AlphaLISA detection kit for type B staphylococcal enterotoxin
A Staphylococcus aureus enterotoxin and detection kit technology, applied in the biological field, can solve the problems of goat antibody troubles, complex preparation of Fab segments, false positive immunological results, etc., and achieve rapid detection methods, good application prospects, and reduced impact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
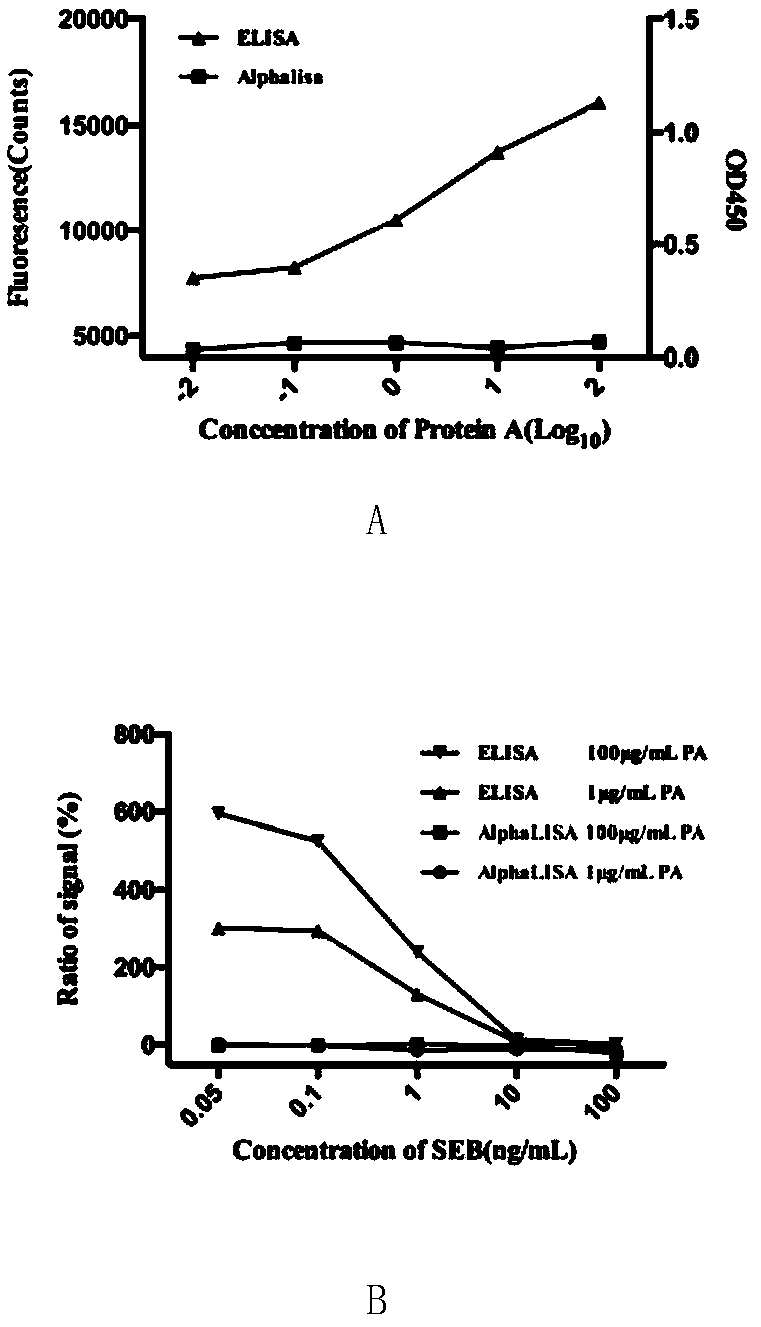
[0045] Embodiment 1, the impact of protein A on the detection of SEB by ELISA and AlphaLISA
[0046] 1. Protein A is used as the sample to be tested
[0047] 1. ELISA detection
[0048] (1) Add 100 μL of SEB capture antibody at a concentration of 1 μg / mL to a 96-well plate, and coat at 4°C overnight.
[0049] (2) Discard the coating solution, and block with 200 μL of PBST (PBS+10% Tween) containing 10% FBS.
[0050] (3) Discard the blocking solution, and add protein A solutions with concentrations of 0.01 μg / mL, 0.1 μg / mL, 1 μg / mL, 10 μg / mL and 100 μg / mL respectively (protein A was purchased from Sigma Company, product number is P6031; protein A The solvent of the solution was PBST) 100 μL, incubated at 37°C for 30 minutes, discarded the supernatant, and washed 3 times with PBST.
[0051] (4) Add 1:2000 biotin-labeled SEB detection antibody, incubate at 37°C for 30 minutes, discard the supernatant, and wash 3 times with PBST.
[0052] (5) Add 1:8000 diluted HRP-labeled str...
Embodiment 2
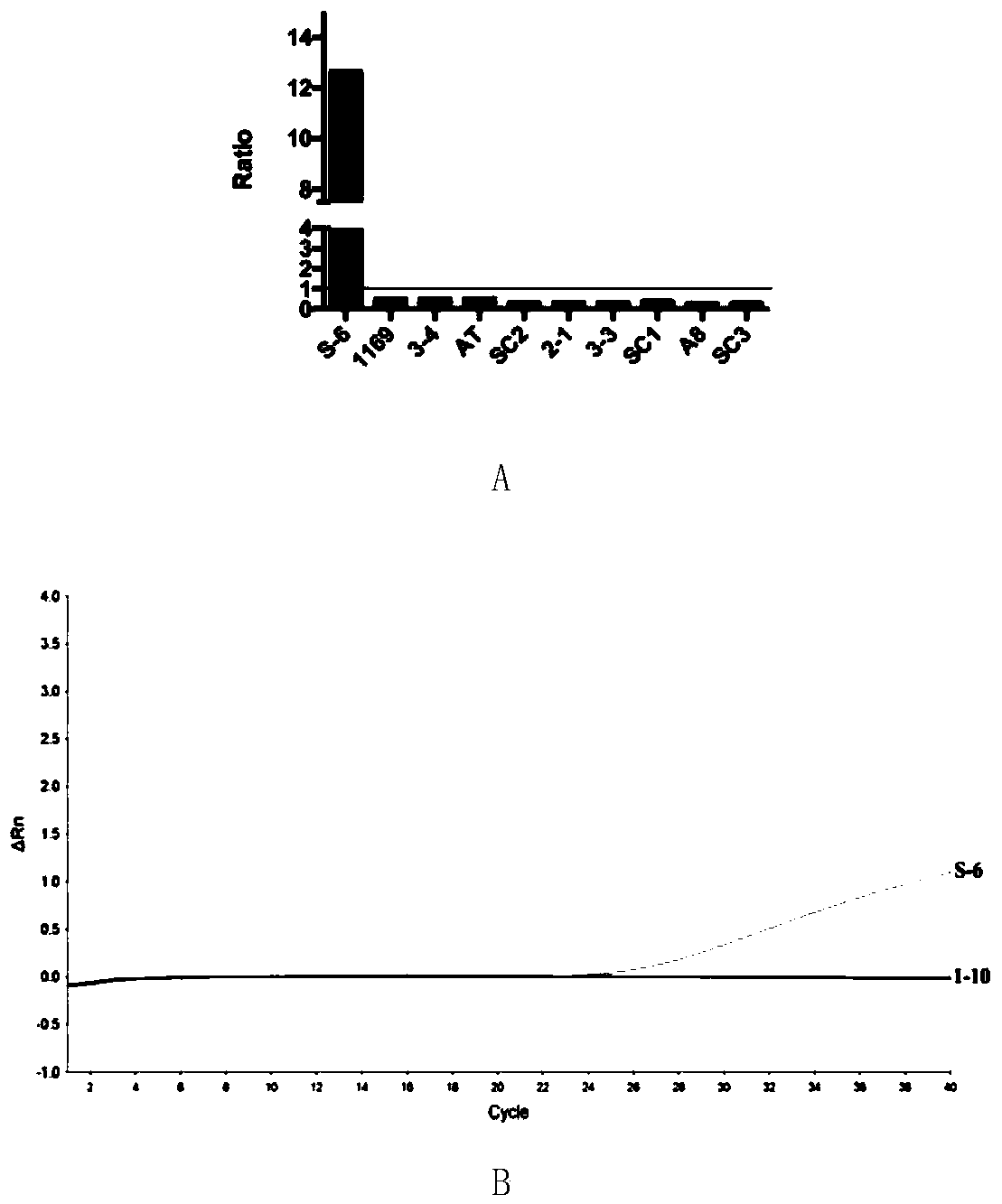
[0066] Embodiment 2, ELISA and AlphaLISA to the detection of SEB in S. aureus culture supernatant
[0067] 1. Detection of SEB-producing strains by qPCR and R-Biopharm RIDASCREEN
[0068] Select 10 strains of Staphylococcus aureus (S-6, 1169, 3-4, AT, SC2, 2-1, 3-3, SC1, A8 and SC3), and use RT-qPCR and R-Biopharm RIDASCREEN to detect 10 strains respectively SEB-producing and non-SEB-producing strains in Staphylococcus aureus.
[0069] 1. Detection of SEB-producing strains by R-Biopharm RIDASCREEN
[0070] Ten strains of Staphylococcus aureus were cultured in the broth medium for 24 hours, centrifuged at 12000rpm for 10min, the supernatant was taken, sterilized by filtration with a 0.22μm filter membrane, and SEB in the supernatant was detected by SEB enzyme-linked method. The SEB enzyme-linked method was operated according to the instructions of R-Biopharm RIDASCREEN (R-Biopharm AG, Darmstadt, Germany).
[0071] R-Biopharm RIDASCREEN test results are as follows: figure 2...
Embodiment 3
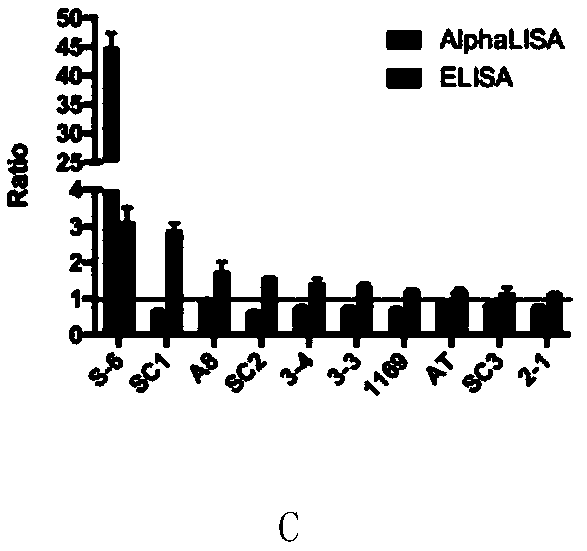
[0088] Example 3, Sensitivity and specificity detection of the AlphaLISA detection kit of Staphylococcus aureus type B enterotoxin
[0089] 1. Sensitivity detection
[0090] SEB solutions of different concentrations were used as samples to be tested, and SEB was detected according to the AlphaLISA detection method in Step 1-2 of Example 1. The SEB solution concentrations of the 4 parameter curves are 0.03ng / mL, 0.05ng / mL, 0.10ng / mL, 0.19ng / mL, 0.39ng / mL, 0.78ng / mL, 1.56ng / mL, 3.13ng / mL, 6.25 ng / mL, 12.5ng / mL, 25ng / mL, 50ng / mL, 100ng / mL, 200ng / mL and 300ng / mL; linear curve SEB solution concentration is 0.03ng / mL, 0.05ng / mL, 0.10ng / mL, 0.19ng / mL, 0.39ng / mL, 0.78ng / mL, 1.56ng / mL, 3.13ng / mL, 6.25ng / mL, 12.5ng / mL, and 25ng / mL.
[0091] The result is as image 3 shown. The results showed that the established AlphaLISA detection system had high sensitivity to SEB detection, the detection limit could reach 25pg / mL, and the linear range was from 25pg / mL to 25ng / mL.
[0092] 2. Spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com