A kind of recombinant superantigen seb mutant, its preparation method and application
A mutant and superantigen technology, applied in the field of medicine and biology, can solve the problems of reduced anti-tumor activity, T-cell incompetence, toxic shock, etc., and achieve the effect of enhanced anti-tumor effect, simple and easy operation, and enhanced activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
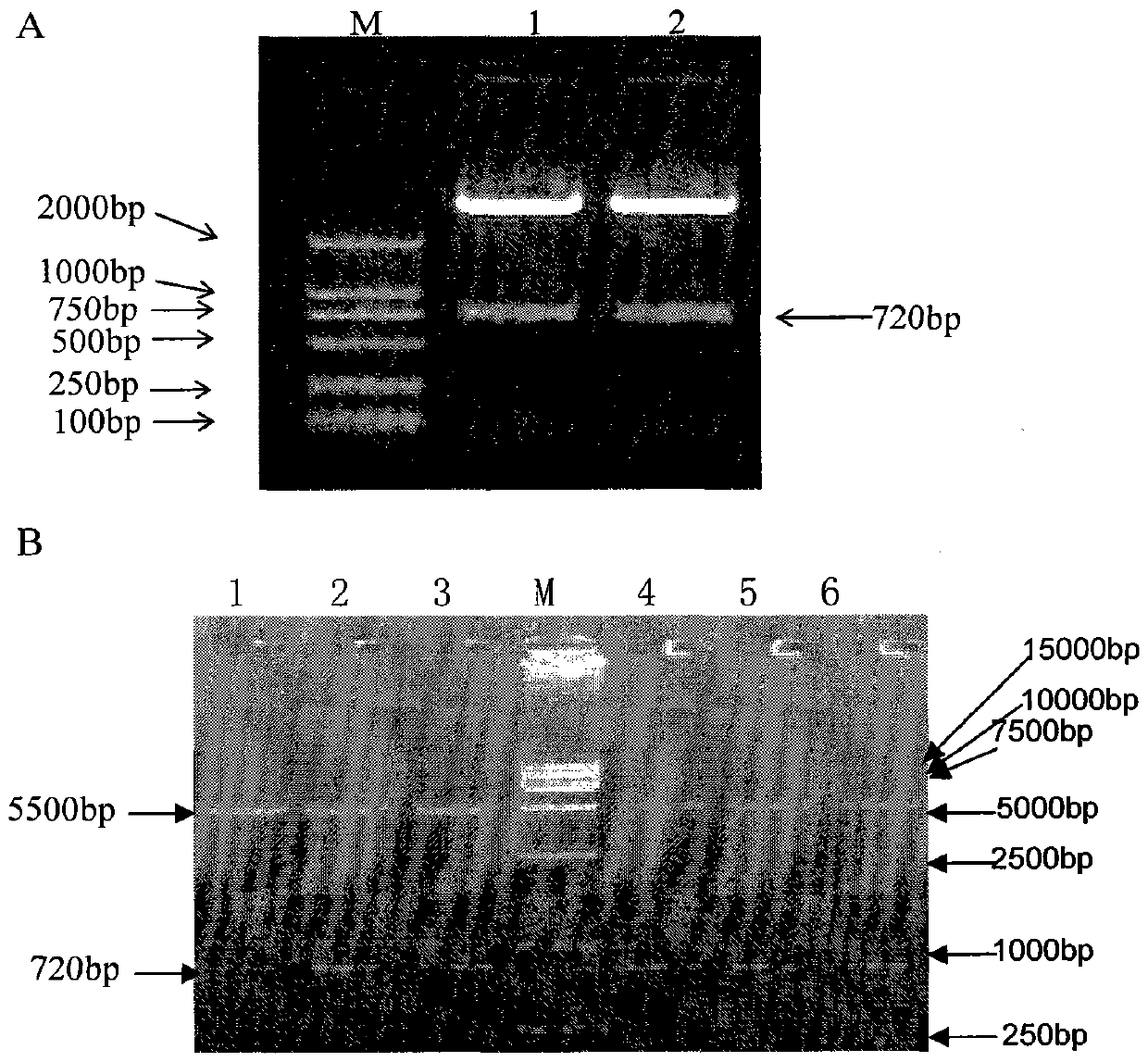
[0025] The preparation of embodiment one recombinant superantigen SEB mutant
[0026] 1. Materials:
[0027] 1.1 Main Instruments
[0028] PCR amplification instrument (Whatman Biometra, Germany), electric thermostatic water bath (Beijing Changfeng Instrument Co., Ltd.), AKTA FPLC protein purification instrument (GE, USA), polyacrylamide gel electrophoresis apparatus (BIORAD, USA)
[0029] 1.2 Strains and main reagents
[0030] Natural Staphylococcus aureus enterotoxin SEB strain ATCC (14458), purchased from ATCC (American Standard Biological Collection); pET-32(a+) expression vector, purchased from Novagen; Escherichia coli DH5α, BL21(DE3) It is the product of Invitrogen Company; gene cloning vector pGEM-T, purchased from Promega Company; NdeI, BamHI, purchased from TaKaRa Biotechnology Company; T4 DNA ligase, purchased from New England BioLabs Company; IPTG, purchased from AMRESCO Company; plasmid extraction kit, Guangzhou Dongsheng Company; Genomic DNA Extraction Kit, Sa...
Embodiment 2
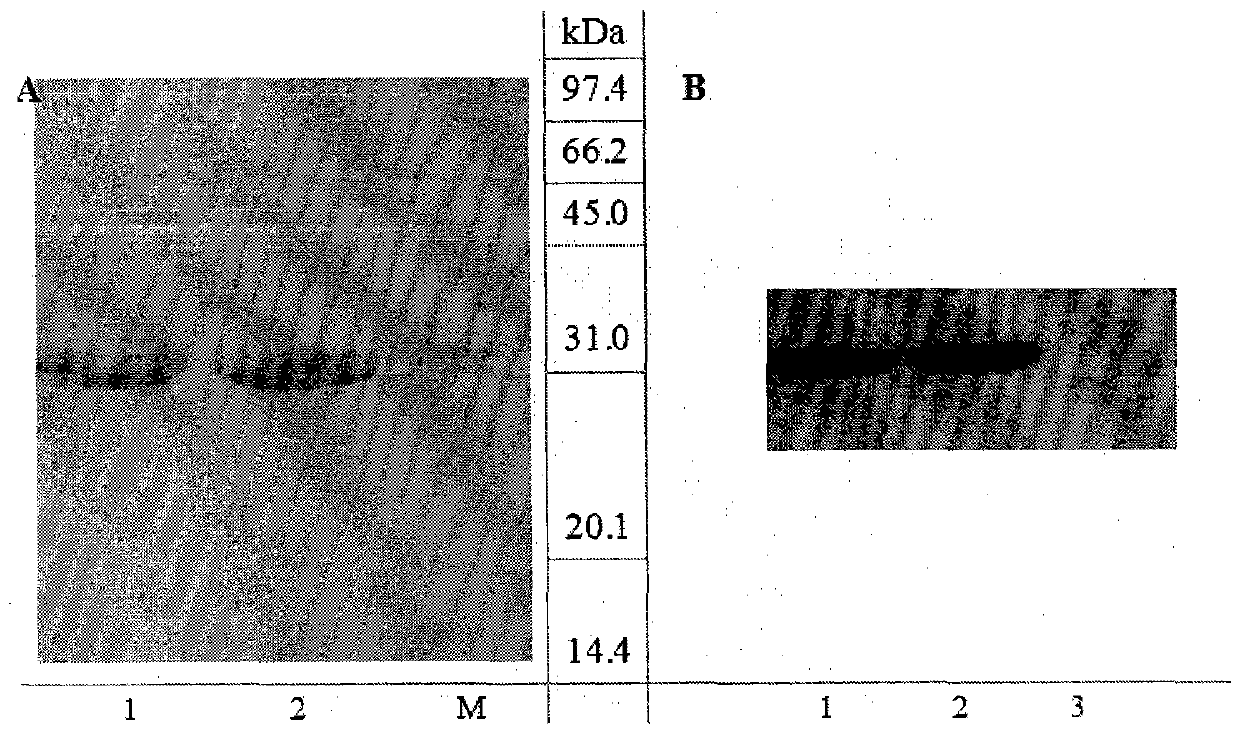
[0060] Western-blot identification of embodiment two purified proteins
[0061] 1. Materials:
[0062] 1.1 Main Instruments
[0063] ST-1 semi-dry transfer electrophoresis tank, Dalian Jingmai Biotechnology Co., Ltd.
[0064] 1.2 Main reagents
[0065] Mouse anti-wt-SEB monoclonal antibody, goat anti-mouse IgG / HRP were purchased from SANTA CRUZ Company; bovine serum albumin (BSA) was purchased from Beijing Biocom Biotechnology Company; SDS and ECL developer were purchased from Sigma Corporation of the United States; Super West PicoTrial Kit was purchased from Thermo Company. The remaining is the same as previous embodiment.
[0066] 2. Method results:
[0067]The prepared protein was electrophoresed by SDS-PAGE and transferred to the membrane by semi-dry method. at 1mA / cm 2 The power, constant current transfer 1h. After the transfer, the nitrocellulose membrane was removed and blocked overnight at 4°C with 5% skimmed milk powder. After the blocking, the blocking sol...
Embodiment 3
[0068] Example 3 Cytotoxic activity test (MTS detection)
[0069] 1. Materials:
[0070] 1.1 Main Instruments
[0071] Bx60-32FB2-E01 fluorescence microscope, Olympus, Japan; ELISA, USA
[0072] National SPECTRA MAX PLUS
[0073] 1.2 Main reagents and consumables
[0074] MTS detection kit was purchased from Promega; cell culture flasks, culture plates and other consumables and RPMI1640 medium were purchased from GIBCO; Ficoll-PaqueTM PLUS lymphocyte separation medium was purchased from GE
[0075] 1.3 Cell lines
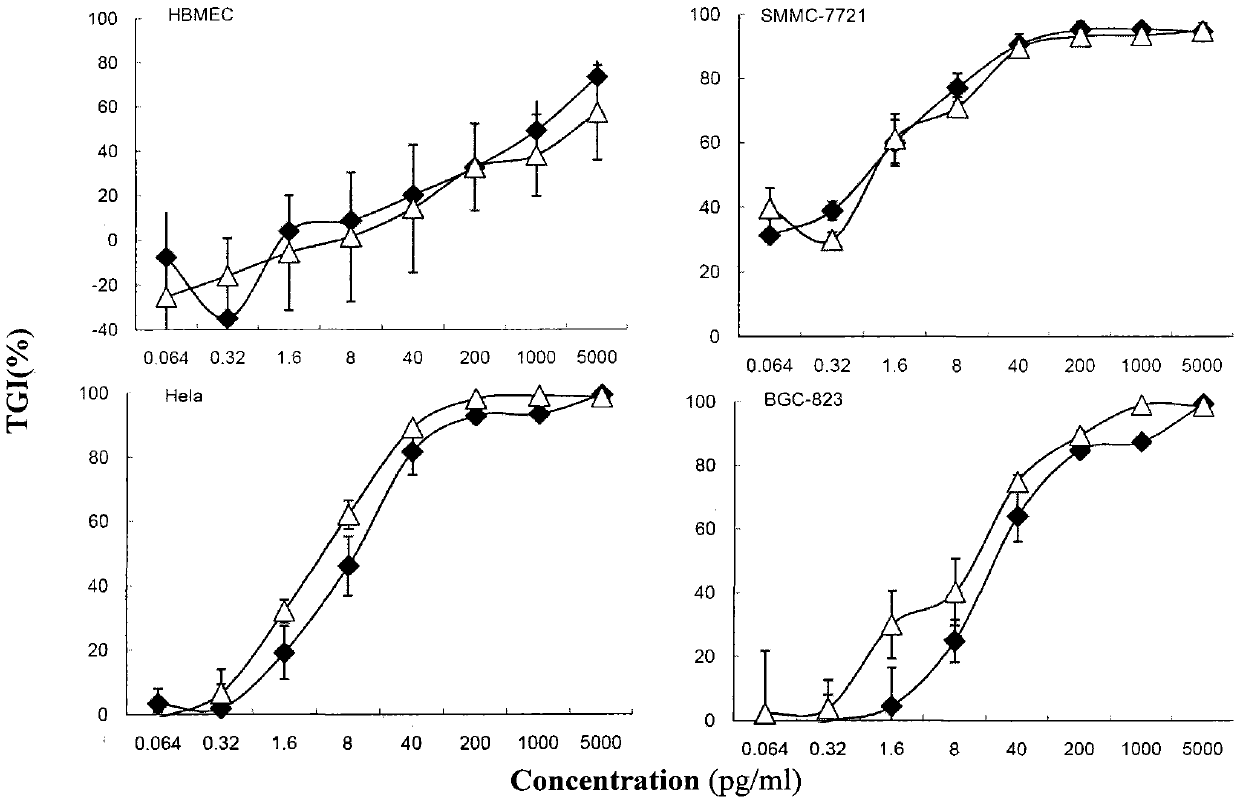
[0076] Human tumor cells (human liver cancer cell line SMMC-7721, cervical cancer cell line Hela, gastric cancer cell line BGC-823) and normal human brain microvascular endothelial cells HBMEC were purchased from the Cell Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The remaining is the same as previous embodiment.
[0077] 2. Method results:
[0078] Take various human cells in good condition, the cell viability mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com