Construction method, expression system and application of multi-union fusion recombinant protein capable of preventing piglet diarrhea
A technology of recombinant protein and expression system, applied in the field of biomedicine, can solve the problem of no vaccine, etc., and achieve the effects of good specificity, good effect and good protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] 1. Find the amino acid sequence (BAA25725.1) and LTIT (BAA25726.1), LTIAA (WP_095374651.1) and LTIAB (WP_095389218.1), LTIAB (WP_095389218.1), LTIAB (WP_095389218.1), LTIAB (WP_095389218.1), LTIAB (WP_095389218.1), LTIAB (ALO79807.1) ) And ltincb (alo79813.1), heat-resistant colorectoxin ST A (CAD87828.1) and ST B (CAD87835.1) and α toxins (AQN80672.1) and beta toxins (Aji77135.1) Relevant information, antigenic analysis is performed by DNASTAR.
[0066] Depending on the amino acid sequence of α toxins LTIA, LTIB, LTICA, LTICB, ST A, ST B, LTICA, LTICB, ST A, ST B, LTICA, LTICB, ST A, ST B, LTLCA, and the amino acid sequence of beta toxins were predicted to predict the antigenicity of antigenicity.
[0067] LTIA's No. 74-113-amino acid residue of LTIAA, 1,1263 amino acid residues of LTIAA, LTIAA, 74th to 263 amino acid residues, LTIABs, 1,263 amino acid residues, LTIAA, 1,163 amino acid residues, LTIAA At the 20th to 50th amino acid residues of LTICA, the 64th to 90 amino a...
Embodiment 2
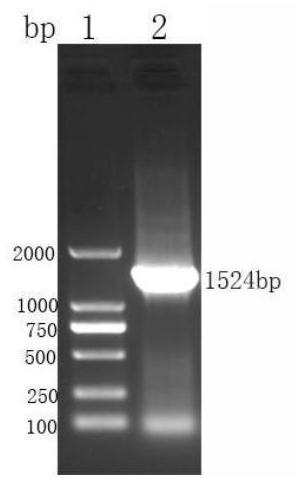
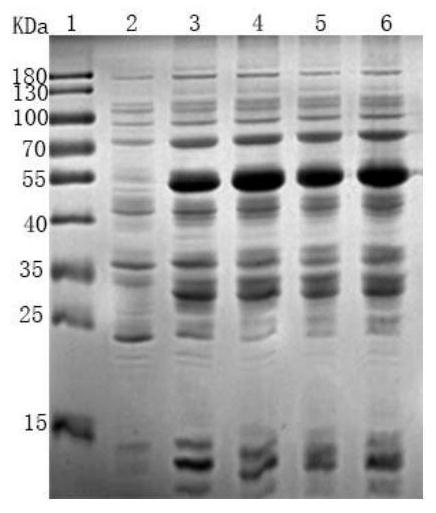
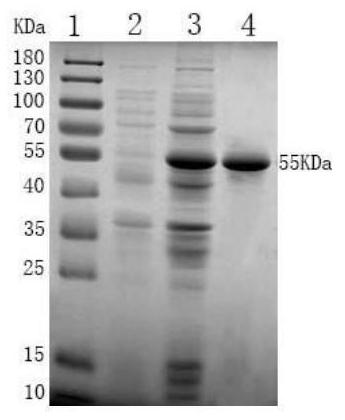
[0105] LTⅠ-LTⅡ-ST-Cp activity verification and purification of recombinant proteins
[0106] 1, Protein Purification
[0107] 1) The LTⅠ-LTⅡ-ST-Cp recombinant E.coli BL21 (DE3) bacteria, before 100μL inoculated in 5ml LB liquid added thousandth of kanamycin penicillin, 37 ℃, 160rpm shaking culture 3h.
[0108] 2) the bacteria in step 1 5ml access 1L sterilized LB added thousandth of kanamycin penicillin, 37 ℃, 160rpm, shaking culture 2h, then added of IPTG, to a final concentration of 1mmol / L, 16 ℃ shaking 160rpm, inducing 24h.
[0109] 3) The bacterial solution from step 2, 8000rpm, 4 ℃ centrifuged 10min, close bacteria, sonicated for PBS, the ice bath was sonicated on ice for 10min bacteria, ultrasonic distance 3s 3s, total 30min, 8000 rpm for ultrasonic completed, centrifuged 4 ℃ 10min, supernatant was discarded, received precipitation. Washed 3 times with PBS, and centrifugation conditions as before.
[0110] 4) Take the resulting precipitate was further weighed wet weight, ...
Embodiment 3
[0152] Recombinant protein LTI-LTITI-ST-CP on the immunocant protection of piglets
[0153] 1, experimental strain and animal
[0154] The new born long white pig, purchased from the Yangchun pig farm (the pig farm did not have a pig diarrhea, and if the pig is not vaccinated after birth), purchase 50 piglets, raised in standard pig houses, professionals according to standards Feeding, each pig is individually crossed, and any antibiotics are not added in the feed.
[0155] 2, the preparation of vaccines
[0156] The recombinant protein was diluted to 1 μg / μL, and the aluminum hydroxide sol adjuvant was slowly added to the recombinant protein (1G adjuvant per piglet), and was slowly shaken at room temperature for 30 min at room temperature.
3,
[0157] 3.1 vaccination and immunization
[0158] 1) Experiment group
[0159] 40 head pigs were randomly divided into 4 groups, and 10 per group was immunized according to the following immunization procedures, and the immunization cycl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com