African swine fever prevention and/or treatment neutralizing antibody and preparation method and application thereof
A monoclonal antibody and carrier technology, applied in the field of neutralizing antibodies, African swine fever prevention and/or therapeutic neutralizing antibodies, can solve the problems of short half-life of antibodies and inability to provide continuous protection, and achieve small virus particles and long-lasting Protective, cost-effective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Another aspect of the embodiments of the present invention provides a method for preparing virus particles, which includes: culturing host cells, waiting for the cells to grow to 70% confluence, adding a transfection reagent, adding the expression vector, pAAV2-RC vector and pHelper The host cells were co-transfected with the vector, cultured at 37°C for 5 hours, and then replaced with fresh medium, then continued to culture and collected cells, and then the collected cells were repeatedly freeze-thawed and lysed, and then post-processed to extract virus particles.
[0054] In some embodiments, the host cells are conventional host cells in the art, preferably eukaryotic cells, more preferably HEK293 cells.
[0055] In some embodiments, the transfection reagent is a conventional transfection reagent in the art, as long as it can transfect the foreign plasmid into the host cell, preferably polyethyleneimine reagent (PEI reagent). The preparation method of the polyethylene...
Embodiment 1
[0072] Example 1 Expression of P54 Protein Recombinant Plasmid Construction
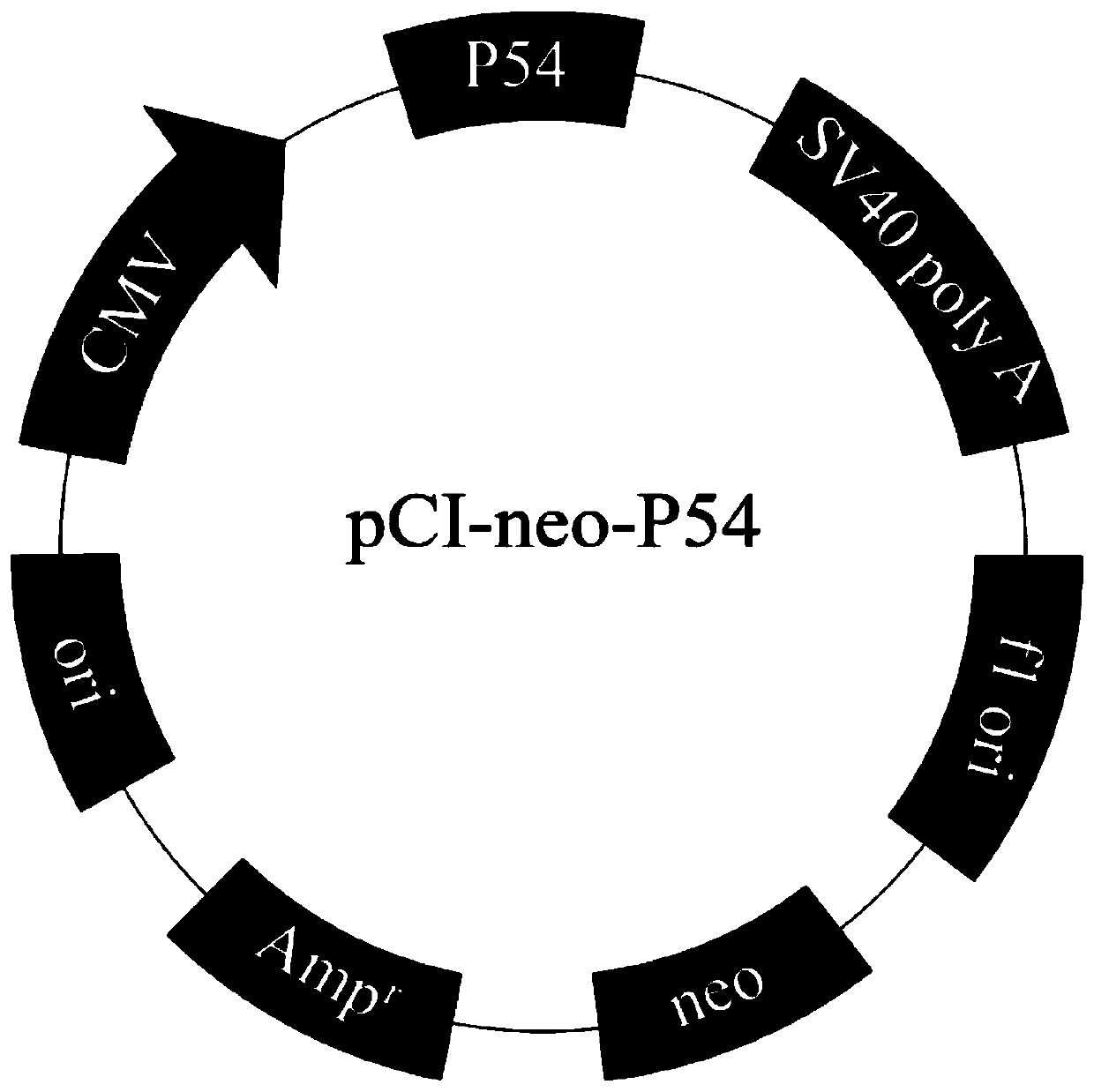
[0073] The amino acid sequence of the P54 protein of an ASFV isolated from China (Gene Bank accession number: MK128995) was codon-optimized (SEQ ID No: 11), and synthesized and cloned into the pCI expression vector at Nanjing GenScript Company. Add a His tag to the C-terminal, insert between the restriction endonucleases MluI and XhoI of the pCI vector to obtain the pCI-P54His plasmid, refer to figure 1 shown. Wherein, the pCI expression vector can also be replaced by various vectors conventional in the art that can be replicated and expressed in the host. The host can be a prokaryotic cell, such as a bacterial cell; a eukaryotic cell, such as an insect, yeast, mammalian cell, etc.; more specifically, it can be a genetically engineered bacterium obtained by transforming the aforementioned recombinant plasmid into Escherichia coli.
Embodiment 2
[0074] Preparation of embodiment 2 immune antigen (ASFV P54 protein)
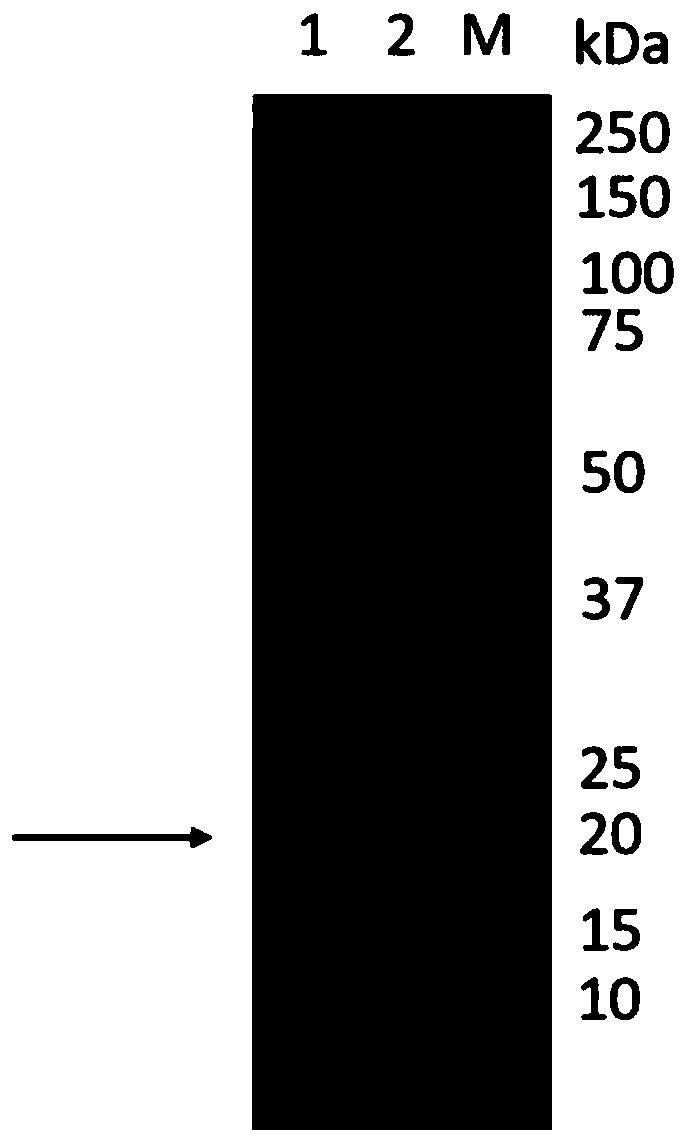
[0075] Escherichia coli was used to amplify the pCI-P54His plasmid, and the plasmid was extracted. HEK293 cells were transfected with PEI and pCI-P54His plasmid, and after 3 days, centrifuged at 3000 r / min for 15 min to obtain the supernatant to obtain P54His protein (SEQ ID No: 12). Harvested cell cultures were subjected to SDS-PAGE, while empty HEK293 cells were used as negative controls. The specific operation is as follows: take 40 μl of harvested cell culture, add 10 μl of 5×loading buffer, bathe in boiling water for 5 minutes, centrifuge at 12000 r / min for 1 minute, take the supernatant and carry out SDS-PAGE gel (12% concentration gel) electrophoresis, After electrophoresis, the gel was stained and decolorized to observe the target band. Such as figure 2 As shown, the target band appears around the molecular weight of about 20kDa, and the negative control has no band at the corresponding position...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com