Protein suspending chip for quantitative detection of staphylococcal enterotoxin B and method for producing the same
A Staphylococcus intestinalis, suspension chip technology, applied in measuring devices, material analysis by observing the influence of chemical indicators, instruments, etc., can solve problems such as interference inspection results, and achieve high sensitivity and wide dynamic detection range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] 2. Preparation of samples to be tested
[0045] 1. Sample preparation
[0046] The target detection sample is staphylococcal enterotoxin B (SEB). Interfering samples or samples used as method-specific tests are other bacteria or other proteins other than the target detection object, including BONT, recombinant HIV P24 antigen, BSA, casein, tryptone, avian influenza virus HA protein, NH protein, etc. The above-mentioned samples to be analyzed were homogeneously dissolved in the sample diluent PB (0.01M, pH7.2) solution, and stored at 4°C. The stock solution concentration of SEB was 1mg / mL. Toxin and recombinant protein samples were diluted just before use. The concentration range of SEB was 10 pg / mL-5 μg / mL. In comparative experiments, the same samples were used for ELISA and suspension chip detection.
[0047] The bacteria to be analyzed were diluted into 10-fold different gradients with PB, and the staphylococcal enterotoxin was diluted into 4-fold different gradi...
Embodiment 1
[0052] Example 1, capture antibody-coated encoded microspheres
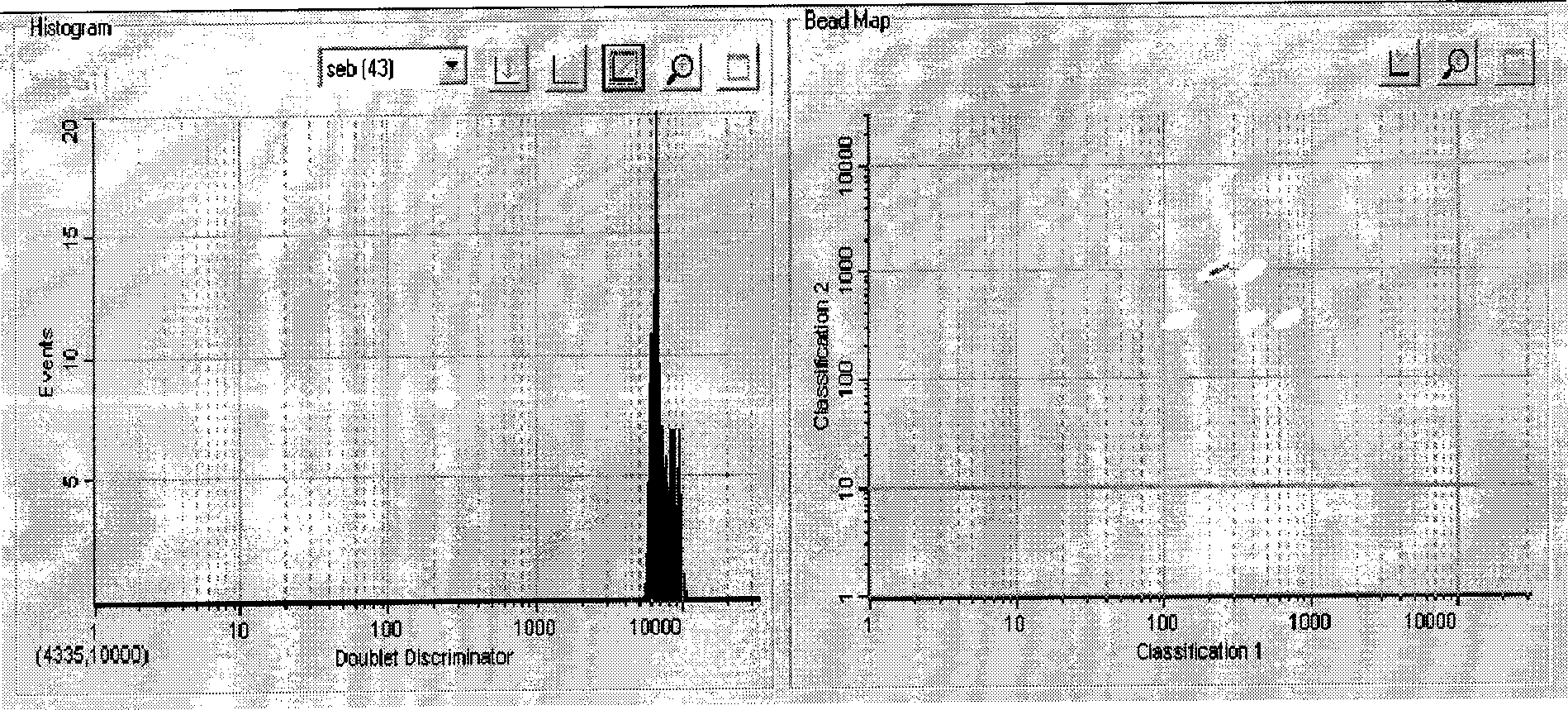
[0053] Rabbit anti-SEB was purified by n-octanoic acid-saturated ammonium sulfate purification. Optionally, one of the encoded microspheres (such as 043) is labeled with an SEB-capturing antibody (SEB antibody).
[0054] A. Activation of encoded microspheres
[0055] Take 100 μL of encoded microspheres into a 1.5 mL centrifuge tube, centrifuge at 14,000 g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere washing buffer to suspend, shake and sonicate, centrifuge at 14000g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere activation buffer, then add 10 μL of freshly prepared EDC (50 mg / mL), then add 10 μL of freshly prepared Sulfo-NHS (50 mg / mL), and shake at room temperature for 20 min. Add 150 μL of PBS (pH7.4), shake, centrifuge at 14000 g, carefully aspirate and discard the supernatant. Add 100 μL of PBS (pH 7.4) to suspend the encoded microspheres.
[0056...
Embodiment 2
[0060] Example 2, Biotin labeling of detection antibody
[0061] Antibodies to be labeled include rabbit anti-SEB antibody, goat anti-SEB antibody, and SEB monoclonal antibody. There are at least two options for detection antibodies labeled with biotin for each analyte. Prepare 10mM biotin solution and 2mg / mL antibody solution to be labeled respectively, add the calculated volume of biotin to the antibody solution to be labeled, shake at room temperature for 30min (or 2 hours on ice), and pass through the column for desalting Aliquot and store at -20°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com