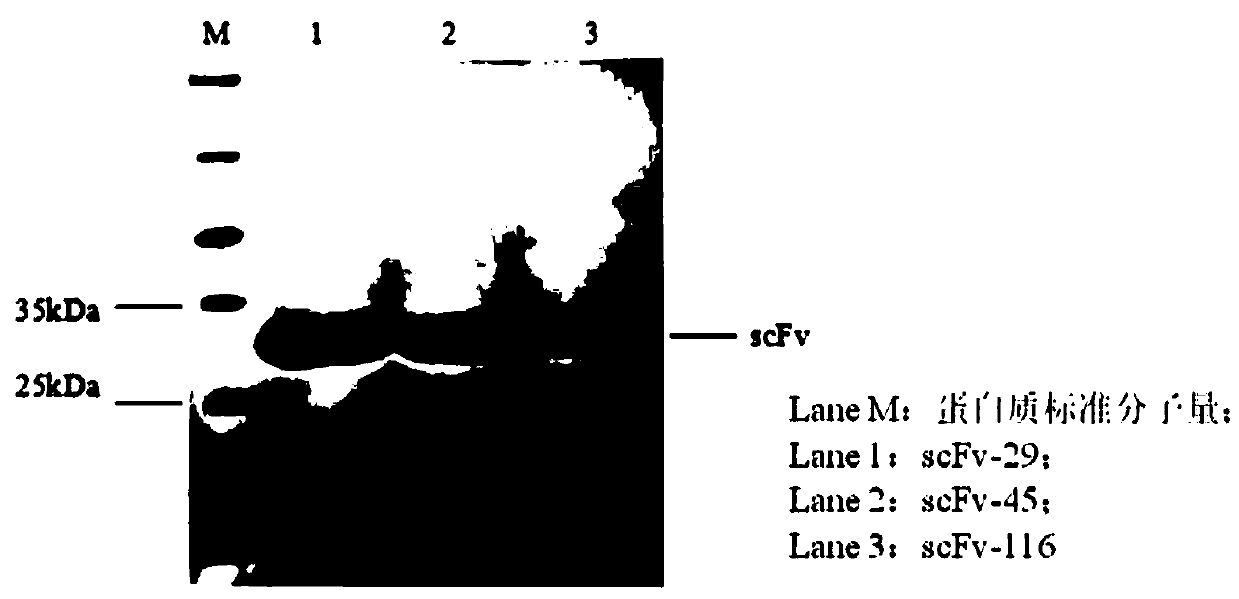
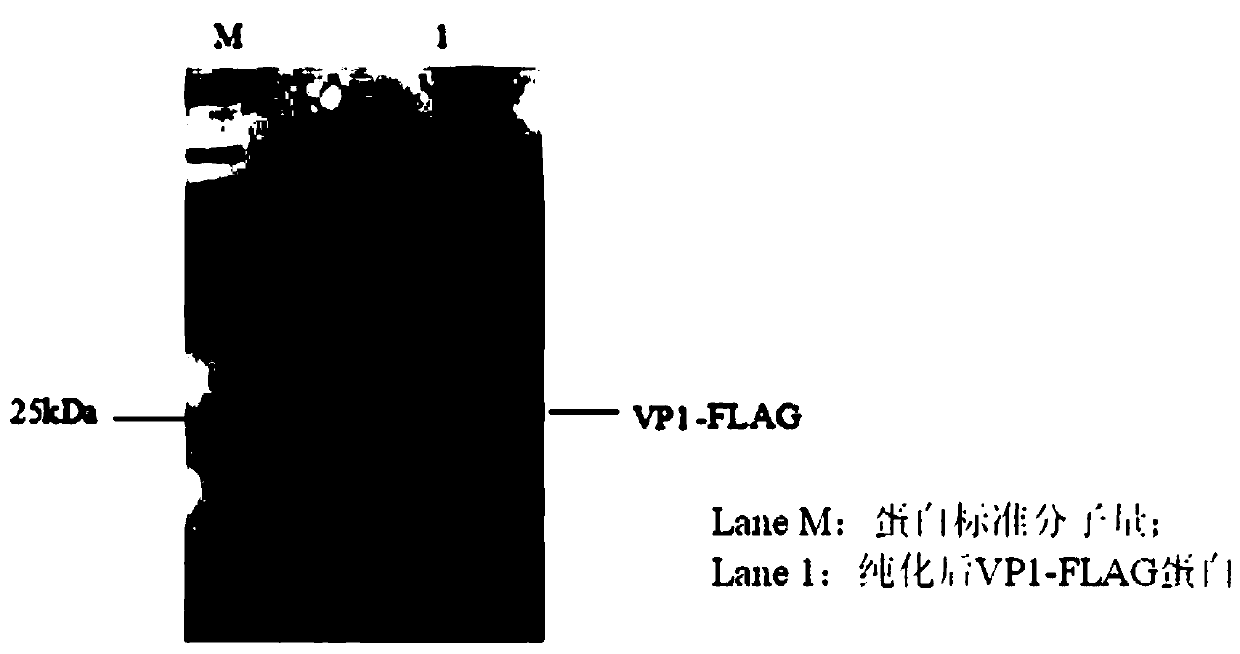
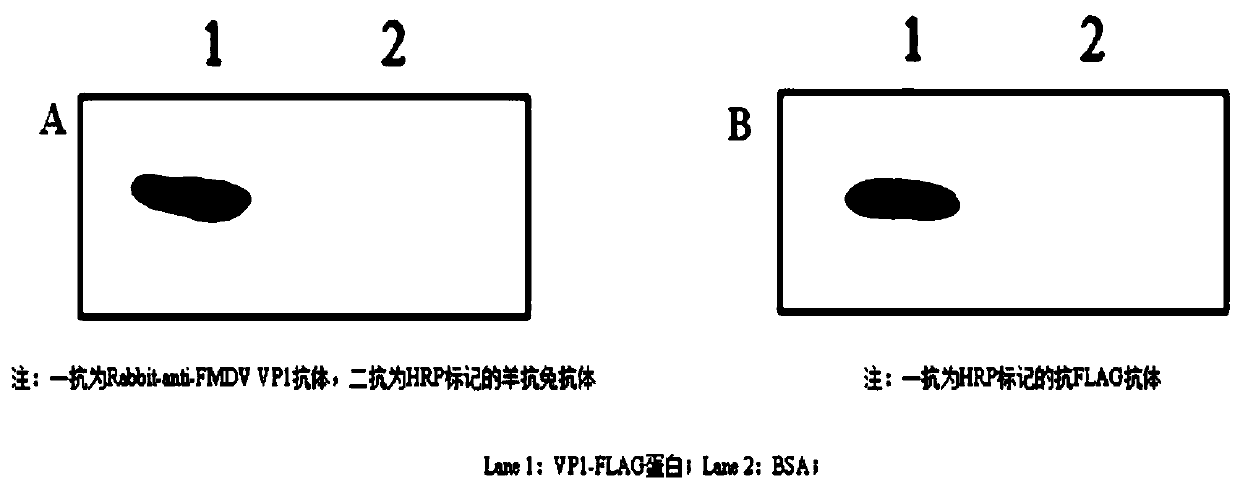
Three scFv antibodies and encoding genes thereof and application of scFv antibodies in preparing preparations for treating or preventing O-type foot-and-mouth disease
InactiveCN110317266AStrong specificityGood treatment effectBiological material analysisImmunoglobulins against virusesSingle-Chain AntibodiesScFv Antibodies
The invention discloses three scFv antibodies and encoding genes thereof and application of the scFv antibodies in preparing preparations for treating or preventing an O-type foot-and-mouth disease. Three single-chain antibodies, namely scFv antibodies are provided; the scFv antibodies have the capability of specifically binding to FMDV structural protein 1 (VP1) and O-type FMDV toxic strains. Byadopting the swine-origin scFv antibodies, defects can be overcome that when an immune serum and an egg yolk antibody are in use, preparation is complicated, the production cost is high, effects are unstable, the quality during industrial production is difficult to control, and horizontally transmitted diseases are caused. The swine-origin scFv antibodies have the advantages of being high in specificity, controllable in quality during industrial production, and the like; the problems of the horizontally transmitted diseases and anaphylactic reaction which are caused by the immune serum are avoided.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com