Light chain and heavy chain variable regions of anti-SEB (Staphylococcal Enterotoxin B) monoclonal antibody FMU-SEB-No.1 with high neutralizing activity
A technology of monoclonal antibody, fmu-seb-no.1, which is applied in the field of biomedicine to achieve strong neutralizing ability and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
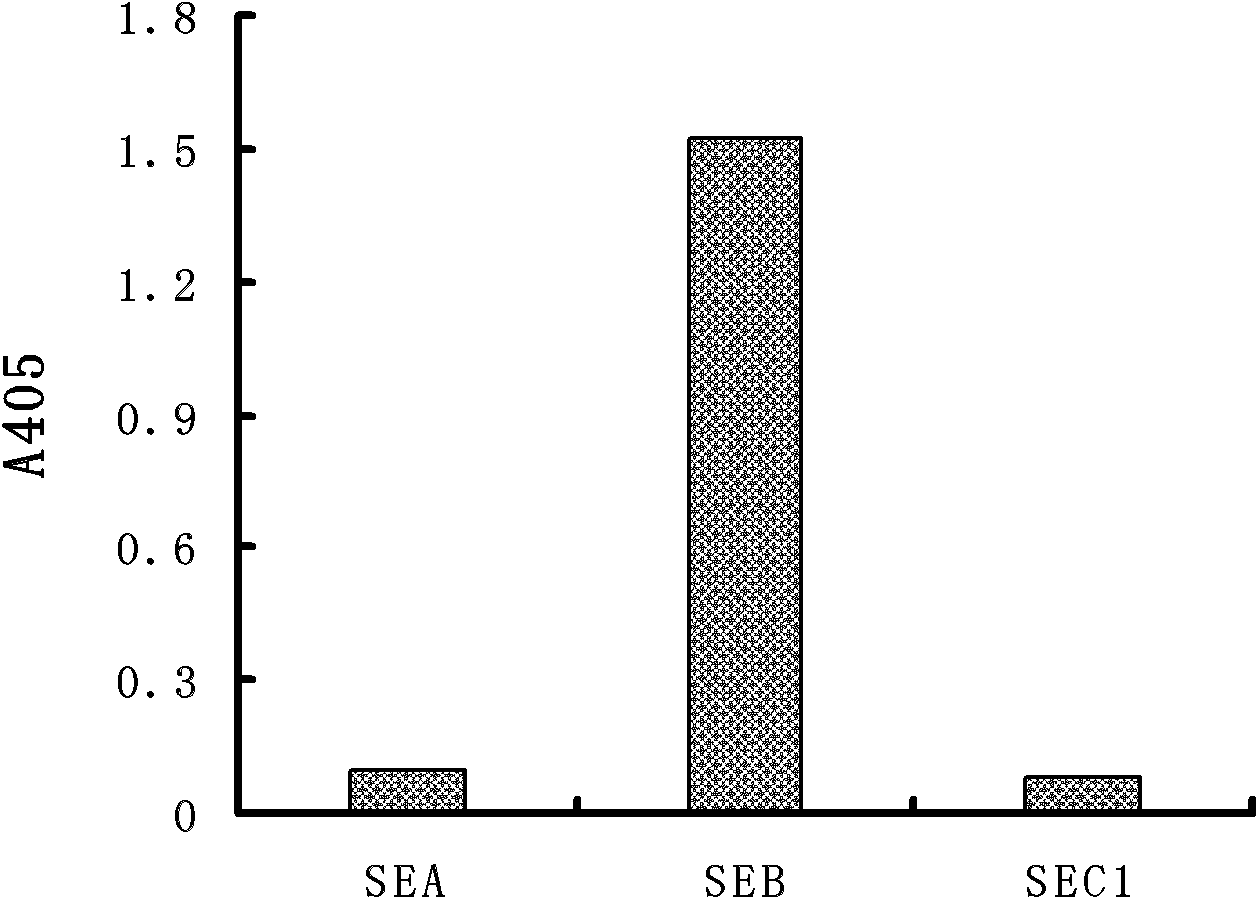
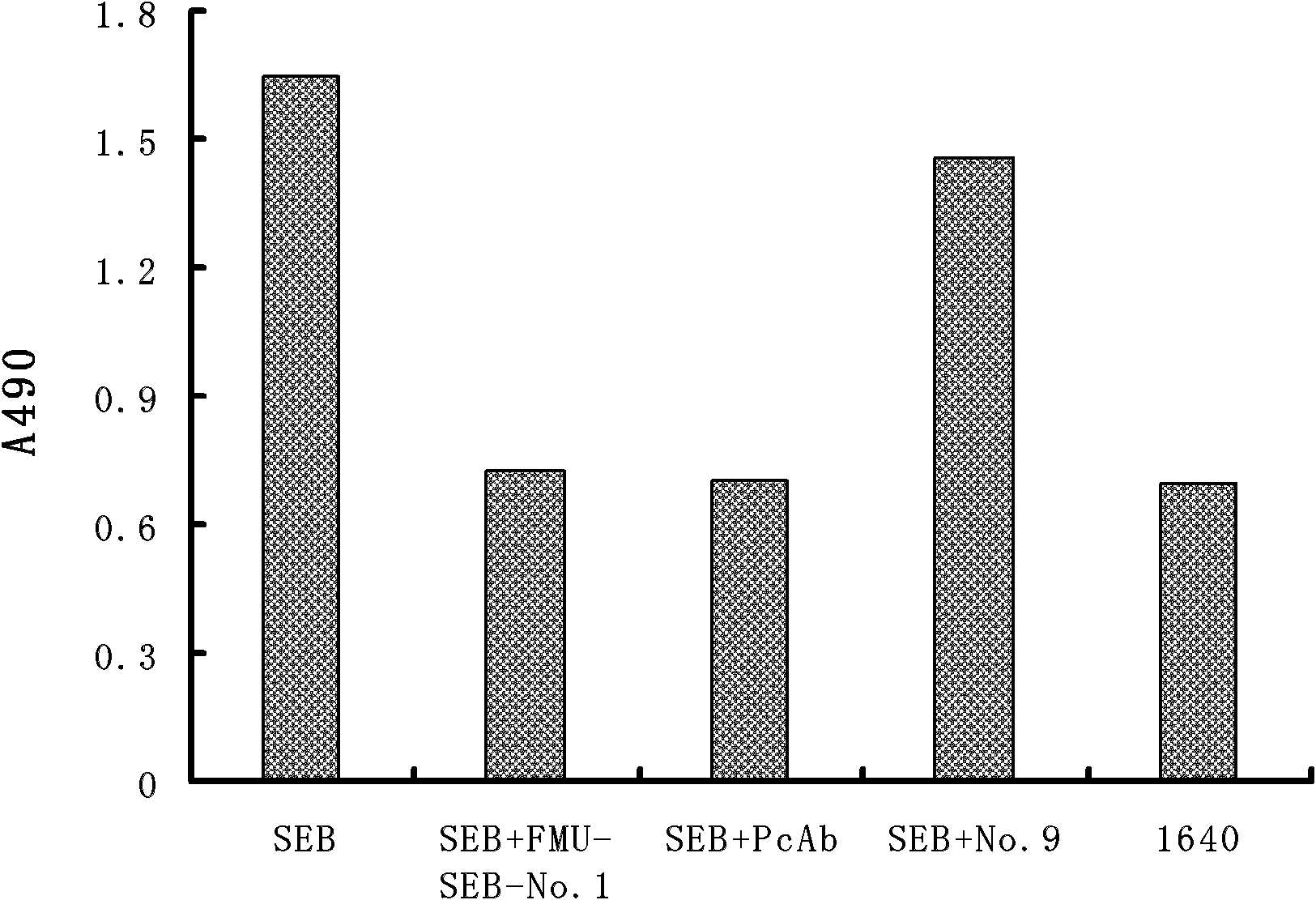
[0030] The present invention immunizes Balb / c mice with natural SEB, prepares a group of mouse anti-SEB monoclonal antibodies, screens out the FMU-SEB-No.1 mAb hybridoma cell line that can stably secrete high-affinity anti-SEB, and prepares Obtain high-affinity anti-SEB mAb from ascites; and confirm the uniqueness of the gene sequence and corresponding protein sequence and its CDR sequence; provide support for anti-SEB chimeric or humanized genetic engineering antibodies. The present invention will be described in detail below in conjunction with the preparation method of specific monoclonal antibodies, antibody activity detection, and sequence detection and uniqueness determination, which is an explanation rather than a limitation of the present invention. The present invention is specifically implemented according to the following steps:
[0031] 1. Preparation of mouse anti-SEB high affinity antibody FMU-SEB-No.1 mAb
[0032] 1.1 Preparation and purification of monoclonal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com