Patents
Literature
100 results about "Staphylococcal Enterotoxins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Detection And Quantification Of Biomarkers Via A Piezoelectric Cantilever Sensor
InactiveUS20090078023A1High sensitivityShort timeMaterial analysis using sonic/ultrasonic/infrasonic wavesMicrobiological testing/measurementEscherichia coliMultiple sensor
Quantification of a target analyte is performed using a single sample to which amounts of the target analyte are added. Calibration is performed as part of quantification on the same sample. The target analyte is detectable and quantifiable using label free reagents and requiring no sample preparation. Target analytes include biomarkers such as cancer biomarkers, pathogenic Escherichia coli, single stranded DNA, and staphylococcal enterotoxin. The quantification process includes determining a sensor response of a sensor exposed to the sample and configured to detect the target analyte. Sensor responses are determined after sequential additions of the target analyte to the sample. The amount of target analyte detected by the sensor when first exposed to the sample is determined in accordance with the multiple sensor responses.
Owner:DREXEL UNIV
Enterotoxin gene cluster (egc) superantigens to treat malignant disease
InactiveUS20090162315A1Good effectPrevent morbidityBacterial antigen ingredientsPeptide/protein ingredientsDiseaseSystemic chemotherapy
The use of classical superantigens for treatment of cancer has resulted in a low response rates and serious toxicity in humans which is attributable, in part, to the presence of preformed superantigen specific antibodies in the plasma of treated patients. The present invention addresses this problem by providing a method for treating tumors comprising the administration of one or a plurality of egc (enterotoxin gene cluster) staphylococcal enterotoxins comprising staphylococcal enterotoxins G, I, M, N, O. These superantigens in native unmodified form can be administered intrathecally, intratumorally, intravenously to humans with advanced lung cancer while resolving pleural effusions and prolonging survival to 300% above control patients treated with talc pleurodesis. Intratumoral egc superantigens induces a significant and sustained reduction of the tumor size. In contrast to classic Sags, the egc superantigens induced minimal toxicity, are rarely associated with the presence of preformed antibodies and are used as a plurality with a broad T cell Vβ profile. Useful egc superantigen compositions for parenteral administration native egc enterotoxins, homologues, fragments and fusion proteins of native egc enterotoxins capable of activating a broad spectrum of T cells expressing T cell receptor / α motifs. T cell survival-enhancing cytokines IL-7, Il-15, Il-23 are used. together with parenteral egc SE therapy. Also disclosed is combined therapy that includes parenteral, intratumoral or intrathecal superantigen compositions in combination with (i) intratumoral low, non-toxic doses of one or more chemotherapeutic drugs or (ii) systemic chemotherapy at reduced and non-toxic doses of chemotherapeutic drugs or (iii) radiation therapy or (iv) anti-angiogenic and tyrosine kinase inhibitors.
Owner:TERMAN DAVID S +4
Function epitopes of staphylococcal enterotoxin B (SEB), monoclonal antibodies specifically bound with function epitopes and application of monoclonal antibodies
InactiveCN102757481AAntibacterial agentsImmunoglobulins against bacteriaEpitopeStaphylococcus enterotoxin B
The invention belongs to the technical field of biology, and particularly discloses function epitopes 195SFWYDMMP202, 12HKSSKFTGL20, 18TGLMEN23 and 43QFLFYFIY51 of staphylococcal enterotoxin B (SEB), monoclonal antibodies 4A3 and 3E2 specifically bound with the function epitopes and application of the monoclonal antibodies in the preparation of drugs for treating SEB-induced toxic shock syndrome.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
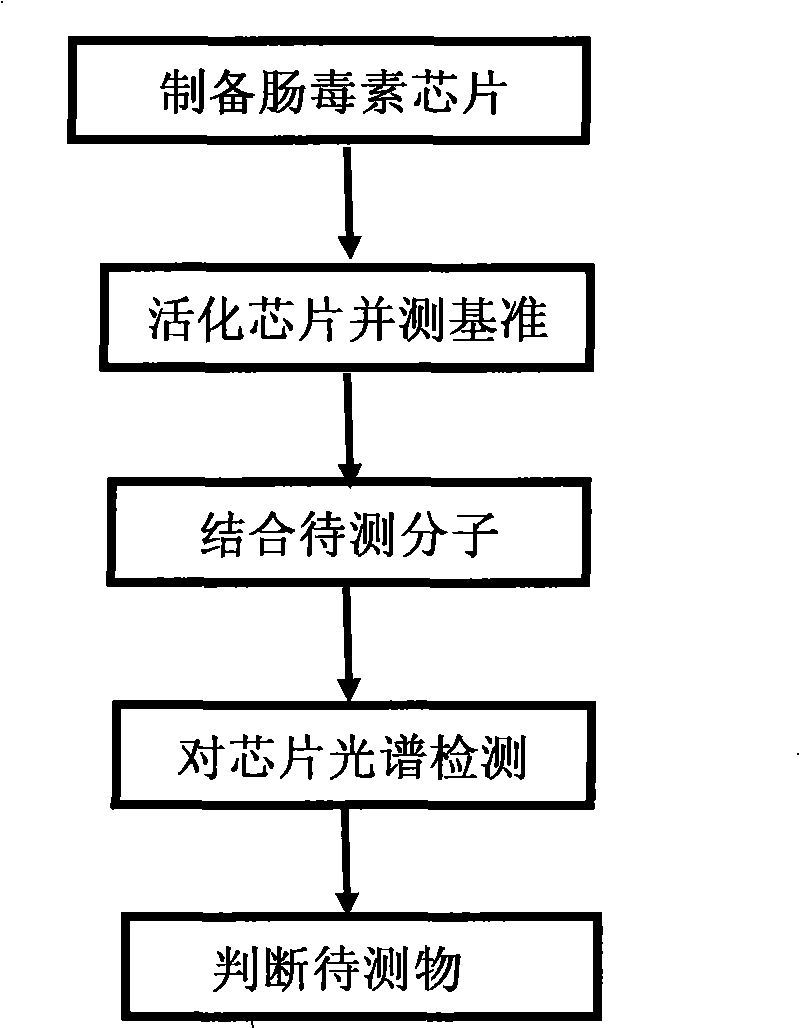
Staphylococcal enterotoxin detection method
InactiveCN101514988AShort detection timeHigh sensitivityMaterial analysis by optical meansStaphylococcusFluorescence
A staphylococcal enterotoxin detection method is characterized in that the detection method comprises the steps as follows: (1) preparing an LSPR detection chip of staphylococcal enterotoxin; (2) activating the surface of the metal structure of the chip to form a specific biological molecular membrane; (3) testing the extinction spectrum of the chip to obtain a reference value before combination; and (4) dripping a to-be-detected sample, putting the sample in an LSPR sensor for detection after the full reaction of the sample, observing spectral shift condition by using the specific reaction among antigen-antibody molecules, and judging whether the to-be-detected sample contains staphylococcal enterotoxin, so as to realize fast detection with high sensitivity and without a label. The method does not need complex equipment and does not use radioactive isotope, enzyme or fluorescence and the like as a label, and has the remarkable characteristics of quickness, high sensitivity, wide application range, security and high stability. The method provides a simple and practical new method for the quick detection of staphylococcal enterotoxin.
Owner:INST OF OPTICS & ELECTRONICS - CHINESE ACAD OF SCI
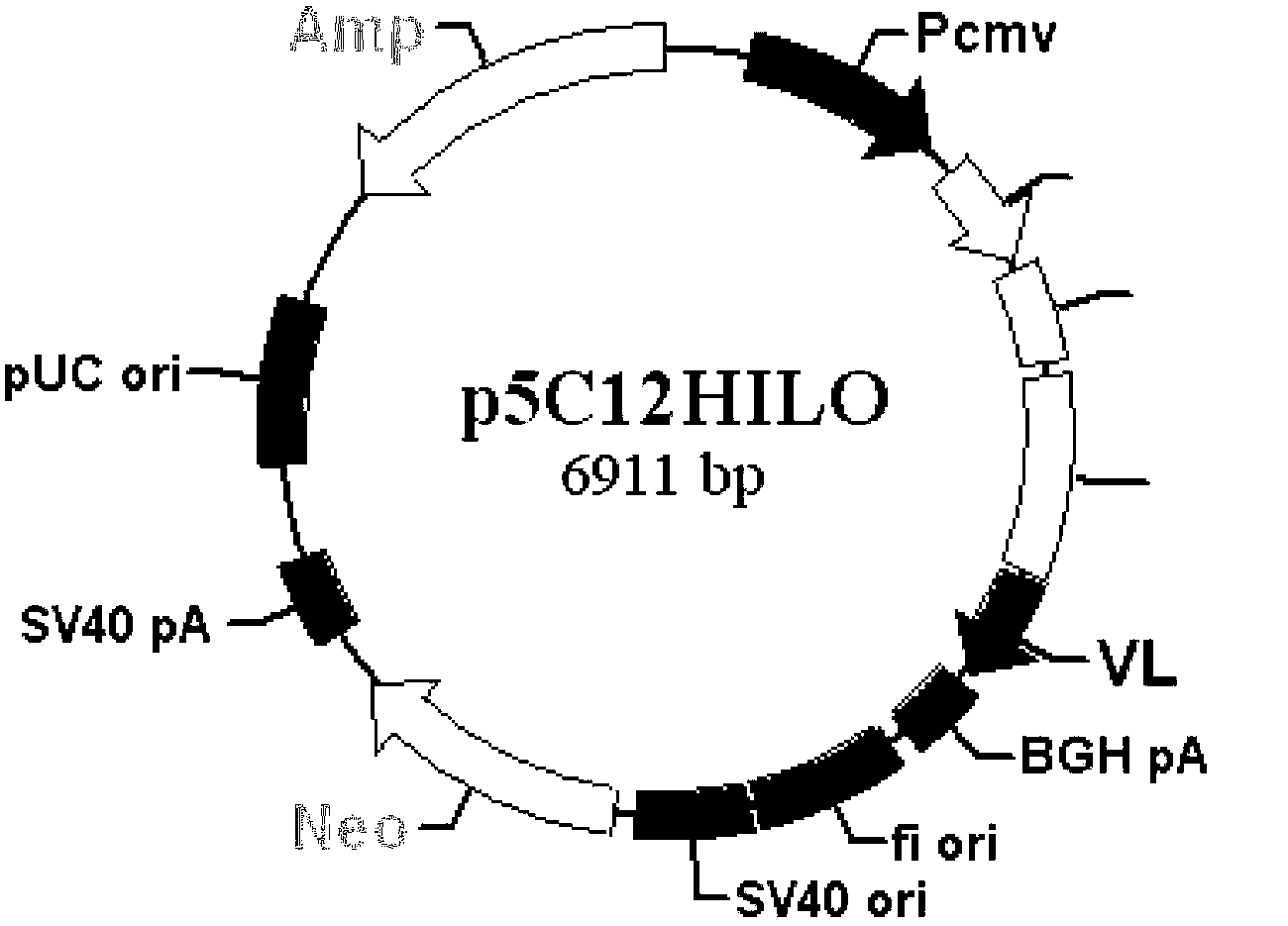
Staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use
InactiveCN103224560AOvercome the defects in the detection meansImmunoglobulins against bacteriaBiological testingAntiendomysial antibodiesGenetic engineering
The invention discloses a staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use. The staphylococcal enterotoxin gene engineering reshaped antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. Through construction of light and heavy chain eukaryotic co-expression vectors of the staphylococcal enterotoxin monoclonal antibody, a high-efficiency expression and stable-secretion mammalian cell line and the gene engineering reshaped antibody having high singularity and strong affinity are obtained. The staphylococcal enterotoxin gene engineering reshaped antibody can be used in staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
Staphylococcal enterotoxin micromolecule antibody and its preparation method and use
InactiveCN103224561AImmunoglobulins against bacteriaVector-based foreign material introductionDisulfide bondingNatural antibody
The invention discloses a staphylococcal enterotoxin micromolecule antibody and its preparation method and use. The staphylococcal enterotoxin micromolecule antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. The preparation method comprises the following steps of designing and constructing eukaryotic mono-promoter co-expression vectors of staphylococcal enterotoxin monoclonal antibody light chain and heavy chain variable region genes, introducing cysteine residues to C- ends of the light chain and the heavy chain to form interchain disulfide bonds by an intracellular peptide fragment self-assembling principle, and simulating antigen binding domain spatial conformation of natural antibodies to obtain high-efficiency expression stable-secretion mammal cell line and the high-singularity strong-affinity micromolecule antibody. The staphylococcal enterotoxin micromolecule antibody can be used for staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
PCR (polymerase chain reaction) synchronous detection kit for staphylococcus aureus enterotoxin A and B genes
InactiveCN102181547ASensitive and accurate detectionReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationNucleotideInverse polymerase chain reaction
The invention discloses a PCR (polymerase chain reaction) synchronous detection primer for staphylococcus aureus enterotoxin A and B genes, a detection kit thereof and a detection method. The nucleotide sequence of the synchronous detection primer is as shown in SEQ (sequence) ID (identity) No. 1 and 2. The invention further provides the PCR detection kit containing the primer. By adopting the detection kit, the staphylococcus aureus enterotoxin A and B in a food can be accurately and sensitively detected, and the lowest detection concentration of DNA (deoxyribonucleic acid) is 3.58ng; furthermore, the detection kit has no cross reaction with other bacteria, and the specificity is good; simultaneously, the pretreatment process of samples is simple, the consumed time is short, a large number of the samples can be detected simultaneously, and the cost is low.
Owner:BEIJING SANYUAN FOOD
Immunochromatographic assay test paper for detecting staphylococcal enterotoxin B and preparation method thereof
InactiveCN1880961ASuitable for useSimple specimen handlingMaterial analysisFiberStaphylococcal Enterotoxins
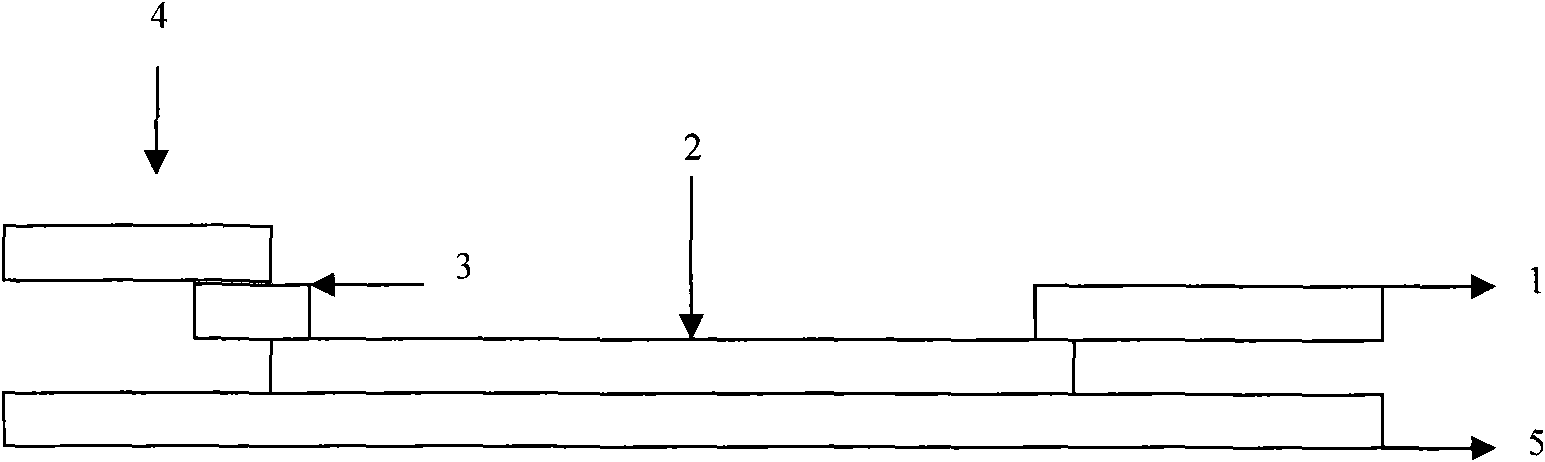
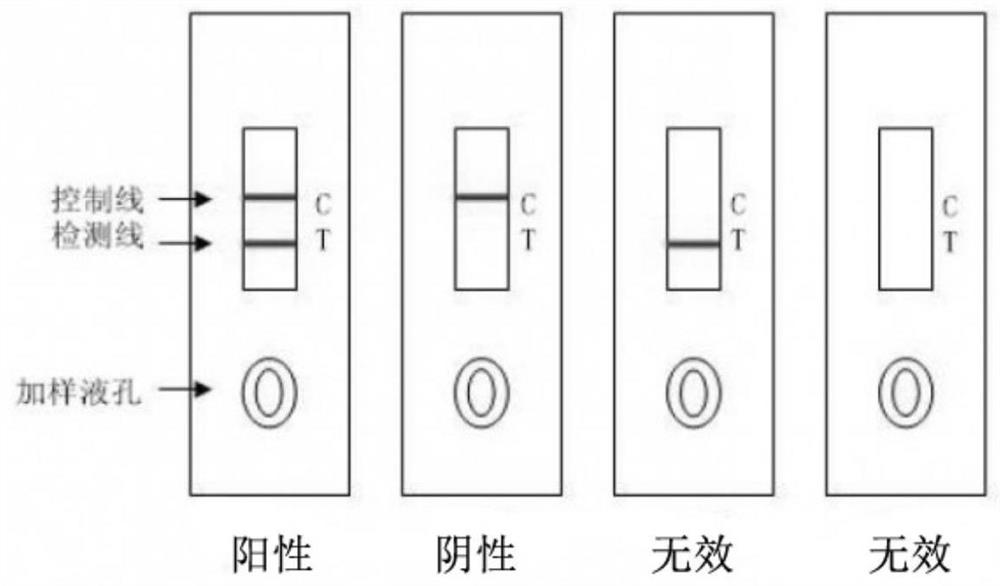
The invention discloses an immune chromatographic indicator paper and making method of B-typed staphylococcus aureus enterotoxin, which comprises the following parts: sample pad 1, metal pad 2 with specific antibody mark colloidal gold probe of B-typed staphylococcus aureus enterotoxin in connection with one end of sample pad tightly, nitric fiber film (NC film) 3 in connection with the other end of metal pad tightly, water-absorbing pad 4 in connection with the other end of nitric fiber film tightly, wherein the nitric fiber film covers mutually separated detecting line 5 and quality control line 6, which is specific antibody of B-typed staphylococcus aureus enterotoxin and sheep-anti-rabbit lgG separately.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Primers useful in polymerase chain reaction for the identification of subtypes c1, c2 and c3 of staphylococcal enterotoxin type c
InactiveUS20060257864A1Sugar derivativesMicrobiological testing/measurementStaphylococcal EnterotoxinsPcr method
The invention provides primers designed on base of the difference of sequences among staphylococcal enterotoxin subtypes, i.e., C1, C2 and C3. The invention also provides a PCR method for detecting subtypes of staphylococcal enterotoxin type C by using the above-mentioned primers. The invention relates also to DNA probes useful for the identification of subtypes C1, C2 and C3 of staphylococcal enterotoxin type C in various food and clinical samples. The primers of the invention comprises following sequences: ENTC15′-ACAGA GTTAT TAAAT GAAGG-3′;ENTC25′-GTATC AGCAA CTAAA GTTAT-3′;ENTC35′-AAGAG ATTAT TTATT TCACGT-3′;ENTCR5′-ATCAT ACCAA AAAGT ATTGC-3′.
Owner:NATIONAL CHUNG HSING UNIVERSITY
Antibody against staphylococcal enterotoxin B and application thereof
ActiveCN110498854AAntibacterial agentsImmunoglobulins against bacteriaStaphylococcus cohniiStaphylococcus enterotoxin B
The invention discloses an antibody against staphylococcal enterotoxin B and application thereof. The heavy chain of the antibody against staphylococcal enterotoxin B has variable regions CDR1, CDR2 and CDR3 of which the amino acid sequences are shown in SEQ ID NO. 5, SEQ ID No. 6 and SEQ ID No. 7, or is composed of CDR variants with equivalent functions; and light chain of the antibody against staphylococcal enterotoxin B has variable regions CDR1, CDR2 and CDR3 of which the amino acid sequences are shown in SEQ ID NO. 9, SEQ ID No. 10 and SEQ ID No. 11, or is composed of CDR variants with equivalent functions. The antibody against staphylococcal enterotoxin B is capable of specifically binding with staphylococcal enterotoxin B or free enterotoxin B; and thus, the antibody can be used fortreating, preventing and diagnosing staphylococcus aureus infection. Therefore, the antibody against staphylococcal enterotoxin B will be an important research direction in the fields of "non-antibiotic" treatment of methicillin-resistant staphylococcus aureus infection and drug resistance development control.
Owner:ARMY MEDICAL UNIV
Rebuild golden staphylococcus enterotoxin N and preparation and application thereof
InactiveCN101037478ATypical superantigen activityGrowth inhibitionPeptide/protein ingredientsDepsipeptidesAntigenStaphylococcus cohnii
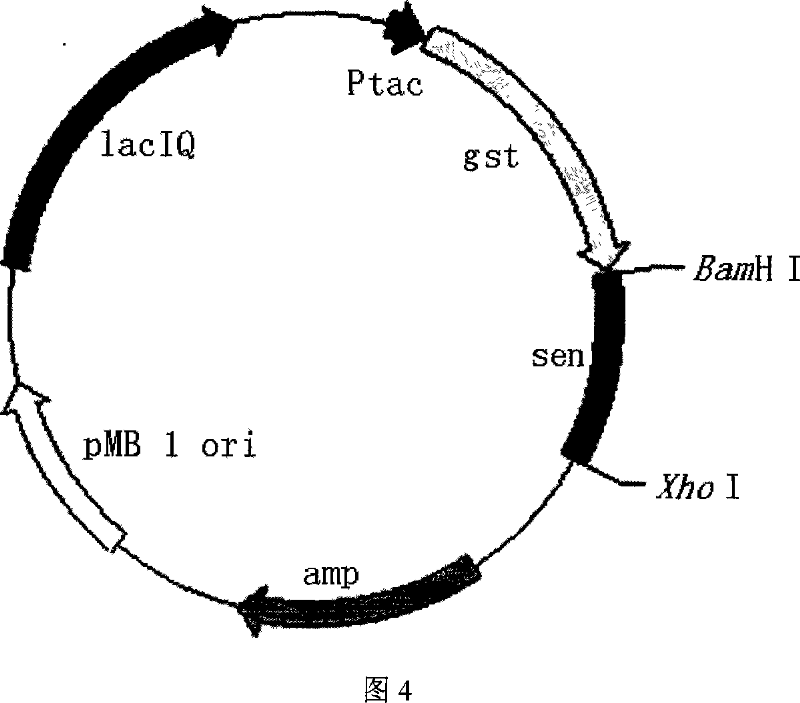
A recombinant Methicillin-resistant Staphylococcus aureus enterotoxin N belongs to a super antigen with SEQ ID NO.1 amino acid sequence, which is expressed by a recombination of a gene coding SEN from the Staphylococcus aureus and a plasmid vector, and a transformation to the proper host and is obtained a recombinant a highly purified SEN protein by affinity purification. The invention proves the recombinant protein has a typical super antigen activity better than SEC and can be applied in the preparation of activating the lymphocytes multiplication and inhibiting the tumor cell proliferation. The product is suitable for preparing the highly purified enterotoxin with the super antigen activity and exploiting super antigen agent. The invention has a wise design, purifies the target protein with affinity chromatography, has a simple process and a high purification speed.
Owner:ZHEJIANG UNIV
Staphylococcus aureus enterotoxin B (SEB) immune preparation and its preparation method and use
InactiveCN103160519AImproving immunogenicityImprove development and utilization valueAntibacterial agentsMicroorganism based processesHigh titerImmunogenicity
The invention provides a staphylococcus aureus enterotoxin B (SEB) immune preparation SEB2-HSP65 and its preparation method and use. The preparation method comprises the following steps of modifying a SEB gene into a SEB2 gene, fusing the SEB2 gene and a gene of a heat shock protein HSP65 to obtain a core gene segment SEB2-HSP65, and carrying out expression of the core gene segment SEB2-HSP65 to obtain a recombinant fusion protein SEB2-HSP65. The recombinant protein SEB2 does not have a TCR cell receptor binding capacity thereby solving the problem that the existing SEB immune preparation produces a large amount of inflammatory factors, and the HSP65 enhances immunogenicity of the recombinant fusion protein SEB2. The recombinant fusion protein SEB2-HSP65 as an immune preparation for animal immunization does not need any immunologic adjuvants, can produce high-titer antibodies, can help animals to resist 5*LD50SEB toxin attack and has a protection rate of 100%. The recombinant fusion protein SEB2-HSP65 has large development and use values.
Owner:张婉茹
Staphylococcus aureus enterotoxin detection kit and preparation and use methods thereof
InactiveCN105652003AStrong specificityHigh sensitivityMaterial analysisStaphylococcal EnterotoxinsToxin detection
The invention discloses a staphylococcus aureus enterotoxin detection kit and preparation and use methods thereof; the kit includes staphylococcus aureus enterotoxin SEA and SEB specific antibodies and enzyme-labeled staphylococcus aureus enterotoxin SEA and SEB antibodies, has the characteristics of high specificity, high sensitivity, high precision, high accuracy and the like and can quickly detect melamine remaining in feeds and animal products.
Owner:HUAAN MAGNECH BIO TECH
Protein G staphylococcus aureus enterotoxin kit and preparation method thereof
InactiveCN105353129AIncrease profitReduce dosageMaterial analysisAbzymeStaphylococcus aureus enterotoxin B
The invention relates to a protein G staphylococcus aureus enterotoxin kit and a preparation method thereof. Protein G is adopted to preprocess an enzyme labeling plate, and quick and accurate detection on staphylococcus aureus enterotoxin A / B is realized by combining with an ELISA double-antibody sandwich method. The kit consists of the 96-hole enzyme labeling plate, a staphylococcus aureus enterotoxin A-resistant protein G recombinant protein monoclonal antibody, a staphylococcus aureus enterotoxin B-resistant monoclonal antibody, a staphylococcus aureus enterotoxin antibody enzyme labeling object containing a horseradish peroxidase label, a sample diluent, a cleaning solution and a stop buffer. The protein G staphylococcus aureus enterotoxin kit can quickly and effectively detect the residual level of tony red in a to-be-detected sample, and also has the characteristics of simple operation, good specificity, high sensitivity, and the like.
Owner:HUAAN MAGNECH BIO TECH
Staphylococcus aureus enterotoxin B nanometer antibody B7, application and reagent kit
ActiveCN110526968ARelatively small molecular massImprove stabilityImmunoglobulins against bacteriaBiological material analysisStaphylococcus cohniiStaphylococcus enterotoxin B
The invention discloses a staphylococcus aureus enterotoxin B nanometer antibody B7, an application and a reagent kit. The obtained nanometer antibody has small relative molecular mass, strong stability and high yield, can specially recognize SEB, and is broader in use and higher in specificity compared with a conventional monoclonal antibody. The invention discloses the nanometer antibody and a gene sequence for coding the nanometer antibody, a method for producing the nanometer antibody, and a reagent kit applying the antibody. The obtained nanometer antibody can prevent combination with surface protein A of the staphylococcus aureus, shows higher specificity, has good stability and small molecular weight, and can realize mass production.
Owner:NORTHWEST A & F UNIV
Genotype inspection method and genotyping inspection method of staphylococcal enterotoxin
InactiveCN101760553AQuick checkSensitive highMicrobiological testing/measurementMicroorganism based processesQuarantineEpidemiology
The invention discloses a genotype inspection method and a genotyping inspection method of staphylococcal enterotoxin. The genotype inspection method of staphylococcal enterotoxin includes the steps of sample treatment, DNA extraction, PCR and PCR amplified product analysis. The amplified sample is put in agarose gel including ethidium bromide for electrophoresis and color development and observed under a transmissive uviol lamp; the inspected sample can be judged as a positive sample including corresponding staphylococcal enterotoxin genotype if the length of the amplified nucleic acid band is the same as the amplified length in table 2. The first method can fast inspect the particular staphylococcal enterotoxin genotype, with high sensitivity, high particularity and high accuracy. The second method can achieve systematic genotyping of staphylococcal enterotoxin genes from different sources and provide technical support for analysis of relationships among different enterotoxin types. The invention is significant in food security quarantine, disease diagnosis and molecular epidemiology research.
Owner:TIANJIN UNIV
Immune chip for detecting staphylococcal enterotoxin and papaverine and its preparing method
The present invention discloses an immune chip for detecting staphylococcal enterotoxin and papaverina dn its preparation process. The immune chip has slide glass with surface treated with silane as aldehydation reagent and crosslinked with glutaraldehyde as double-function crosslinking reagent, and connected via covalent bond with the molecule of at least one kind of antibody staphylococcal enteriotoxin A antibody, staphylococcal enterotoxin B antibody, staphylococcal enterotoxin C antibody and papaverine antibody. The detection with the immune chip is simple, effective and low in cost.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Novel prophylactics/remedies for immunopathy
InactiveUS20060024322A1Low toxicityBacterial antigen ingredientsPeptide/protein ingredientsWild typeBULK ACTIVE INGREDIENT
A prophylactic / remedy for immunopathy for immunopathy comprising, as an active ingredient, modifications of Staphylococcal enterotoxin B (SEB) with substitution of at least one amino acid residues within the amino acid sequence of natural type SEB, or derivatives thereof, wherein the SEB modifications or derivatives thereof have inhibitory activity on T cell activation wherein they interact with specific Vβ component of T cell receptor (TCR) but are reduced in their immunological responsiveness to SEB without inducing elimination of T cells having specific Vβ component, the elimination being normally induced by natural type SEB or recombinant wild-type SEB.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES & KOWA COMPANY
Protein suspending chip for quantitative detection of staphylococcal enterotoxin B and method for producing the same
InactiveCN101498720ACoating volume improvementQuick checkMaterial analysis by observing effect on chemical indicatorProtein insertionBacterium L
The invention aims to provide a protein suspension chip capable of quantificationally detecting bacterial toxin and the preparation method thereof, and particularly relates to a protein suspension chip which is suitable for quantificationally detecting and analyzing staphylococcal enterotoxin. The method has high sensitivity and high particularity, good detection capacity and wide dynamic range and builds a novel detection modularity platform.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Preparation method of monoclonal antibody for simultaneous detection of staphylococcal enterotoxins A and B
InactiveCN102329391AImmunoglobulins against bacteriaTissue cultureStaphylococcus cohniiStaphylococcal Enterotoxins
The invention relates to a preparation method of a monoclonal antibody for simultaneous detection of staphylococcal enterotoxins A and B (referred to as SEA and SEB). The SEA and SEB commonly caused staphylococcal poisoning are used as immunogens, and the monoclonal antibody for simultaneous detection of SEA and SEB is prepared via a mixed immunization method, a hybridoma technology and screening in an indirect ELISA (enzyme-linked immunosorbent assay, referred to as ELISA) method. The monoclonal antibody can be used for simultaneously detecting the SEA and SEB in food via one-step reaction, and thus the workload of analysis of staphylococcal food poisoning can be reduced.
Owner:王 小红 +2
Quick detection agent of staphylococcus intestinal toxin
InactiveCN1955739AShorten detection timeSimplify operating proceduresBiological testingAntigenBacterial enterotoxins
A reagent bar used for quickly detecting staphylococcus enterotoxin consists of reagent, reagent bar and micro-scale pipet. It is featured as using cell clasmatosis solution as reagent and carrying 20 micron L of specific single clone antibody of anti-staphylococcus enterotoxin, collaurum labeled single clone antibody of anti-staphylococcus enterotoxin, collaurum labeled quality control antigen and antibody of relevant antigen on reagent bar.
Owner:吴斌 +2
Colloidal gold test paper for staphylococcal enterotoxin B (SEB), preparation method thereof and application thereof
InactiveCN101561435AEasy to detectSave manpower and material resourcesBiological testingAntibody adsorptionStaphylococcal Enterotoxins
The invention discloses a detection test paper. The test paper comprises (1) a reaction support, (2) a water absorption pad, (3) a nitrocellulose membrane, (4) a gold-labeled antibody adsorption membrane and (5) a sample pad, wherein the nitrocellulose membrane is coated with a detection strip and a quality control strip of a staphylococcal enterotoxin B antibody and a quality control antibody; and the gold-labeled antibody adsorption membrane contains the staphylococcal enterotoxin B antibody labeled by colloidal gold. The test paper can be matched with a small instrument to carry out semi-quantitative detection, does not need professional training, has clear and identifiable result, can objectively store data, is simple to operate, easy to popularize, suitable for a basic level, suitable for field detection of emergency and suitable for epidemiological investigation, and plays an auxiliary role in diagnosis of staphylococcal enterotoxin B infection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Application of SEC, antiviral vaccine adjuvant and composition
InactiveCN105983096AEnhance immune responseIncrease secretion levelAntiviralsAntibody medical ingredientsTiterEnterotoxin
The invention belongs to the field of biological products, and relates to a vaccine adjuvant, in particular to application of staphylococcus enterotoxin (SEC2) C2 to serving as a vaccine adjuvant and preparing a vaccine composition and a method. The SEC2 is adopted as the vaccine adjuvant, and experiments prove that the SEC2 can serve as a foot-and-mouth disease vaccine adjuvant. The SEC2 serves as the adjuvant, and compared with an existing commercial vaccine, the titer of an antibody and expression of cell factors IL-4 and IFN-gamma can be remarkably improved, and humoral immunity and cellular immune responses are stimulated more effectively. Meanwhile, the adjuvant is convenient to produec and transport and low in cost, and is convenient and easy to popularize when used for vaccine preparation.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Indirect competitive ELISA (Enzyme Linked Immunosorbent Assay) method based on multi-epitope tandem peptides and used for synchronously detecting staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin G (SEG)
ActiveCN103412122AStrong specificityHigh sensitivityMaterial analysisStaphylococcal EnterotoxinsELISA unit
The invention belongs to the technical field of immunoassay and particularly relates to an indirect competitive ELISA (Enzyme Linked Immunosorbent Assay) method based on multi-epitope tandem peptides and used for synchronously detecting staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin G (SEG). The method mainly comprises preparation of SEA and SEG multi-epitope tandem peptide peptides (including antigens), preparation of anti-rabbit SEA and SEG antibodies, sample pretreatment and establishment of the indirect competitive ELISA detection method based on multi-epitope tandem peptides. The indirect competitive ELISA method comprises the following steps: (1) enveloping; (2) plate washing; (3) sealing; (4) plate washing; (5) adding standard substances, samples to be detected and specific antibodies in sequence; (6) plate washing; (7) adding ELIAS secondary antibodies; (8) plate washing; (9) developing and ending reaction. The method provided by the invention has the characteristics of high specificity, high sensitivity and good repeatability, is suitable for quick, synchronous and quantificational detection of SEA and SEG in foods such as milk and dairy products.
Owner:ANHUI AGRICULTURAL UNIVERSITY
AlphaLISA detection kit for type B staphylococcal enterotoxin
ActiveCN108872598AReduce the impactFast detection methodBiological testingBiotin-streptavidin complexMicrosphere
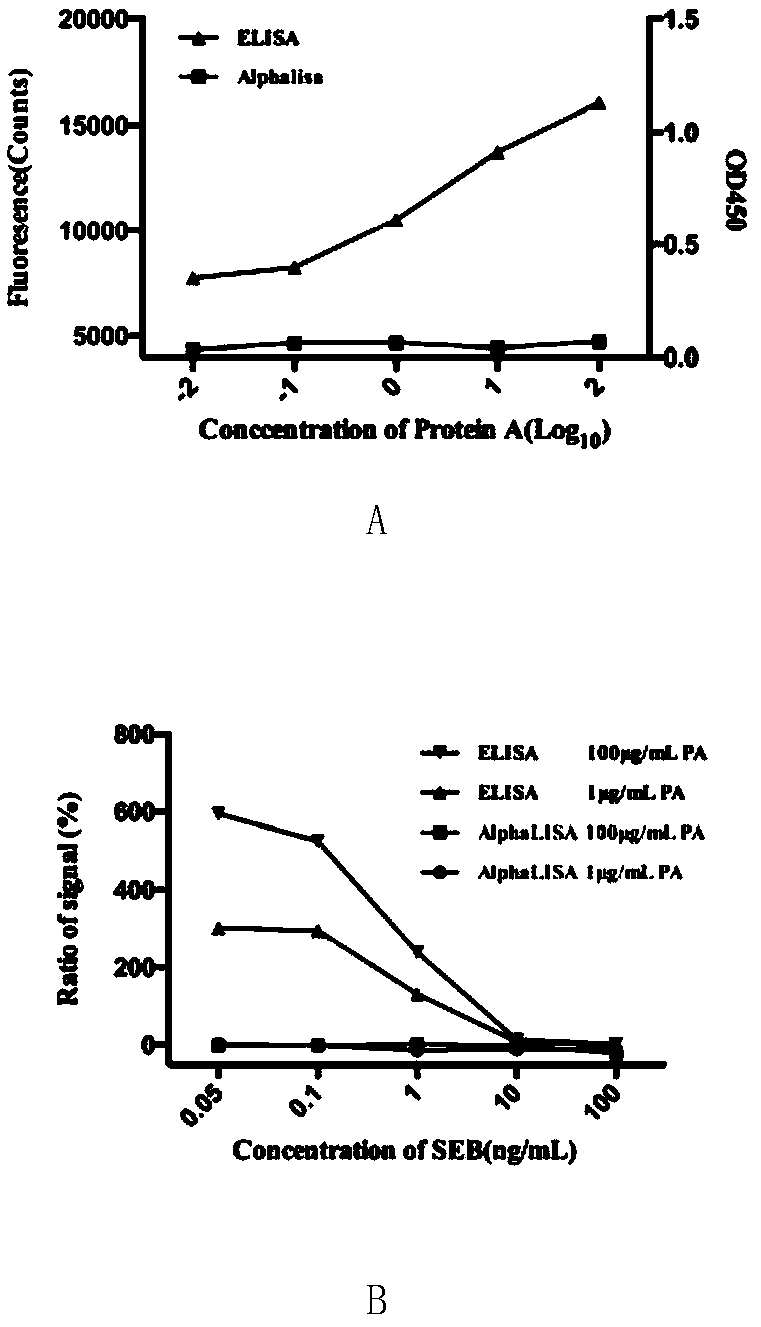
The invention discloses an AlphaLISA detection kit for type B staphylococcal enterotoxin (SEB). The AlphaLISA detection kit provided by the invention comprises an SEB capturing antibody labeled receptor microsphere, a biotin labeled SEB detection antibody and a streptavidin labeled donor microsphere. The invention further provides a detection method of the type B staphylococcal enterotoxin; the detection method of the type B staphylococcal enterotoxin, provided by the invention, is used for detecting the type B staphylococcal enterotoxin by adopting an AlphaLISA technology. An experiment proves that the detection method of the type B staphylococcal enterotoxin can be used for greatly reducing influences on protein A; the detection method provided by the invention is rapid, accurate and reliable and has a good application prospect.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Magnetic particle chemiluminescence assay kit and method for detecting staphylococcal enterotoxin A (SEA)
ActiveCN103383354AEasy to useStrong specificityChemiluminescene/bioluminescenceBacterial enterotoxinsStaphylococcus
The invention discloses a magnetic particle chemiluminescence assay kit and a method for detecting SEA, and belongs to the technical field of food safety detection. The method is a magnetic particle chemiluminescence assay method for fast detecting SEA in foods and preparations. The method comprises the following steps of diluting a standard substance, adding a marker into the dilute standard substance, carrying out uniform mixing, warm bath, sedimentation and washing, adding a luminous substrate into the mixture, determining chemiluminescence intensity, drawing a standard curve, wherein an OD value of a detected sample is in the range of the standard curve. The magnetic particle chemiluminescence assay kit has the advantages of simple operation, fast speed, high sensitivity, strong singularity and low cost.
Owner:北京泽诚生物技术有限公司
Staphylococcus aureus enterotoxin B resistant antibody, test paper and kit
ActiveCN112028994AImmunoglobulins against bacteriaFermentationBiotechnologyStaphylococcus aureus enterotoxin B
The invention discloses an antibody or an active fragment thereof specifically combined with staphylococcus aureus enterotoxin B, colloidal gold immunochromatographic detection test paper or a kit containing the antibody, and a preparation method and application thereof. The detection test paper or kit is convenient to produce, stable in effect, free of non-specific reaction, low in coating amountand beneficial to mass production. The detection method of the staphylococcus aureus enterotoxin B provided by the invention has the advantages of rapidness, sensitivity, simplicity, convenience, specificity and the like, and has wide application in food safety detection and monitoring.
Owner:北京弘进久安生物科技有限公司
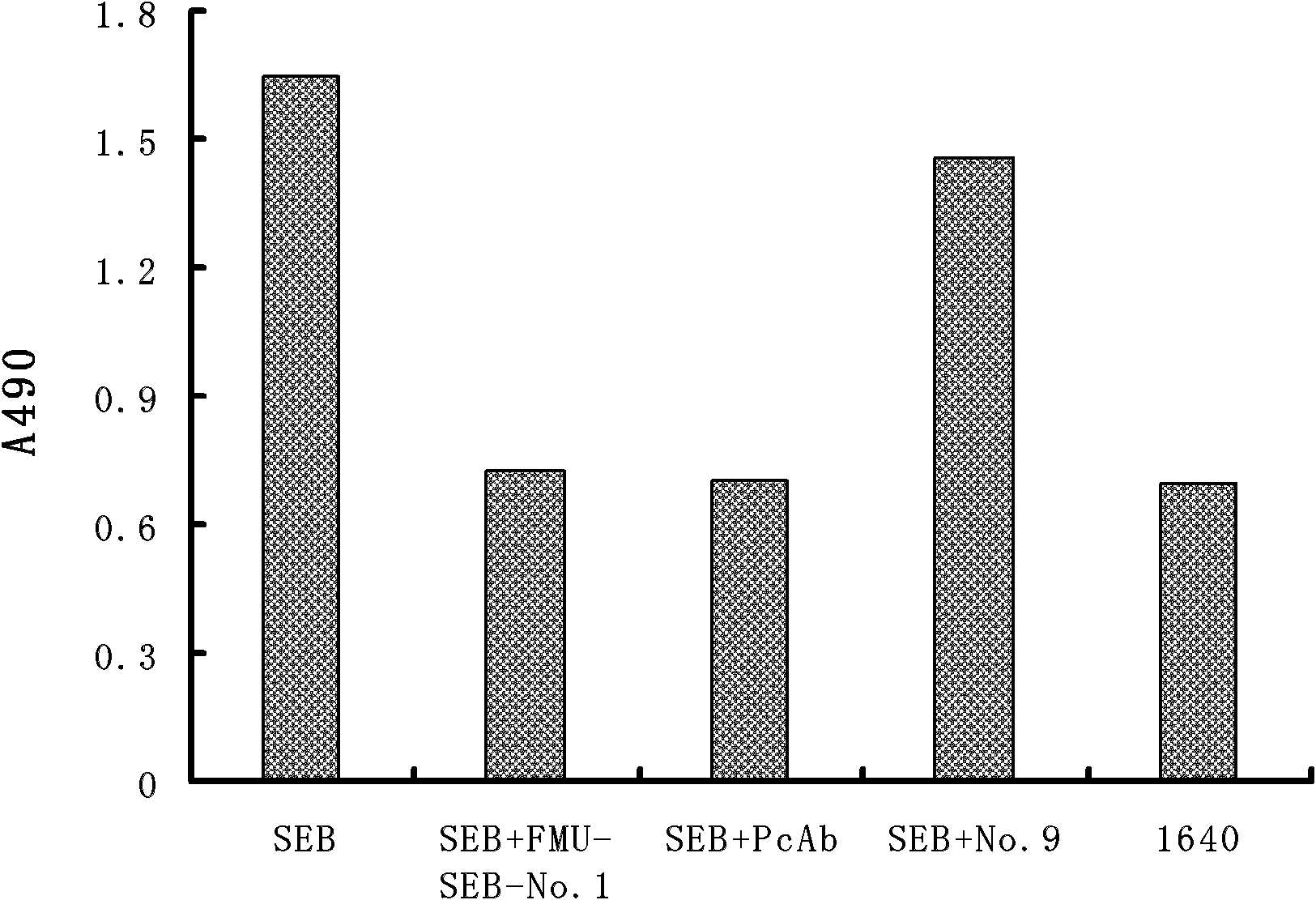
Light chain and heavy chain variable regions of anti-SEB (Staphylococcal Enterotoxin B) monoclonal antibody FMU-SEB-No.1 with high neutralizing activity
ActiveCN101955533AHigh affinityStrong neutralization abilityImmunoglobulins against bacteriaMaterial analysisBALB/cGenetic engineering
The invention discloses light chain and heavy chain variable regions of an anti-SEB (Staphylococcal Enterotoxin B) monoclonal antibody FMU-SEB-No.1 with a high neutralizing activity. The amino acid sequence of an FMU-SEB-No.1 mAb light chain variable region is shown in SEQ.ID.NO.1, and the amino acid sequence of an FMU-SEB-No.1 mAb heavy chain variable region is shown in SEQ.ID.NO.2. A method for the invention comprises the following steps of: preparing a group of mouse anti-SEB mAbs by using a natural SEB immune Balb / c mouse, screening hybridoma cell lines which can stably secrete high-affinity anti-SEB mAb; and preparing ascitic fluid to obtain a high-affinity anti-SEB mAb FMU-SEB-No.1. The invention is beneficial to confirming the uniqueness of a gene sequence and a corresponding protein sequence as well as a CDR sequence thereof, and providing support for researching and developing an anti-SEB mosaic or humanized genetic engineering antibody.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Non-toxic immune stimulating enterotoxin compositions
InactiveUS20050026272A1Low toxicityUseful biological propertyBacteriaDepsipeptidesBiological propertyStaphylococcal Enterotoxins
Pyrogenic toxins, such as staphylococcal enterotoxins, modified in the disulfide loop region are provided. The modified toxins retain useful biological properties but have substantially reduced toxicity compared to the corresponding unmodified native toxin. The native pyrogenic toxins are typically modified by deletions within the disulfide loop region to produce modified enterotoxins having 100-fold or greater decrease in toxicity.
Owner:IDAHO RESARCH FOUNDATION INC
Recombinant superantigen SEB mutant, preparation method and applications thereof
ActiveCN103965302AHigh activityImprove anti-tumor effectBacteriaPeptide/protein ingredientsAntigenAntineoplastic Immunotherapeutic
The present invention discloses a recombinant superantigen staphylococcal enterotoxin B (SEB) mutant, and a preparation method and applications thereof. According to the present invention, a SEB genome derived from natural staphylococcus aureus is extracted, and after the SEB genome is obtained, site-specific mutagenesis at toxicity-related amino acid sites is performed to obtain the SEB mutant. The SEB mutant has characteristics of both enhanced activity and weakened toxicity, and can be used as anti-tumor immune therapy drugs or immune system modulating drugs. The recombinant superantigen SEB mutant has good market prospects.
Owner:军事科学院军事医学研究院微生物流行病研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com