Antibacterial peptide and application thereof
A technology of antimicrobial peptides and drugs, applied in the direction of cationic antimicrobial peptides, applications, antibacterial drugs, etc., can solve the problems of low antibacterial activity and hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of porcine-derived antimicrobial peptide PMAP-36 mutant
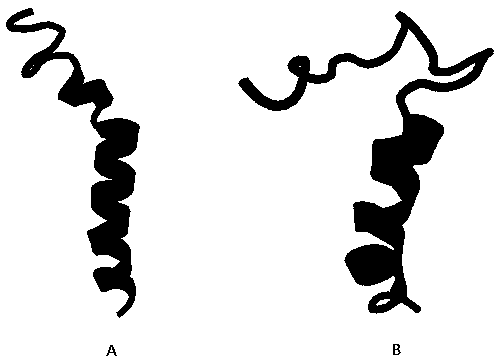
[0019] First, the porcine antimicrobial peptide PMAP-36 (SwissProt ID: P49931) is an antimicrobial peptide composed of 37 amino acids. However, structural analysis revealed that the antimicrobial peptide has multiple folded structures (see figure 1 B), it is not conducive to the expression of antimicrobial peptides and membrane penetration during the antibacterial process. In order to further improve its antibacterial activity, the spatial structure analysis of the amino acid sequence of the porcine antimicrobial peptide PMAP-36 was finally completed. Five amino acid mutations in 36 (L7V, T11S, I18E, I25A and I32A) were used to obtain the porcine antimicrobial peptide PMAP-36 mutant, whose amino acid sequence is SEQ ID NO:1.
[0020] Second, entrust a related peptide synthesis company to synthesize and prepare the porcine antimicrobial peptide PMAP-36 mutant with the amino acid sequence of...
Embodiment 2
[0021] Embodiment 2: antibacterial test
[0022] Taking the test strain Escherichia coli after activation in the laboratory as the test object, using the antibacterial operation method, setting a positive control (ampicillin solution), a negative control (distilled water), and studying the antimicrobial peptide PMAP-36 before transformation and Example 1 The antibacterial effect of the porcine antimicrobial peptide PMAP-36 mutant whose modified amino acid sequence is SEQ ID No.1 is prepared. Among them, Escherichia coli is used as the test bacterium, and the bacteriostatic test of antimicrobial peptides on Escherichia coli is carried out, and the culture condition is cultured in a constant temperature incubator at 37°C. Among them, 50 μL of the sample to be tested was added dropwise to each well, the amp concentration of the positive control group was 1 M, and the modified porcine antimicrobial peptide PMAP-36 mutant and PMAP before modification were added dropwise at a concen...
Embodiment 3
[0024] Example 3: Minimal Hemolytic Concentration Determination (MHC)
[0025] Referring to Example 5 of the invention patent application CN201410669038.8, the minimum hemolytic concentration of the antimicrobial peptide was determined. The specific method is as follows:
[0026](1) Add 100 μL of 8% porcine red blood cells resuspended in PBS to a 96-well plate, and then add 100 μL of antibacterial peptides serially diluted in PBS, so that the concentrations of antimicrobial peptides in each well are 100 μg / mL, 50 μg / mL, and 25 μg / mL, 12.5μg / mL, 6.25μg / mL, 3.12μg / mL, 1.56μg / mL and 0.78μg / mL. Add 100 μl 0.2% Triton X-100 to positive control wells, add 100 μL PBS to negative control wells, incubate at 37°C for 1 hour, centrifuge at 3000 rpm for 5 minutes, pipette 100 μL supernatant from each well into another 96-well plate, measure OD value at 550 nm wavelength Calculate hemolysis percentage=[(OD value of experimental well-OD value of negative well) / (OD value of positive well-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com