Three scFv antibodies and encoding genes thereof and application of scFv antibodies in preparing preparations for treating or preventing O-type foot-and-mouth disease
A coding and gene technology, applied in the field of scFv antibodies, can solve the problems of uneven quality and easy transmission of blood-borne diseases, etc., achieve good therapeutic effect, controllable production quality, and avoid the effect of horizontal transmission of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Discovery of scFv antibody (single-chain antibody) and its coding gene
[0046] 1. Construction of antibody library
[0047] The pigs immunized with FMD VP1 virus particles were tested for their antibody titers by FMD O-type liquid phase blocking ELISA antibody detection kit. The pig spleen with the highest antibody titer (1:256) was used to extract total RNA from the spleen tissue by Trizol method . Refer to the porcine antibody gene sequence registered in GenBank, compare and analyze the sequence, design primers for the amplification of the variable regions of the heavy chain and light chain of the porcine antibody library, and insert Sfi I and Hind III into the upstream of the heavy chain in sequence Insert the NheI restriction site downstream; insert the BamH I restriction site upstream of the light chain, and insert Xho I and Sfi I restriction sites downstream in turn. The VH and VL fragments were respectively inserted into the upstream and downstream ...
Embodiment 2
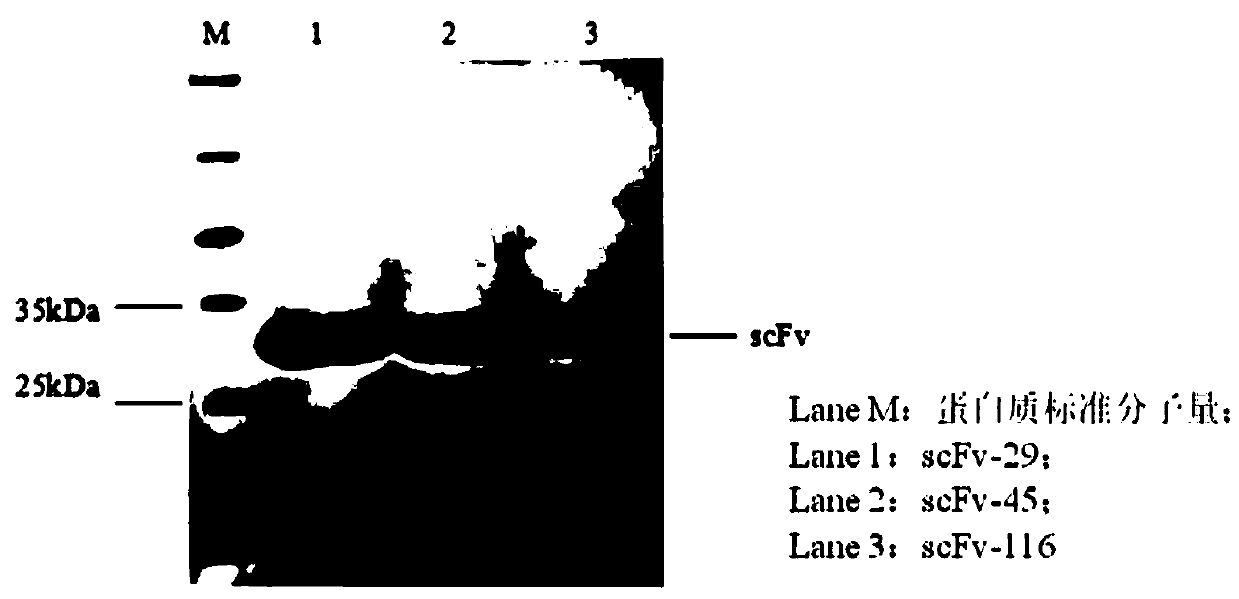
[0057] Embodiment 2, preparation of scFv antibody
[0058] 1. Using the plasmids pBSD-scFv-29, pBSD-scFv-45, and pBSD-scFv-116, which have been proved to be positive clones by FACS, as templates, design primers to clone the three strains of scFv. The upstream and downstream primers for cloning scFv-29 are P3 and P4 respectively; the upstream and downstream primers for cloning scFv-45 are P5 and P6 respectively; the upstream and downstream primers for cloning scFv-116 are P7 and P8 respectively; the upstream primers are all introduced into Nde I, the downstream primers are introduced into the Xho I restriction site, synthesized by Shanghai Sangon Sequencing Company. The scFv gene sequence is amplified by PCR, and the PCR amplified product is separated by nucleic acid electrophoresis.
[0059] The restriction endonuclease Nde I site is marked with an underline, and the Xho I restriction site is marked with a wavy line:
[0060] scFv-29 primer:
[0061] P3: CATATGATGAAGCTTGGGT...
Embodiment 3
[0074] Embodiment 3, the preparation of VP1 protein
[0075] 1. Construction of recombinant plasmids
[0076] 1. Synthesize the FMDV VP1 double-stranded DNA molecule shown in sequence 7 of the sequence listing.
[0077] 2. Using the double-stranded DNA molecule synthesized in the step as a template, the primers composed of P1 and P2 are used to perform PCR amplification on vp1 to obtain a PCR amplification product.
[0078] The restriction endonuclease BamH I site is underlined, the Bsa I restriction site is double underlined, and the FLAG sequence is obliquely bolded and marked with wavy lines:
[0079] P1: GGTCTCTAGG TATGACCACTTCGACGGGCGAGT
[0080] P2:
[0081] 3. The PCR amplified product of step 2 was double-digested with restriction endonucleases BsaI and BamHI, and the digested product was recovered.
[0082] 4. Digest the plasmid pHisSUMO with restriction endonucleases BsaI and BamHI to recover a vector backbone of about 5700 bp.
[0083] 5. Ligate the digeste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com