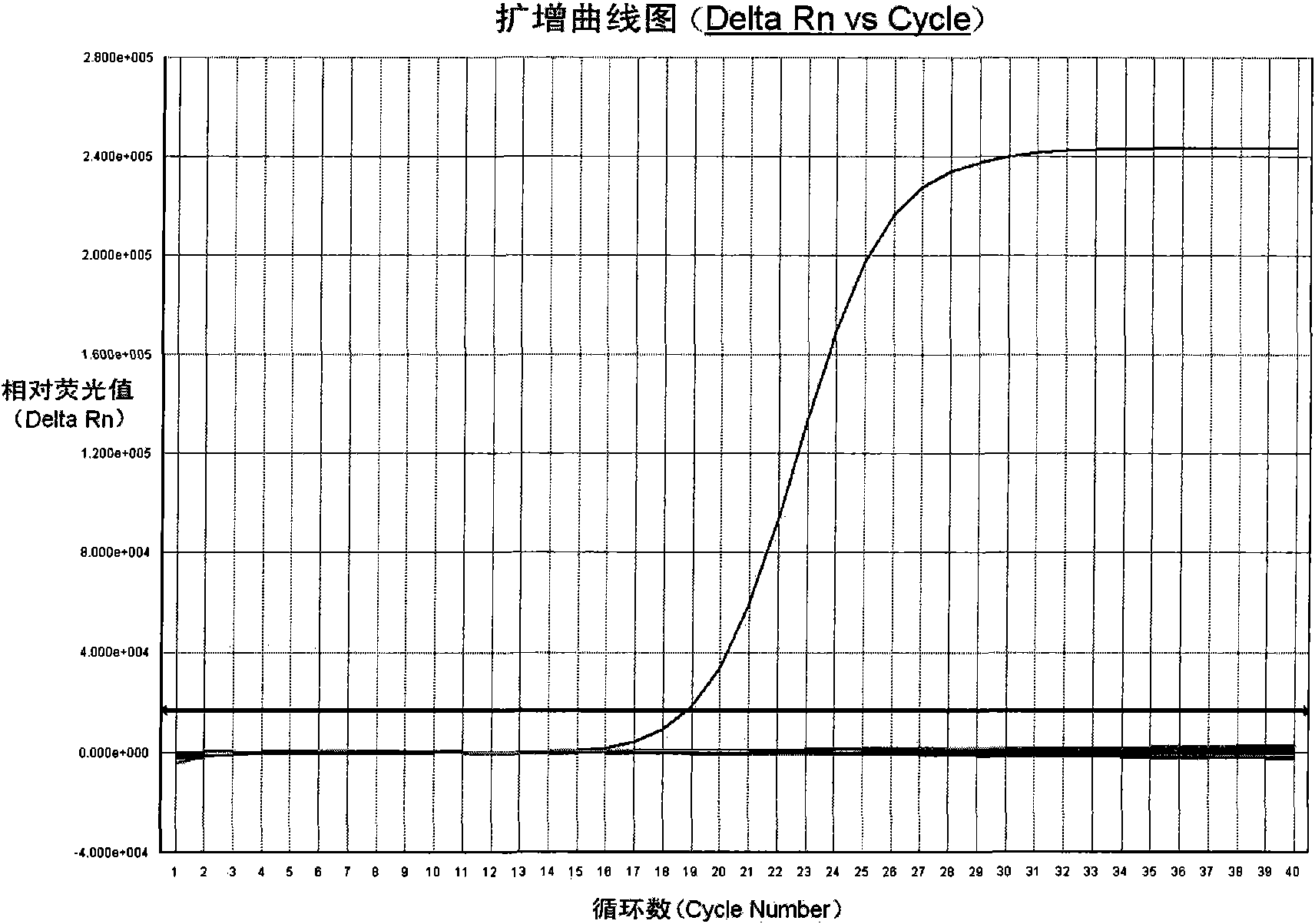
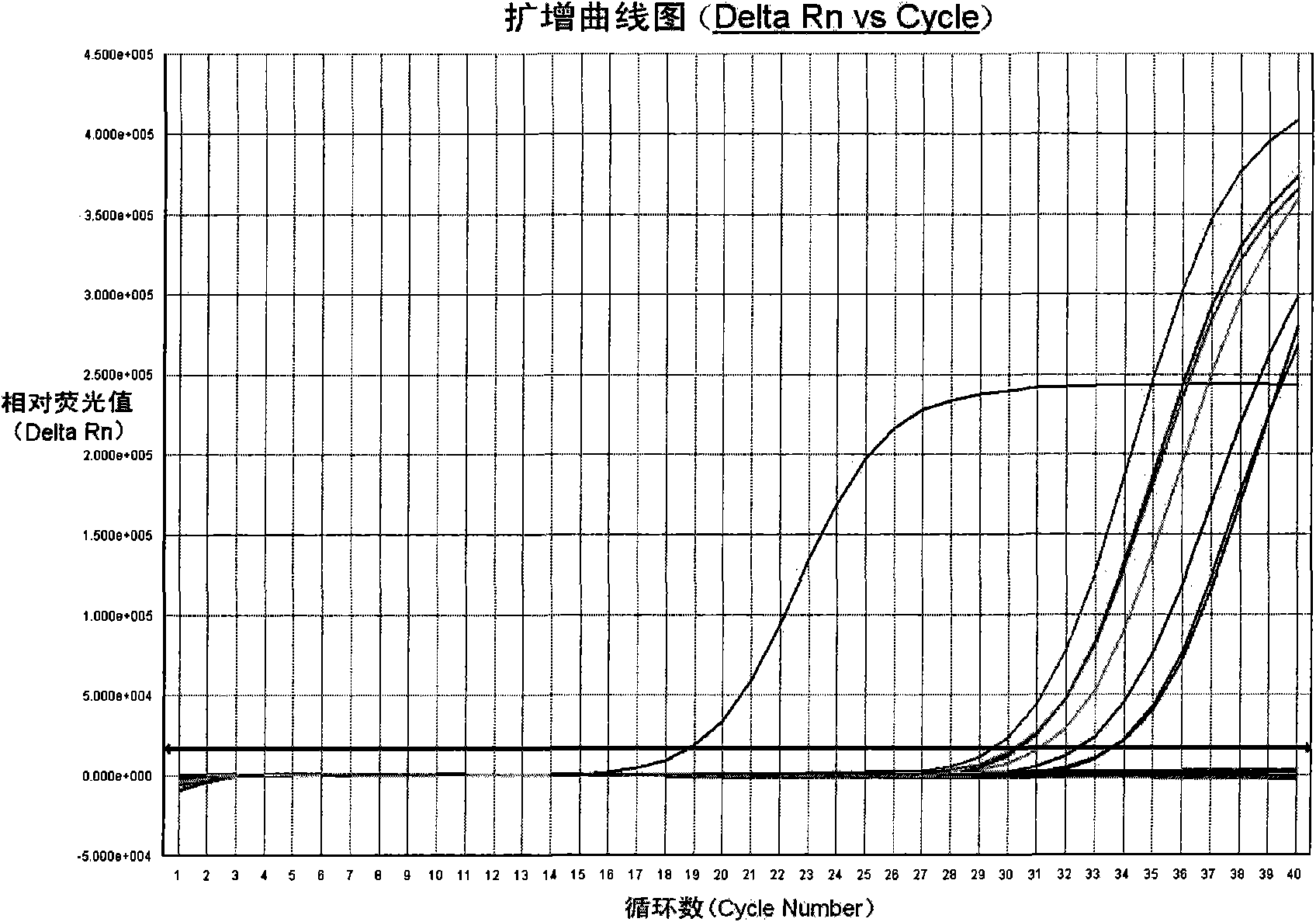
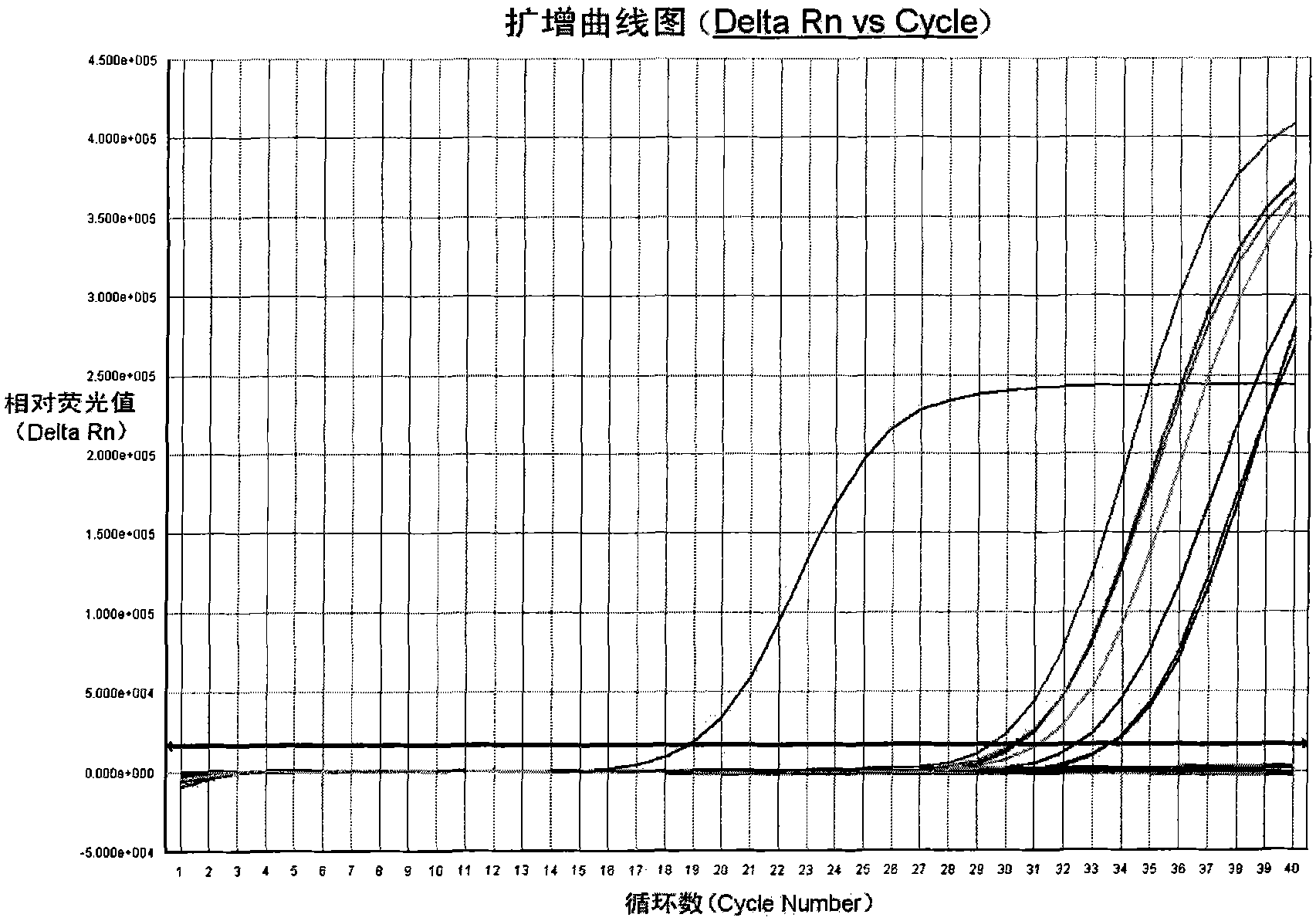
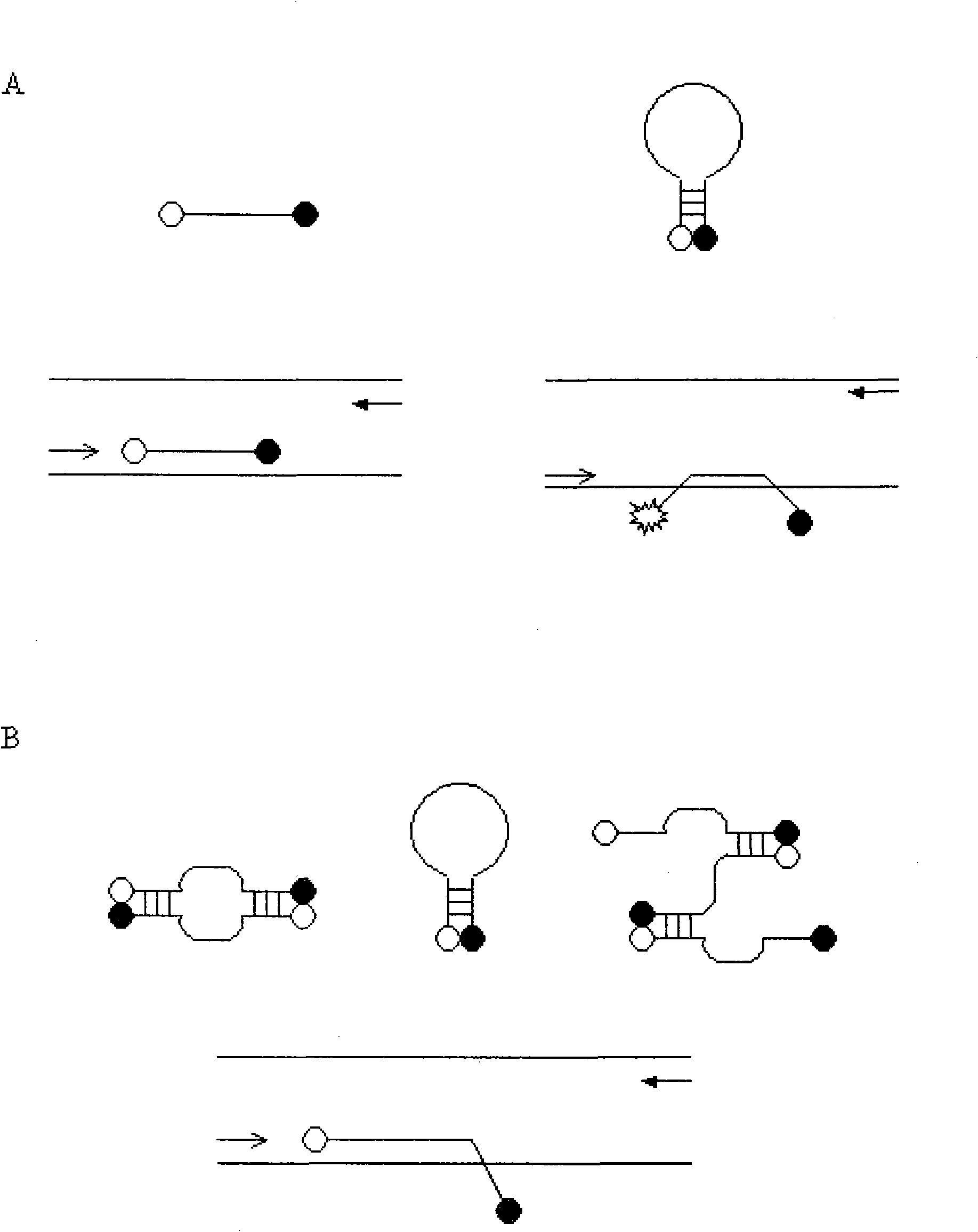
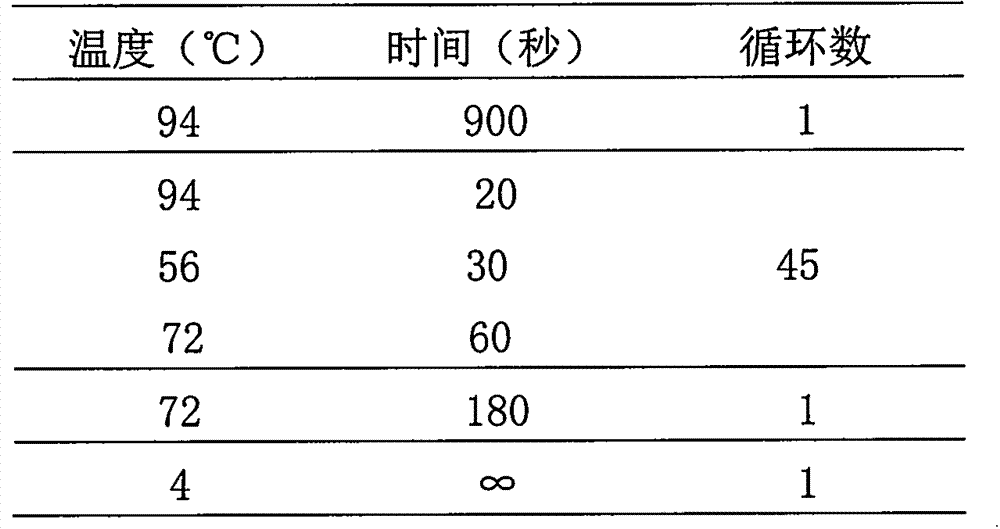
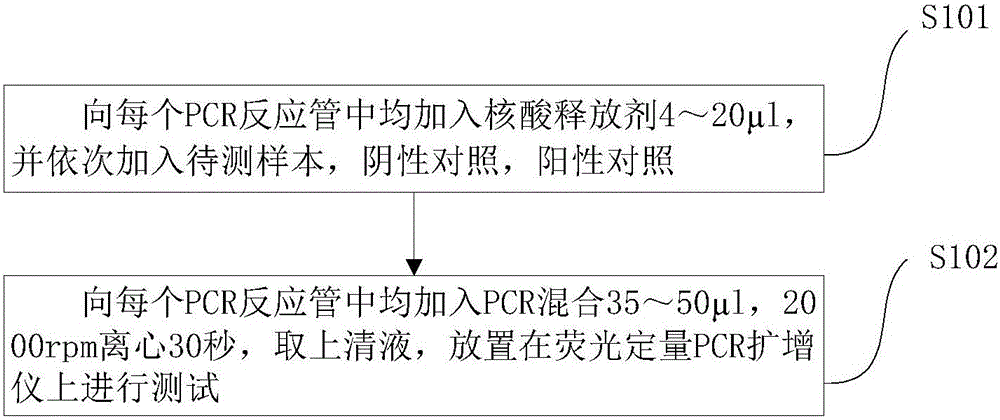
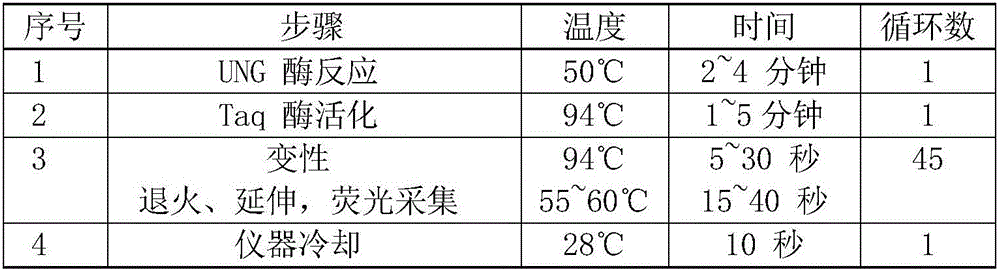
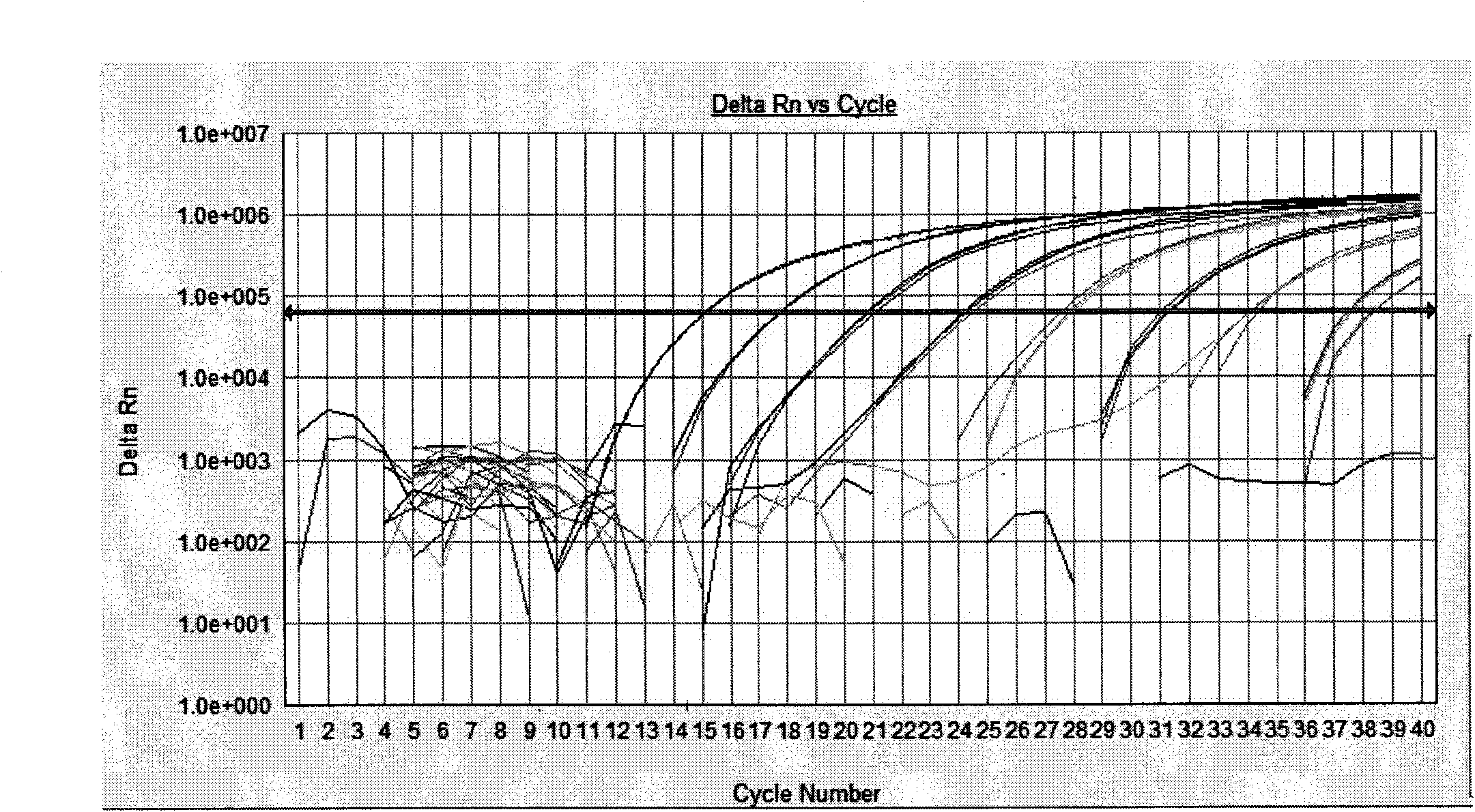
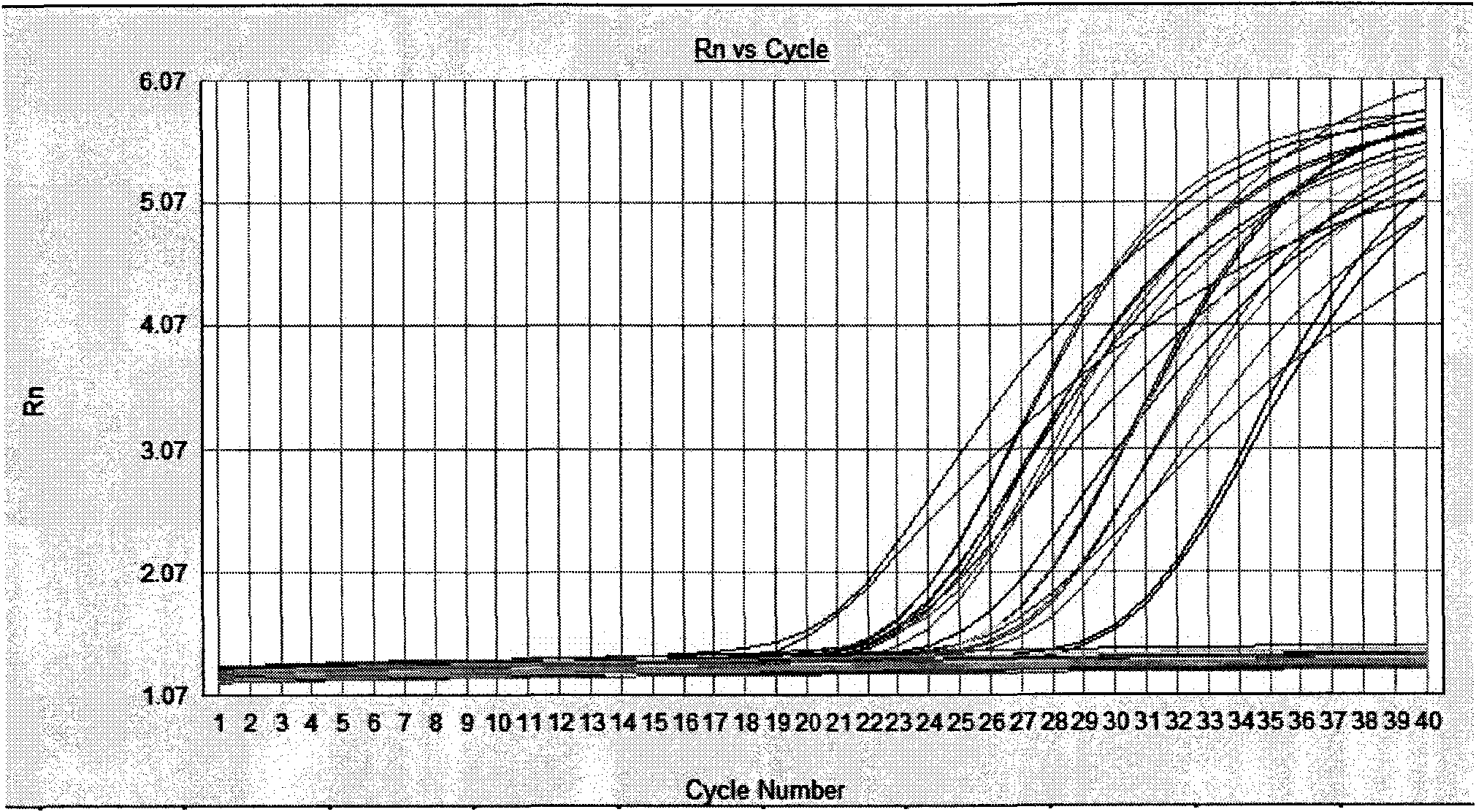
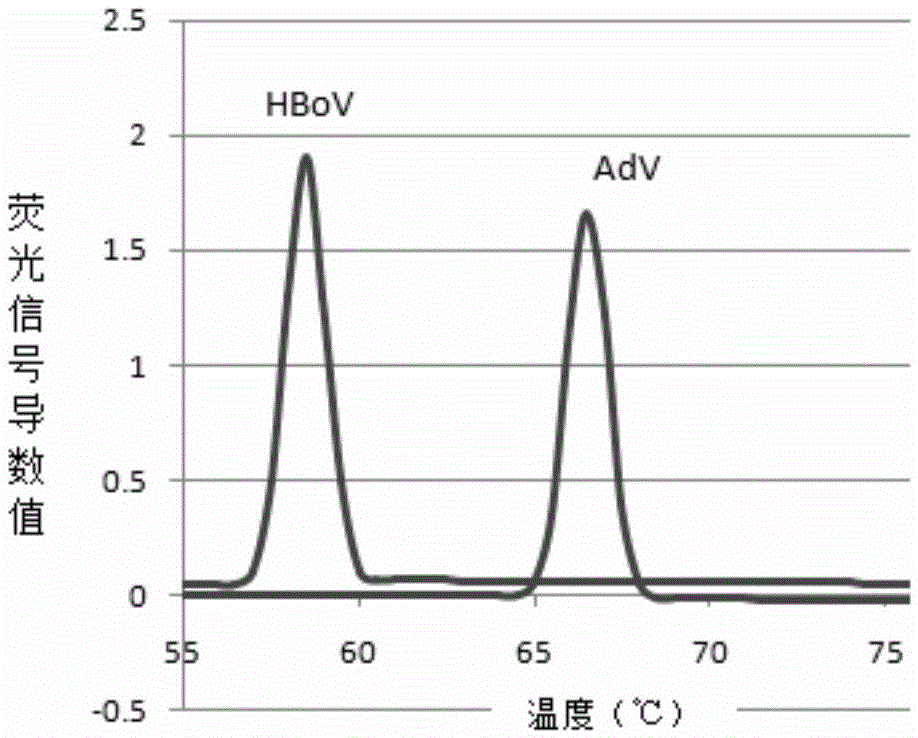
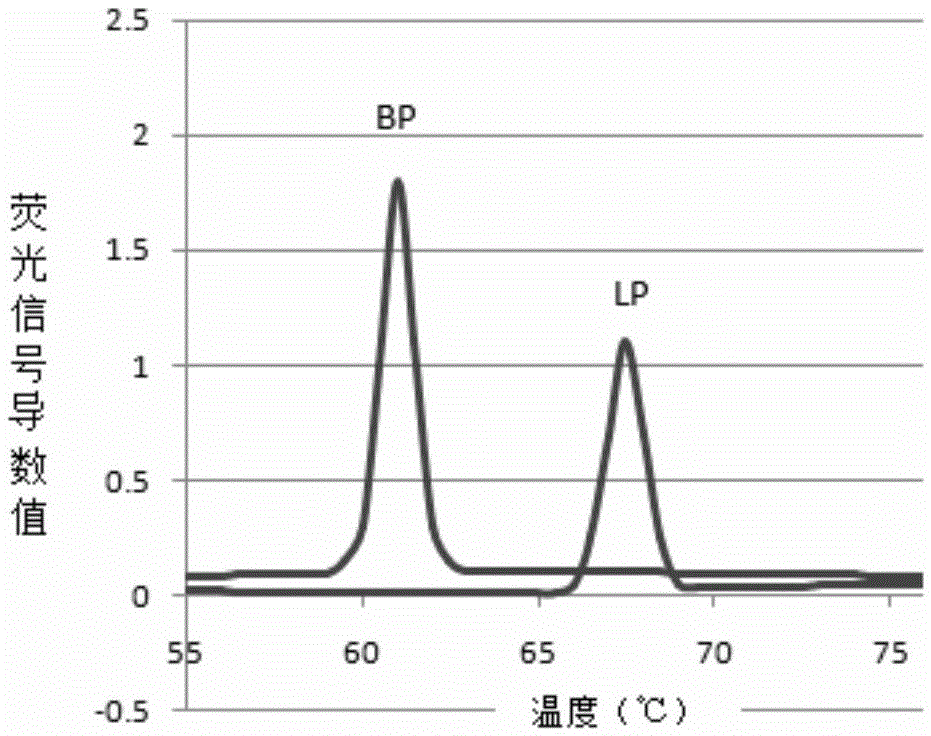
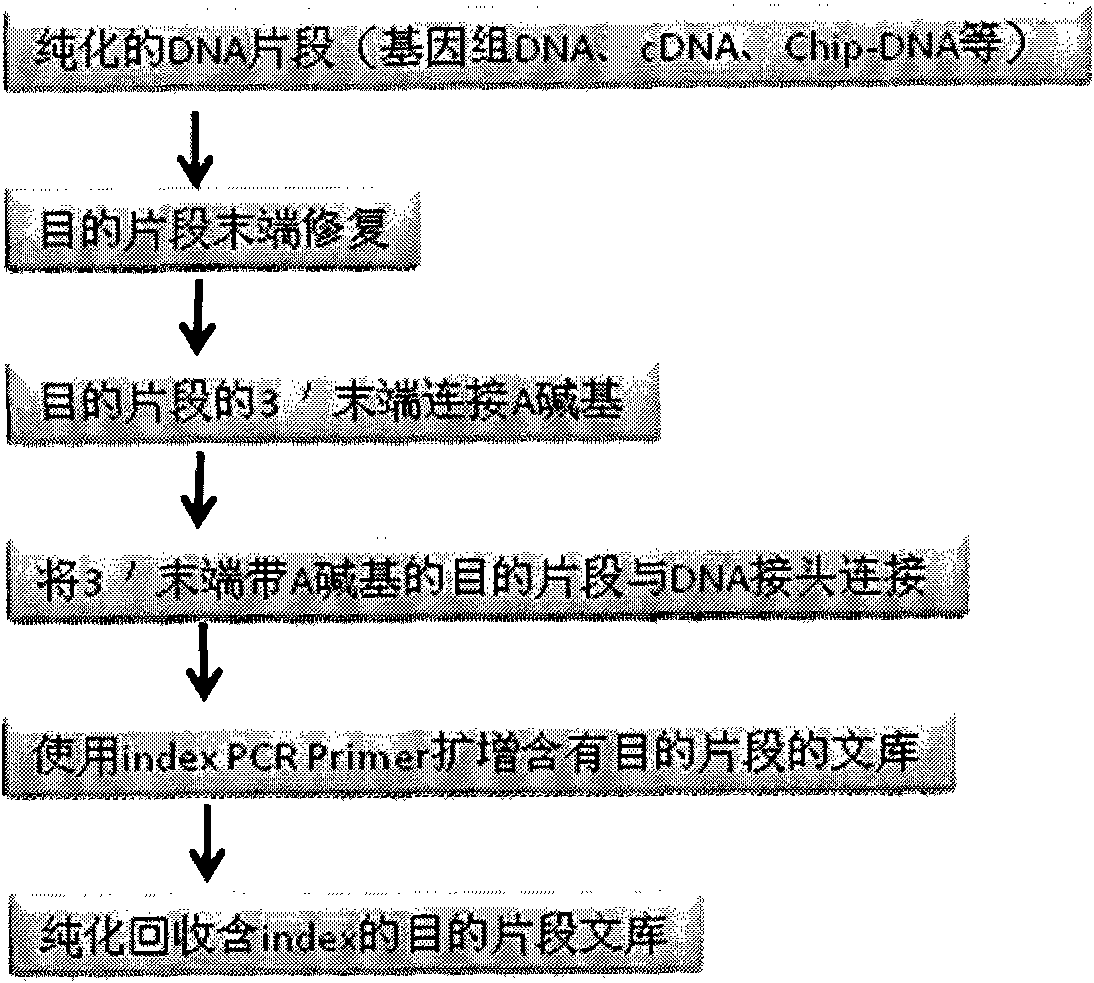Patents
Literature
131 results about "Inverse polymerase chain reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inverse polymerase chain reaction (Inverse PCR) is a variant of the polymerase chain reaction that is used to amplify DNA with only one known sequence. One limitation of conventional PCR is that it requires primers complementary to both termini of the target DNA, but this method allows PCR to be carried out even if only one sequence is available from which primers may be designed.
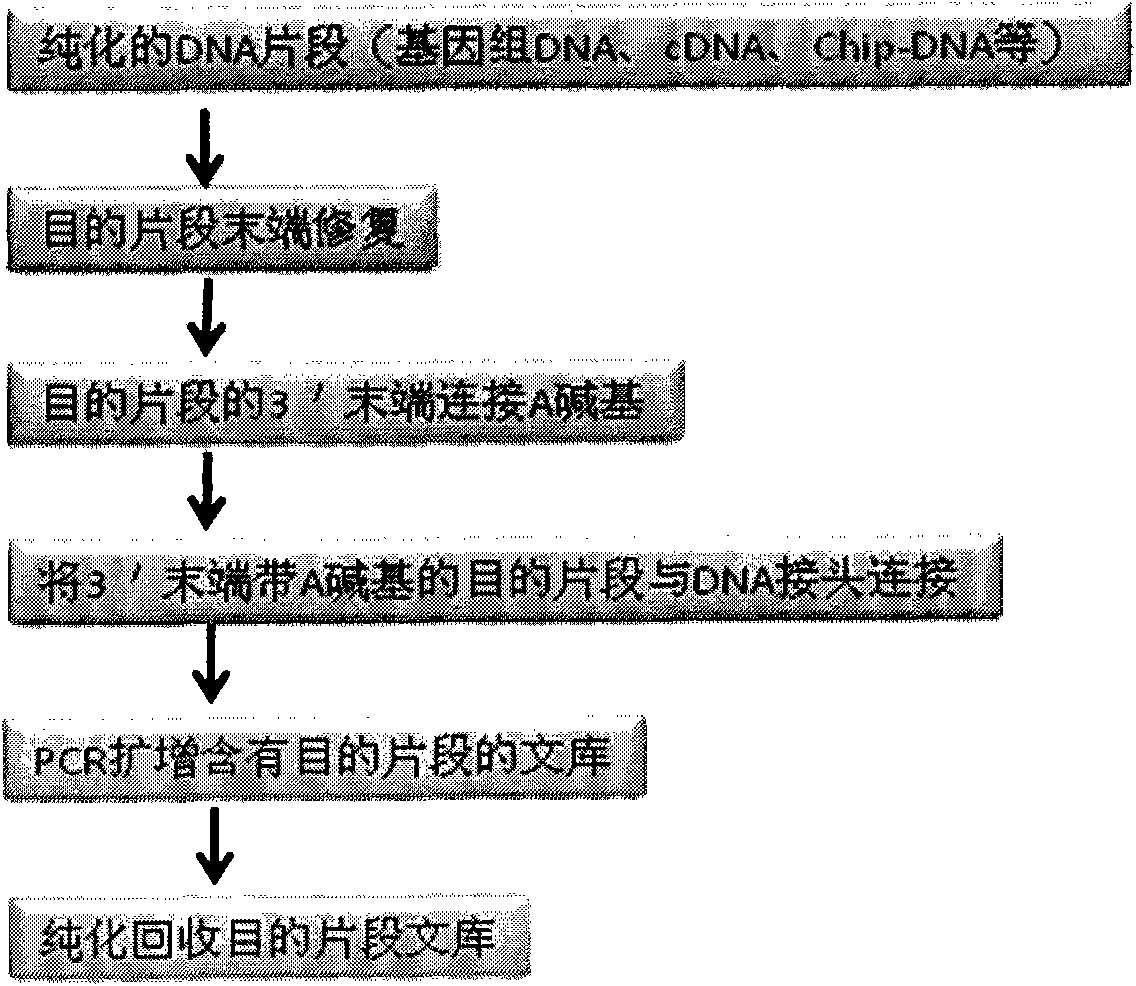
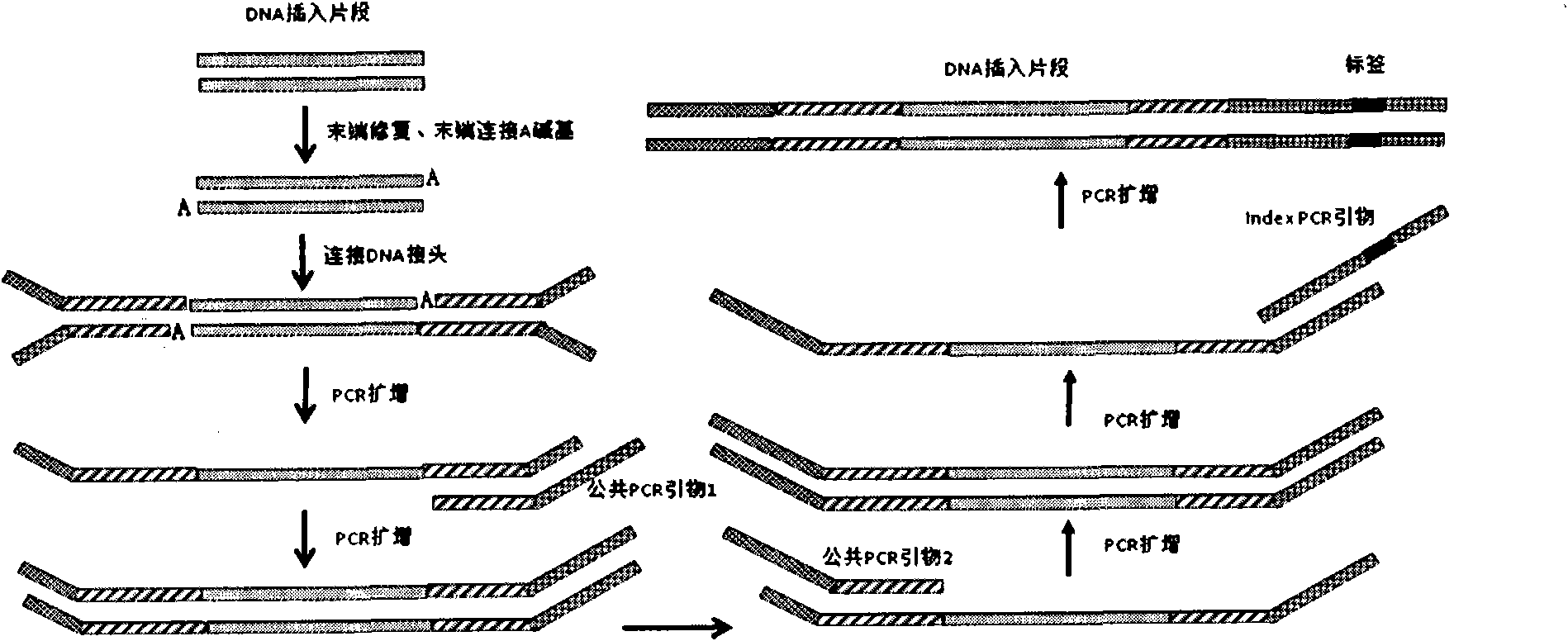
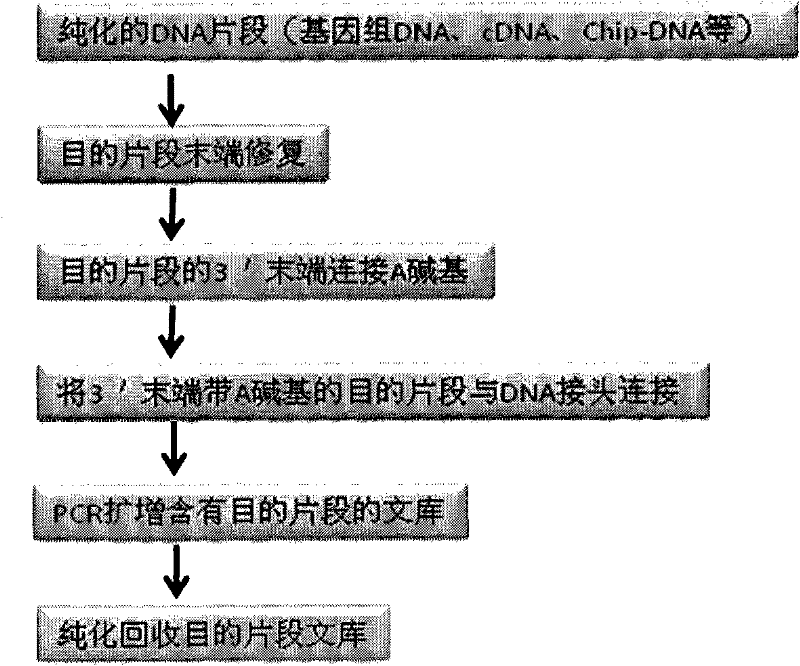
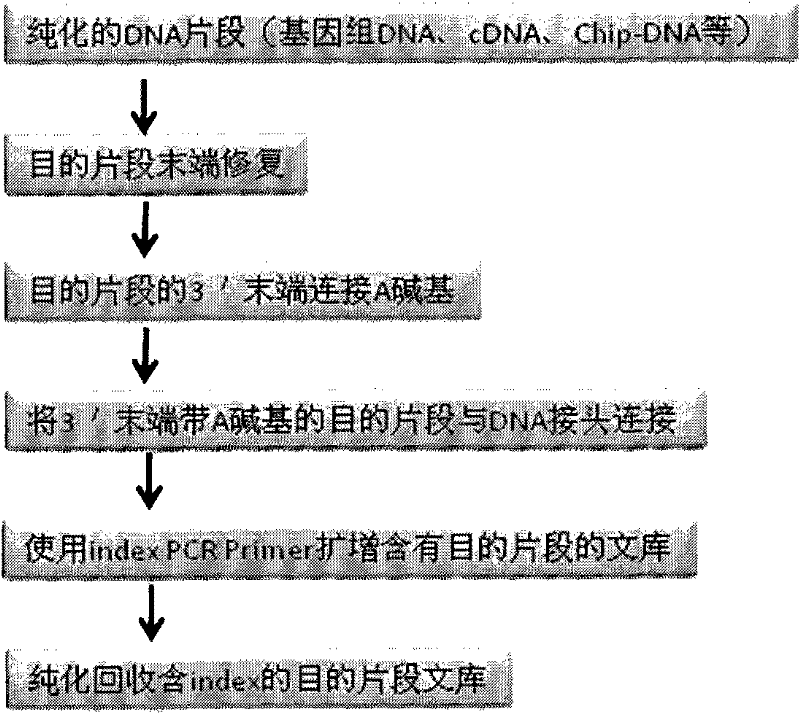
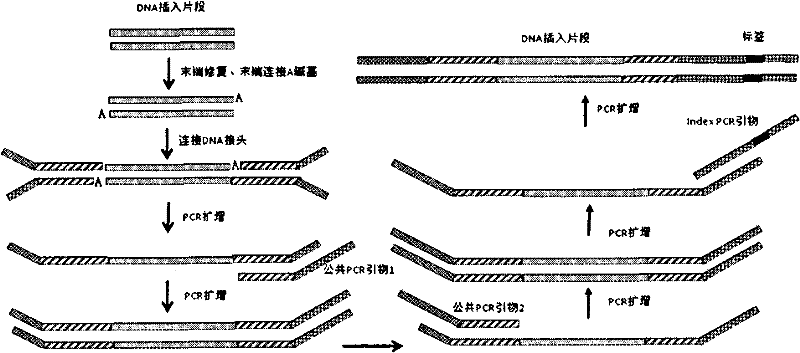
Joint connection-based deoxyribonucleic acid (DNA) polymerase chain reaction (PCR)-free tag library construction method
The invention designs 161 unique tag sequences with the lengths of 8 bp. Tags are embedded into a deoxyribonucleic acid (DNA) joint so as to form a DNA polymerase chain reaction (PCR)-free tag joint. The invention also provides a DNA tag library which introduces the tag sequence by connecting the DNA PCR-free tag joint without a PCR and is applied to solexa DNA sequencing. After a library construction method is optimized, the DNA tag library is constructed without the PCR.
Owner:BGI TECH SOLUTIONS
DNA(deoxyribonucleic acid) index library building method based on PCR (polymerase chain reaction)
ActiveCN102409049AImprove production efficiencyHigh sequencing throughputNucleotide librariesMicrobiological testing/measurementBiotechnologyInverse polymerase chain reaction
The invention designs 161 unique index sequences with a length of 8bp, and the indexes are embedded into DNA PCR (deoxyribonucleic acid polymerase chain reaction) primers to form DNA PCR index primers, thus being capable of importing the index sequences through PCR. The invention successfully establishes a method for building a DNA index library, and the method is applied to solexa DNA sequencing.
Owner:BGI TECH SOLUTIONS
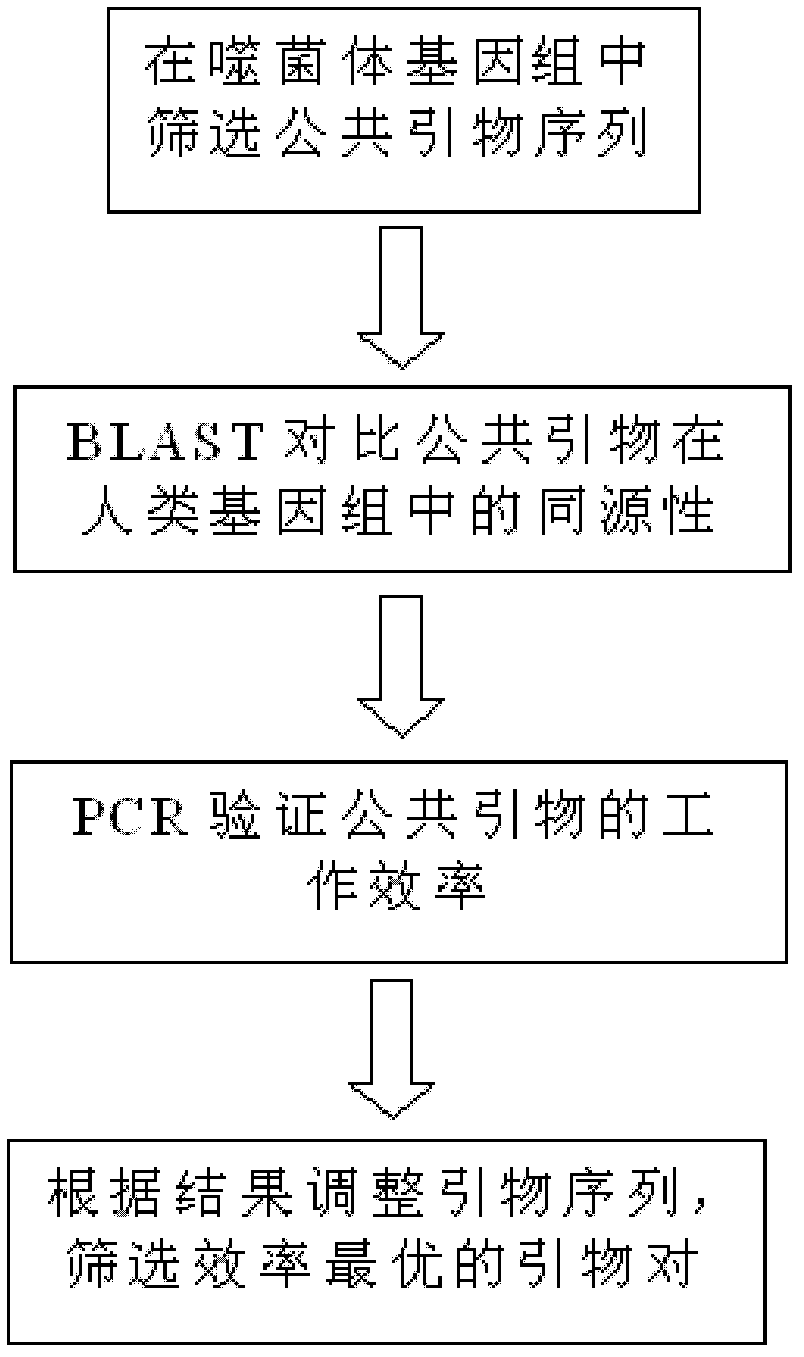
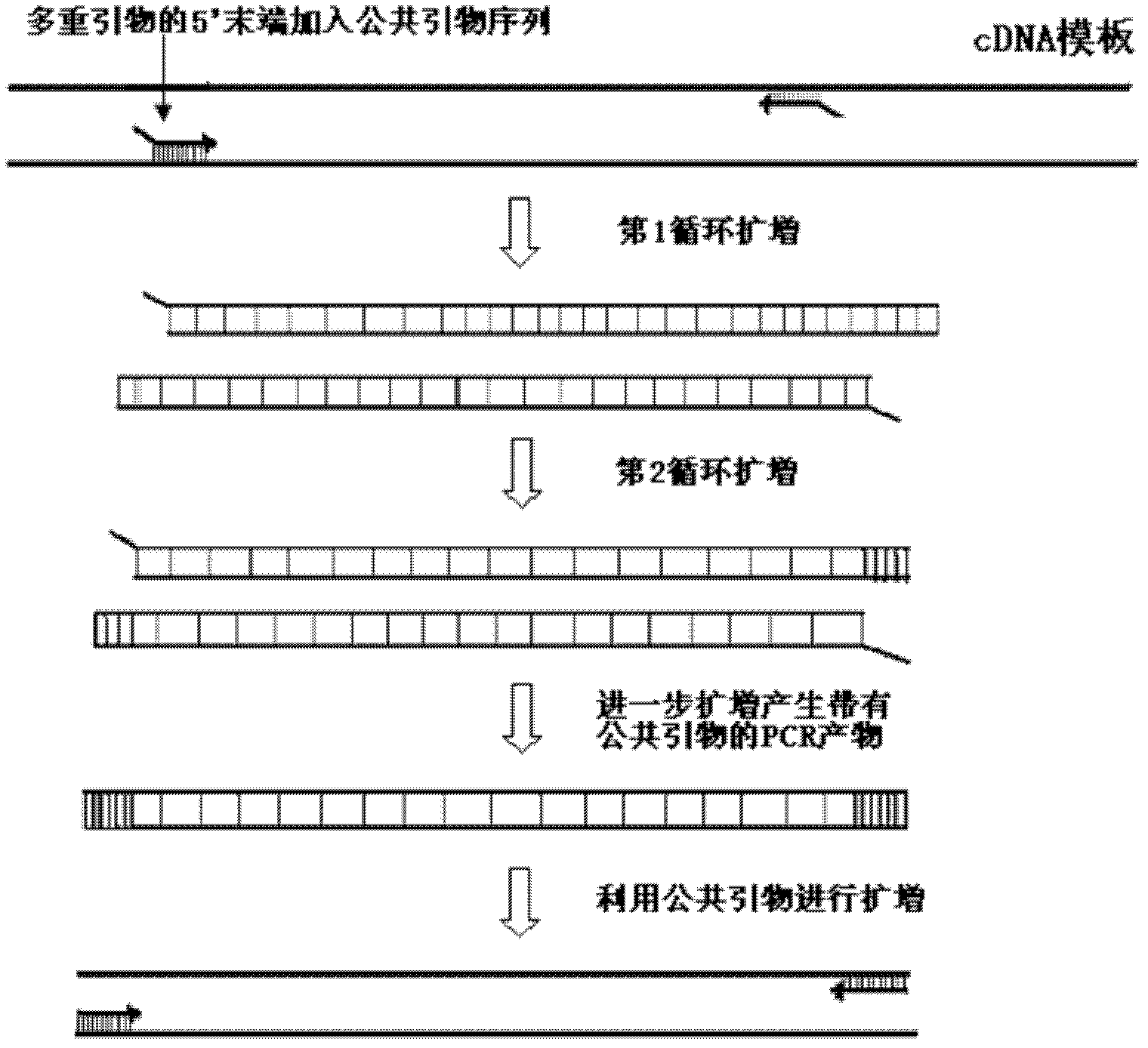
Multiplex polymerase chain reaction (PCR) amplification method and reagent kit
ActiveCN102352411AReduce the chance of interactionSolve mutual interference, a technical bottleneck in multiplex PCRMicrobiological testing/measurementInverse polymerase chain reactionBottle neck
The invention discloses a multiplex polymerase chain reaction (PCR) amplification method and a reagent kit for multiplex PCR amplification. The multiplex PCR amplification method comprises the step of designing public primers and multiplex primers with the public primers. The public primer design needs to follow two rules that: (1) under any optimized amplification conditions, no specific products are produced during the amplification of the public primers and templates to be measured; and (2) when the specific products are obtained through the amplification of the multiplex primers with the public primers, the public primers have the capability of amplifying the specific products, and then, a multiplex PCR system is configured for amplification. Because of the introduction of the public primers, the sequence specificity primer pair concentration in the reaction system is reduced by the level of more than four pairs, the possibility of the multiplex primers in the low concentration togenerate the mutual action in the same reaction system is greatly reduced, the problem of the technical bottle neck in the multiplex PCR of mutual interference of different primer pairs can be solved, and the precondition can also be created for adding new primer pairs and increasing the detection gene number.
Owner:北京凡知医学科技有限公司
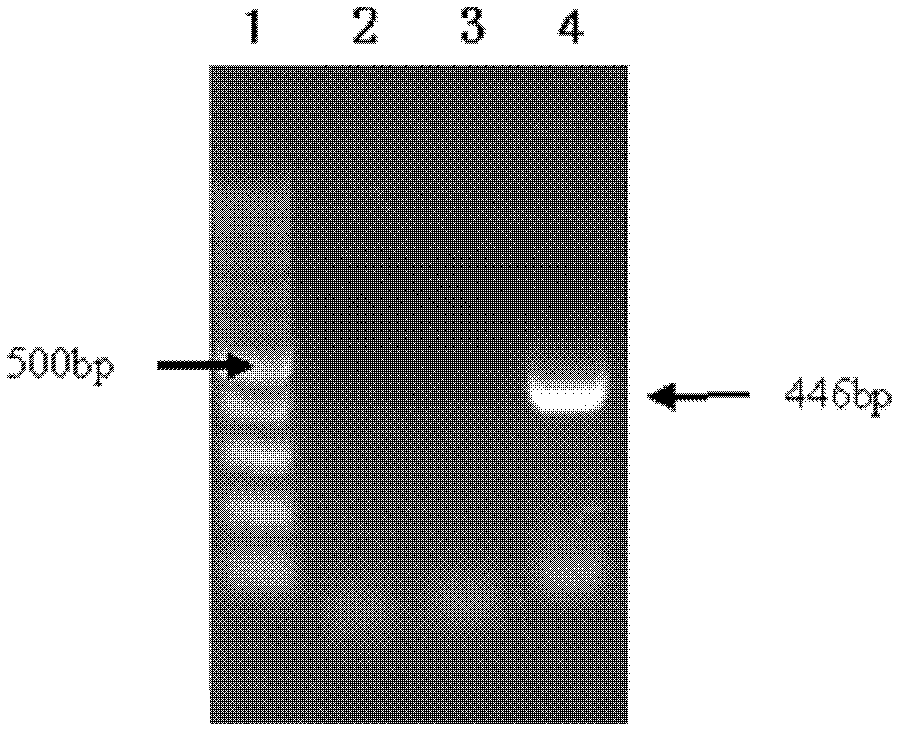
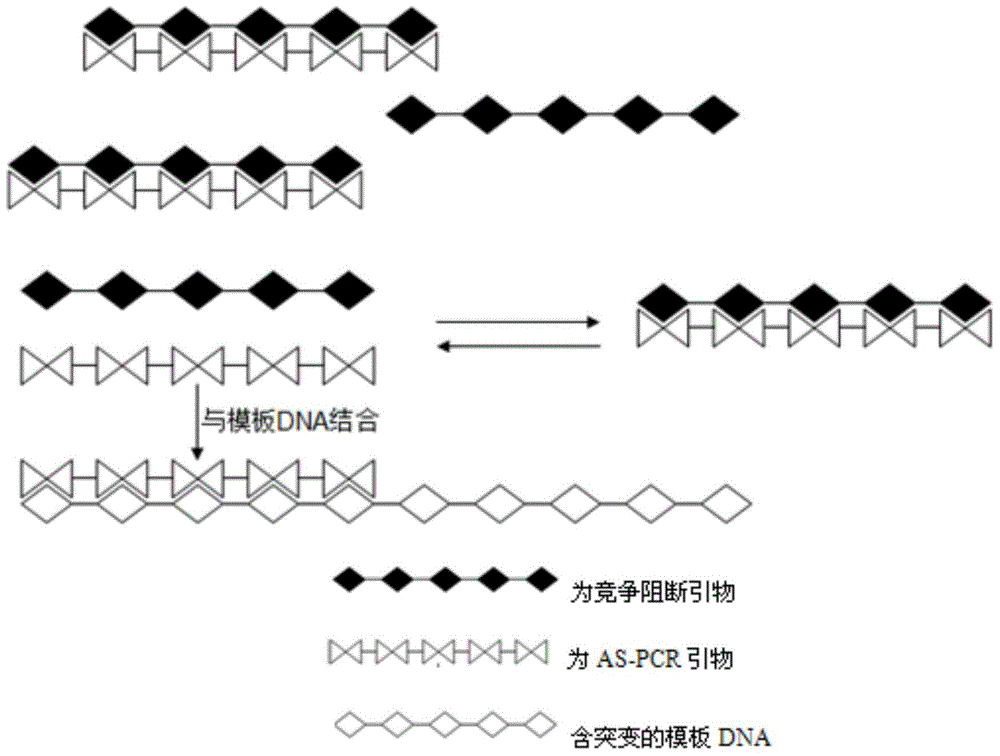
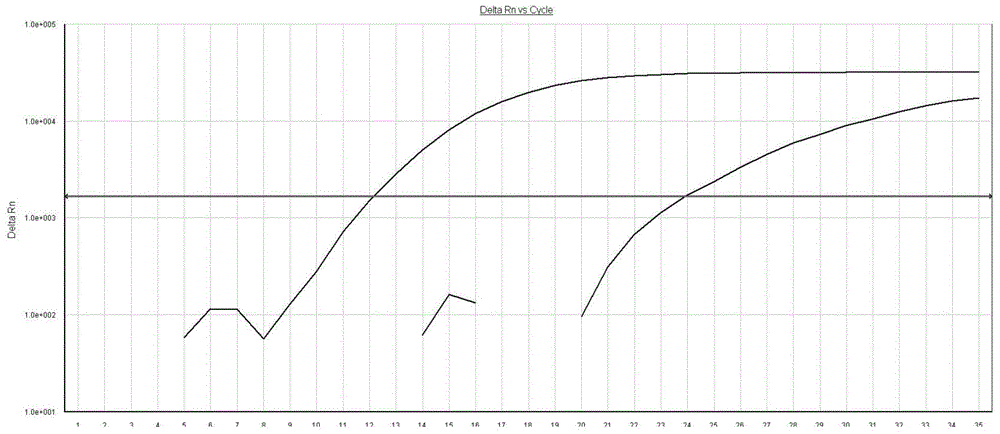
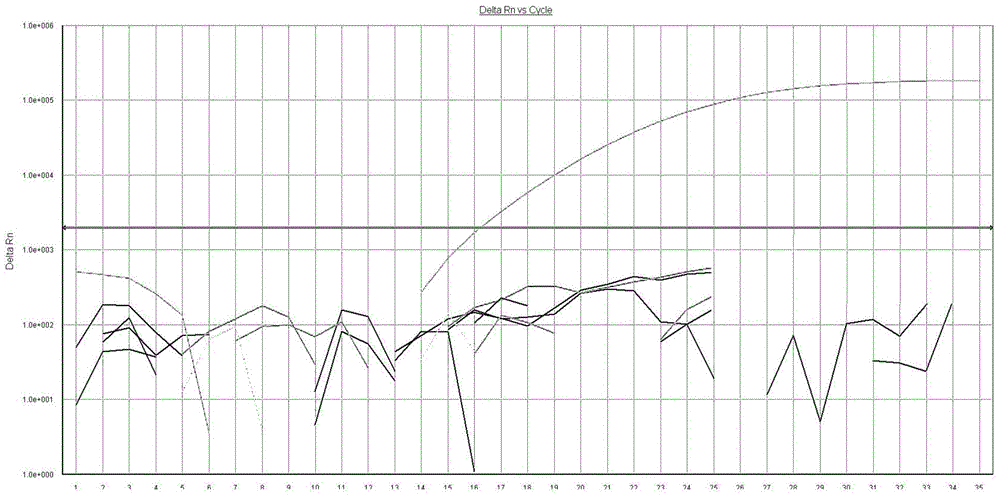
AS-PCR (allele-specific polymerase chain reaction) primer design method, gene mutation detection method and kit
InactiveCN104611427AStrong specificityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationPolymerase LOligonucleotide
The invention relates to the field of molecular biology, in particular to an AS-PCR (allele-specific polymerase chain reaction) primer design method, a gene mutation detection method and a kit. The AS-PCR primer design method comprises the following steps: (1), an AS-PCR primer is designed for a target sequence containing a to-be-detected allele mutation region; (2), a competition blocking primer is designed for the AS-PCR primer and adopts oligonucleotide in reverse complement with the AS-PCR primer. According to the gene mutation detection method, the AS-PCR primer and the competition blocking primer have a PCR amplification reaction. The kit comprises the AS-PCR primer, the competition blocking primer, a TaqMan probe, an internal control primer, an internal control probe, polymerase, dNTP (deoxynucleotide) and a buffer solution. The AS-PCR primer design method, the gene mutation detection method and the kit have the advantage that the difference of specificity of the AS-PCR primers on mutation sites due to strong and weak mismatch of the mutation sites is reduced effectively.
Owner:江苏宏泰格尔生物医学工程有限公司
Nucleic Acid Amplification Method
InactiveUS20100015617A1Novel and effective nucleic acid amplification methodSimple and rapid detectionAnalysis using chemical indicatorsSugar derivativesOrigin of replicationNucleotide
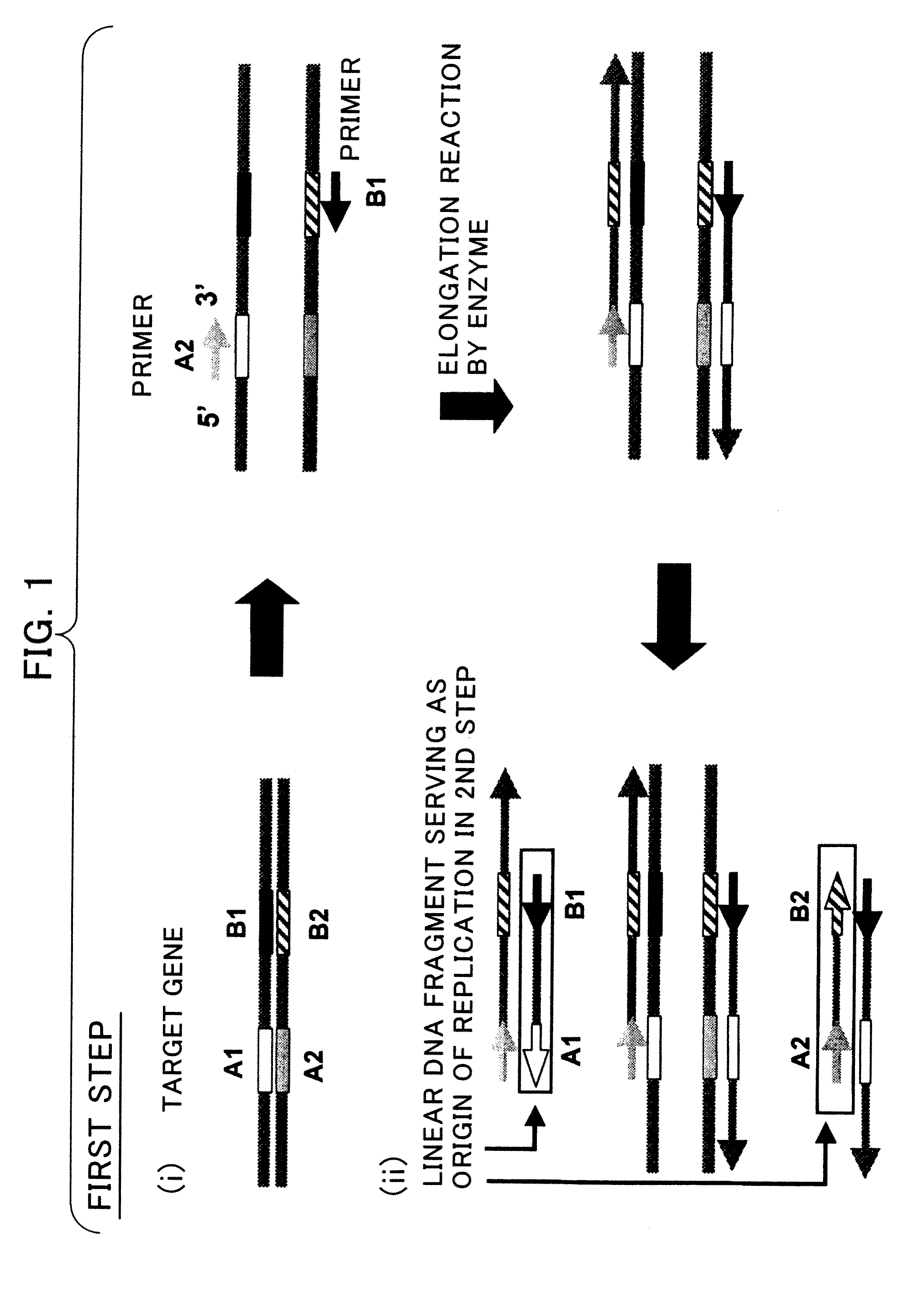
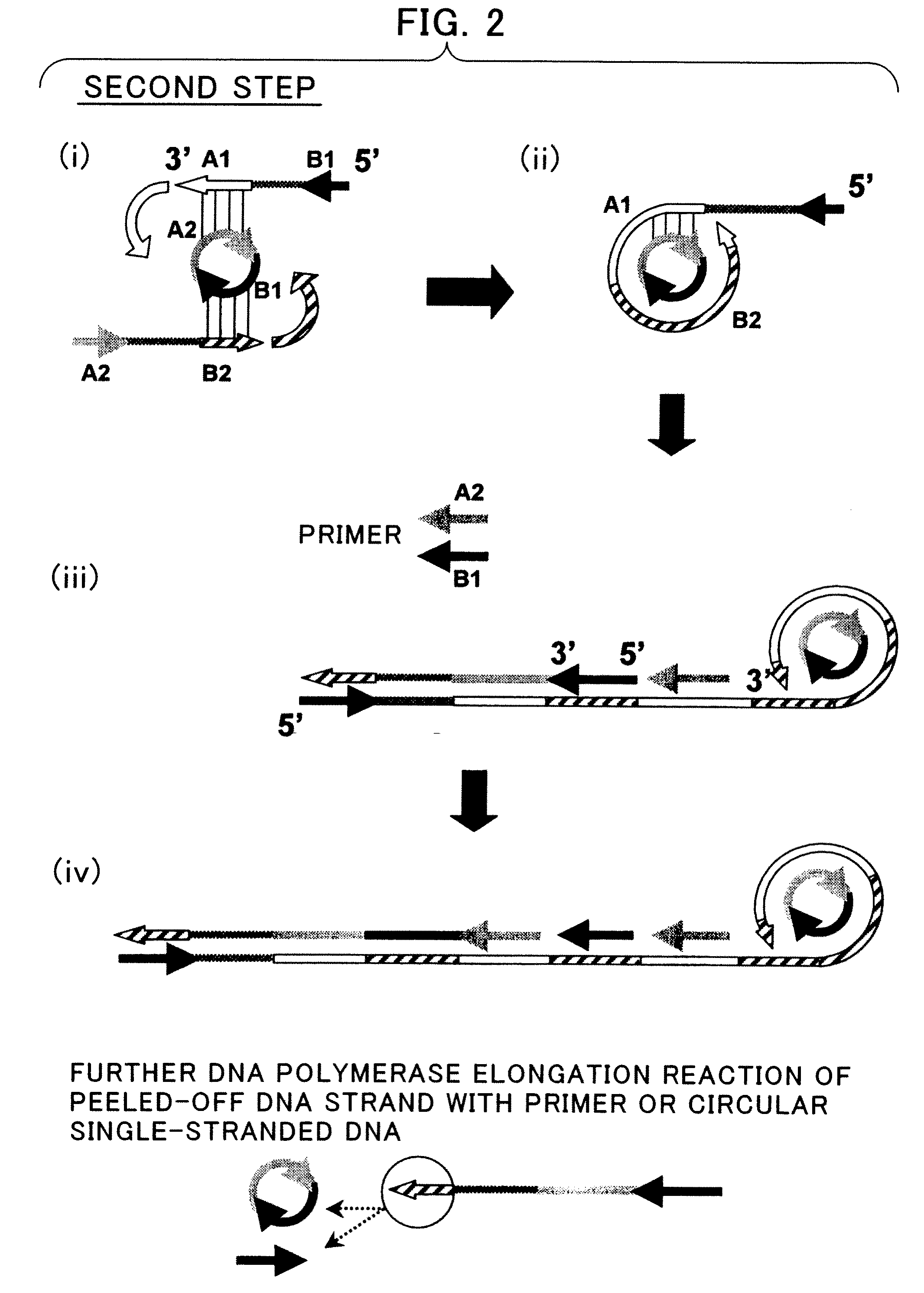
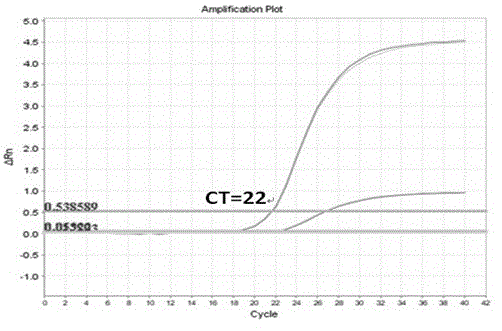
Disclosed is a nucleic acid amplification method which is based on a new principle and enables to amplify a nucleic acid having a specific nucleotide sequence in a simple manner, within a short time and with efficiency. The nucleic acid amplification method comprises the steps of: (a) conducting a DNA polymerase chain reaction by using, as a template, DNA comprising a nucleotide sequence to be amplified and using a primer pair having a nucleotide sequence complementary to the nucleotide sequence to be amplified, thereby producing a linear DNA fragment; and (b) conducting a chain-substituting DNA polymerase chain reaction in a chaining manner by using cyclic single-stranded DNA comprising the same nucleotide sequence as that of at least one of the primer pair as a template and employing the 3′-terminus of the linear DNA fragment produced in step (a) as the replication origin.
Owner:TOYO SEIKAN KAISHA LTD
Human K-ras (K-rat sarcoma) gene mutation parting type fluorescent quantitation PCR (polymerase chain reaction) detecting reagent kit and detecting method
InactiveCN102747158AGrowth inhibitionAdvantages and Notable ImprovementsMicrobiological testing/measurementFluorescence/phosphorescenceWild typeMinor groove
The invention discloses a human K-ras (K-rat sarcoma) gene mutation parting type fluorescent quantitation PCR (polymerase chain reaction) detecting reagent kit and a detecting method. The detecting reagent kit comprises PCR mixed reaction liquid, a peptide nucleic acid probe, a taqman-MGB (minor groove binder) probe, an inner control forward and reverse primer and probe, an external control forward and reverse primer and probe and a positive reference article, wherein the inner control forward and reverse primer and probe is used for K-ras gene mutation detection, and the external control forward and reverse primer and probe is used for the K-ras gene mutation detection. The PNA (peptide nucleic acid) technology and the taqman-MGB technology are combined, relevant ARMS (amplification refractory mutation system) primers are designed, PNA can be stably combined with wild K-ras genes 12 or 13 codon sequences, only mutation specimens can be amplified, and the K-ras gene mutation can be detected. The method has the advantages that the speed is high, simplicity and convenience are realized, the specificity is good, the sensitivity is high, and the method can be used for the clinical K-ras gene mutation screening.
Owner:武汉海吉力生物科技有限公司
Real time polymerase chain reaction (PCR) detection primer, real time PCR probe and kit for detecting duck adenovirus A and duck adenovirus 2
InactiveCN107043831ASimplify operating proceduresLow costMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceInverse polymerase chain reaction
The invention provides a real time polymerase chain reaction (PCR) detection primer, a real time PCR probe and a kit for detecting duck adenovirus A and duck adenovirus 2. The sequences of the primer and the probe are as shown in SEQ ID No. 1-6, and the primer and the probe have very high specificity and sensitivity. At present, the research reports about primers and probes that can detect the duck adenovirus A and the duck adenovirus 2 simultaneously by means of a double-TaqMan real-time fluorescence quantitative PCR detection method are not seen at home and abroad. The establishment of the primer, the probe and the kit appear for the first time in related fields at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
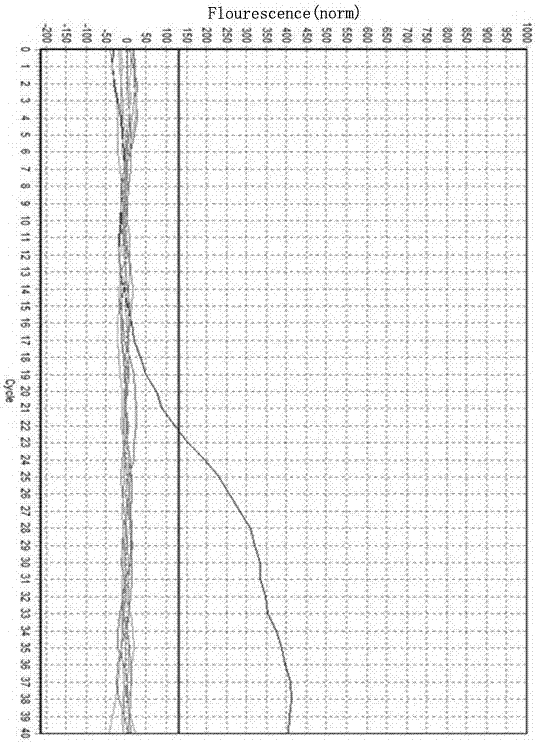
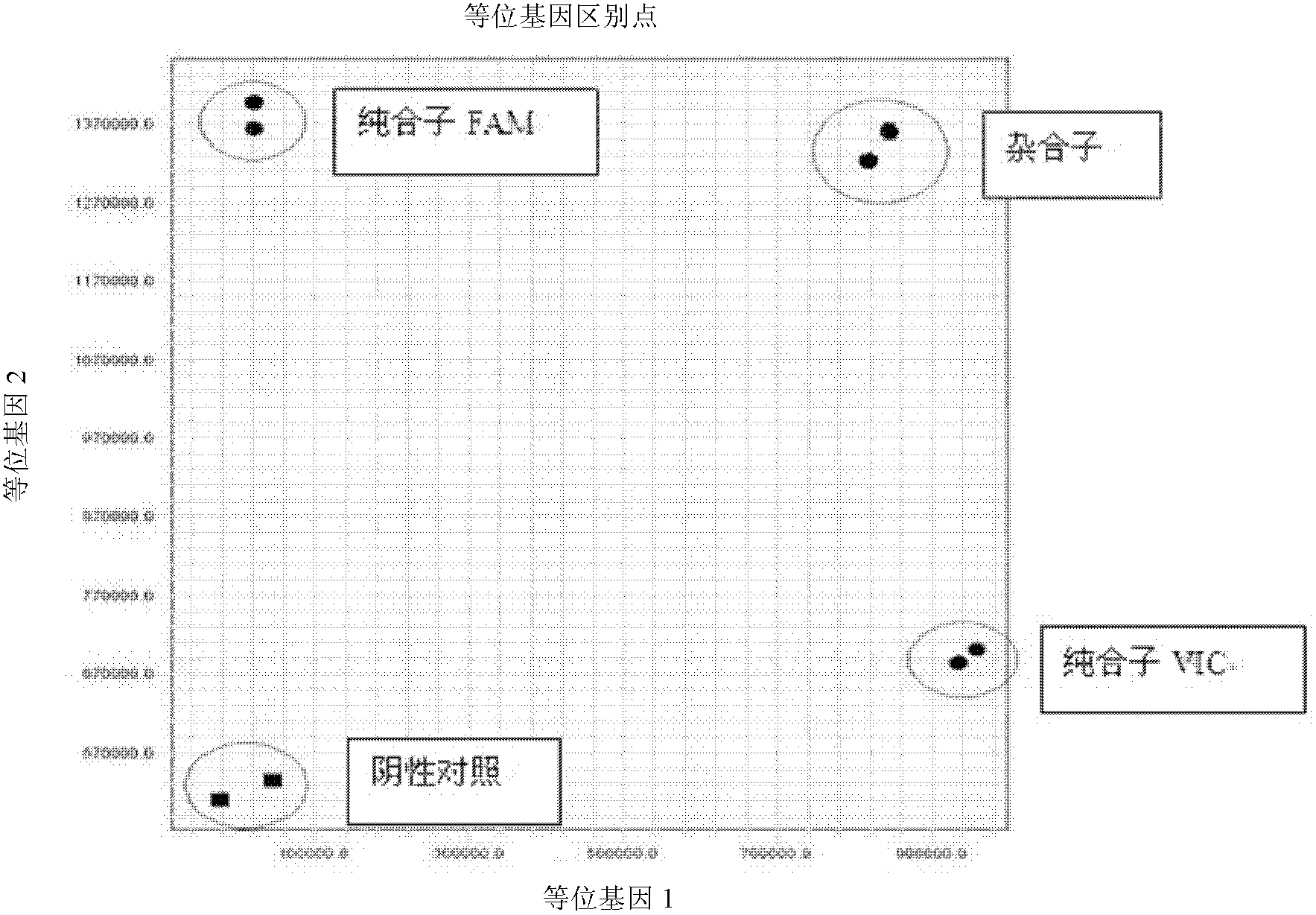
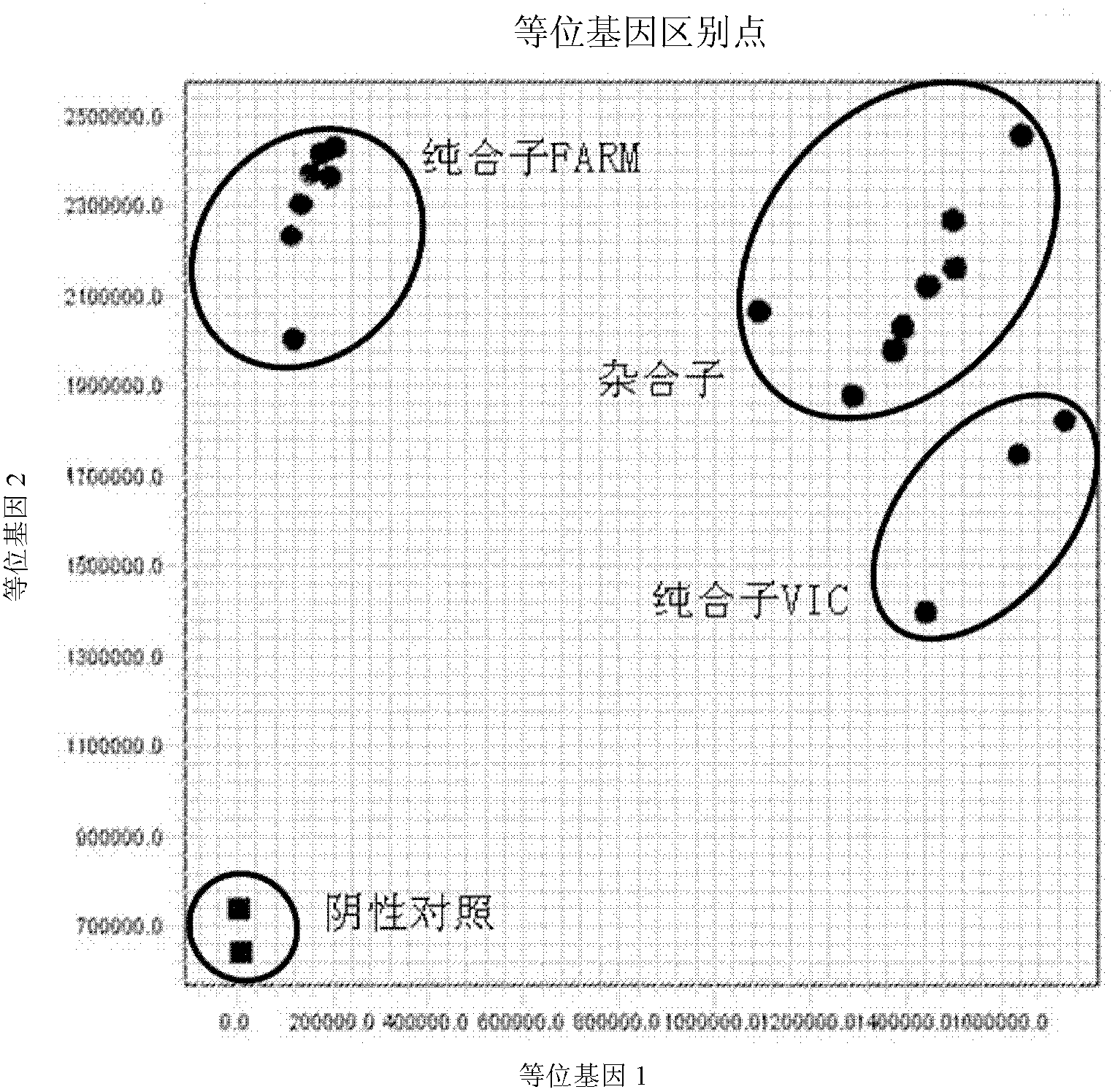
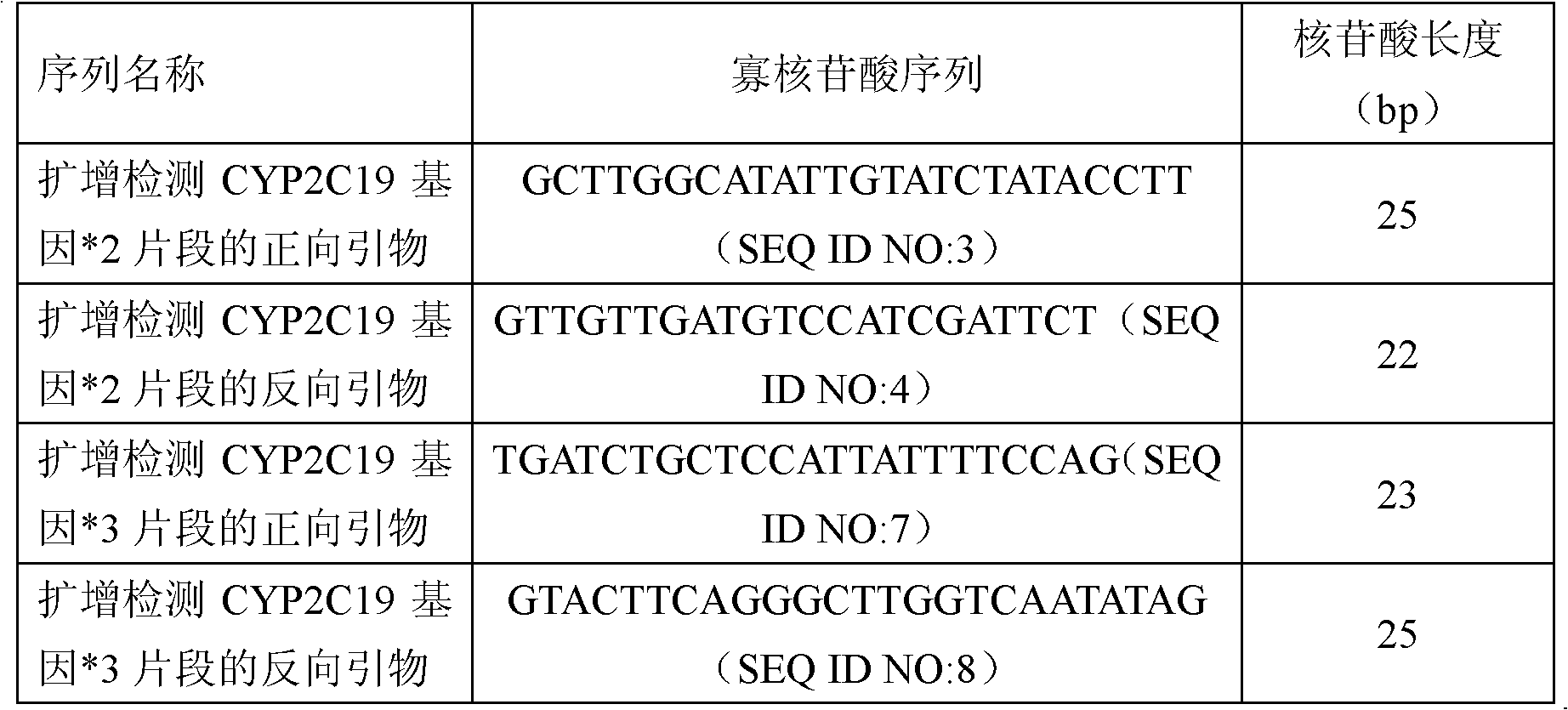
Fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes
InactiveCN102534005AIncreased sensitivityImprove featuresMicrobiological testing/measurementProtein detectionNucleotide
The invention provides a fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes, and belongs to the field of in-vitro nucleic acid testing. The fluorescent PCR kit comprises PCR reaction liquids for detecting CYP2C19*2 and CYP2C19*3 genotypes, Taq DNA polymerase, and uracil-DNA glycosylase, wherein the PCR reaction liquids for detecting the CYP2C19*2 and CYP2C19*3 genotypes respectively comprise PCR amplification primers, minor groove binder (MGB) probes and the like; and nucleotide sequences for detecting the CYP2C19*2 and CYP2C19*3 genotypes are shown as SEQ ID NO:3-4 and SEQ ID NO:5-6 respectively. The kit has high sensitivity and specificity, can monitor the reaction progress in real time, ensures short reaction time, avoids subsequent treatment, can avoid reaction product pollution to the greatest extent, and can replace the traditional protein detection or the common PCR detection to diagnose the CYP2C19 genotypes.
Owner:CHANGSHA 3G BIOTECH
PCR (polymerase chain reaction) amplification system for genotyping of three SNP (single-nucleotide polymorphism) loci related to human folic acid metabolism and detection kit
ActiveCN106701987AEnables direct detectionAvoid confusionMicrobiological testing/measurementInverse polymerase chain reactionDNA extraction
The invention belongs to the technical field of human nucleic acid in vitro detection, and specifically relates to a PCR (polymerase chain reaction) amplification system for genotyping of three SNP (single-nucleotide polymorphism) loci related to human folic acid metabolism and a detection kit. A complex PCR amplification system comprising three SNP loci and three sample molecular marker sites is firstly designed, the three SNP loci adopt allele-specific primers, the three molecular marker sites adopt length polymorphic primers, and the primers are mutually compatible and can react in the same PCR amplification system. The detection kit comprises a primer composition container, a PCR reaction mother solution container, and a PCR auxiliary solution container, wherein the primer composition container comprises a primer SEQ ID No. 1 to SEQ ID No. 15 composition stock solution. In the case of DNA-free extraction, the three SNP loci are simultaneously amplified and typed by a PCR reaction, so that the cost, manpower and time can be obviously saved, and the working efficiency can be improved.
Owner:上海五色石医学科技有限公司
Multiply-fluorescence PCR (polymerase chain reaction) detection kit for identifying strong and weak strains of mycoplasma gallisepticum
ActiveCN102605072AGood fast sensitivityImprove scalabilityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInverse polymerase chain reaction
The invention discloses a multiply-fluorescence PCR (polymerase chain reaction) detection kit for identifying strong and weak strains of mycoplasma gallisepticum. Primers used consists of a first primer, a second primer, a third primer and a fourth primer, the sequences of the first primer, the second primer, the third primer and the fourth primer in the sequence table are respectively a sequence1, a sequence 2, a sequence 4 and a sequence 5. The experiment indicates that on one hand, high amplification and excellent specificity of the PCR technology and quick sensitivity of the fluorescencedetection technology are sufficiently used in the agent and detection method; on the other hand, primer pairs universal to the strong and weak strains of the mycoplasma gallispticum and primer pairs special for weak strains and probes are assembled into a fluorescent PCR system for multiply-fluorescence PCR detection, and mutual interference is avoided, and detection sensitivity and specificity are improved further.
Owner:GUANGXI VETERINARY RES INST
Multiplex fluorescence PCR (polymerase chain reaction) universal adapter for microsatellite detection, and detection method and application thereof
InactiveCN104178566ALow costSave moneyMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceNucleotide
The invention discloses a multiplex fluorescence PCR (polymerase chain reaction) universal adapter for microsatellite detection, which comprises an adapter 1, an adapter 2, an adapter 3 and an adapter 4, wherein the nucleotide sequences of the adapters 1-4 are disclosed as SEQ ID:1-4. The universal adapter has the advantages of high PCR amplification efficiency and no interference, and can not interfere with microsatellite primers to be amplified. The invention also discloses a method for carrying out microsatellite multiplex fluorescence PCR detection by using the multiplex fluorescence PCR universal adapter for microsatellite detection. In the multiplex PCR process, primer screening and typing experiments can be performed only after the four or three fluorescence adapters are synthesized. The method has the advantages of simple steps, short experimental period and low experimental cost.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Fluorescent PCR kit for detecting herpes simplex virus type II
InactiveCN102102131AIncreased sensitivityReduce the risk of contaminationMicrobiological testing/measurementFluorescencePolyethylene glycol
The invention discloses a fluorescent polymerase chain reaction (PCR) kit for detecting herpes simplex virus type II, and belongs to the field of in-vitro diagnostic kit for nucleic acid. The kit comprises a positive reference, a negative reference, fluorescent polymerase chain reaction liquid, PCR primers and a specific fluorescent probe, polyethylene glycol (PEG) precipitation solution and lysis solution. The invention comprises a PCR system based on a fluorescent PCR technology, contains forward and reverse primers and the fluorescent probe for detecting the gene sequence of the herpes simplex virus type II, can detect the nucleotide sequence of the gene of the herpes simplex virus type II under proper PCR condition, can easily and quickly detect the infection of the HSV II in clinical samples and is high in specificity.
Owner:上海裕隆医学检验所股份有限公司
Taqman real-time fluorescence PCR (polymerase chain reaction) capable of reducing polymerization with primers
InactiveCN102373267ANot easy to missAccurate detection specificityMicrobiological testing/measurementFluorescence/phosphorescenceFluoresceinInverse polymerase chain reaction
The invention provides a Taqman real-time fluorescence PCR (polymerase chain reaction) capable of reducing polymerization with primers. The Taqman real-time fluorescence PCR is improved Taqman and real-time fluorescence PCR which is in a hybrid stem structure combining a panhandle shape at the tail end of a molecular beacon and improves self combination. The Taqman real-time fluorescence PCR is characterized in that: fluorescein is labeled conventionally at 5'-terminal of a template probe sequence, complementary sequences of 5'-terminals of 5 to 6 probes are added to 3'-terminal of the template probe sequence, and a quencher is labeled at the tail terminal so as to form a stem structure similar to the tail terminal of the molecular beacon by hybridizing with the sequence at 5'-terminal of the probe. Two terminals of the probe are self-hybridized and combined, so that the quenching effect is increased, and the background is reduced; the probe is difficultly polymerized with a PCR primer due to self combination, so that primer-probe nonspecific polymerization amplification is eliminated, the detection sensitivity of the Taqman real-time fluorescence PCR is improved, and especially the relevance ratio of a weak-positive specimen is increased.
Owner:BEIJING TAG ARRAY MOLECULAR TEST
Primer group and kit for detecting seven kinds of diarrhea viruses through multiple PCR (polymerase chain reaction), and detecting method of seven kinds of diarrhea viruses
ActiveCN106191316AGet test results quicklyShorten the timeMicrobiological testing/measurementMicroorganism based processesReverse transcriptasePolymerase L
The invention provides a primer group for detecting seven kinds of diarrhea viruses through multiple PCR (polymerase chain reaction), wherein the primer group comprises primers shown as SEQ ID NO.1 and 3-16. The invention also provides a kit for detecting seven kinds of diarrhea viruses through multiple PCR. The kit comprises a multiple PCR primer group, revertase and DNA (deoxyribonucleic acid) polymerase. Through the technical scheme, the sensitivity, the specificity and the simplicity and convenience of the simultaneous detection on the seven kinds of diarrhea viruses are obviously improved.
Owner:CHINA NAT CENT FOR FOOD SAFETY RISK ASSESSMENT +1
Polymerase chain reaction (PCR) fluorescence detection kit and detection method for candida albicans
ActiveCN102417931AEasy extractionEasy to operateMicrobiological testing/measurementMicroorganism based processesFluorescenceNucleotide
The invention relates to the field of microbe detection, in particular to a polymerase chain reaction (PCR) fluorescence detection kit for candida albicans. According to the technical scheme, the fluorescence detection kit for the candida albicans comprises a PCR reaction solution; the PCR reaction solution comprises primers A and a fluorescence probe A; the primers are divided into an upstream primer A and a downstream primer A; and the nucleotide sequence of the upstream primer A is shown as SEQ ID NO.1, the nucleotide sequence of the downstream primer A is shown as SEQ ID NO.2, and the nucleotide sequence of the fluorescence probe A is shown as SEQ ID NO.3. The kit has the advantages of high sensitivity and specificity, stability, timeliness, convenience for operation and the like.
Owner:泰普生物科学(中国)有限公司
Multiple-fluorescent PCR (polymerase chain reaction) amplification reagent and kit for detecting instability of microsatellite
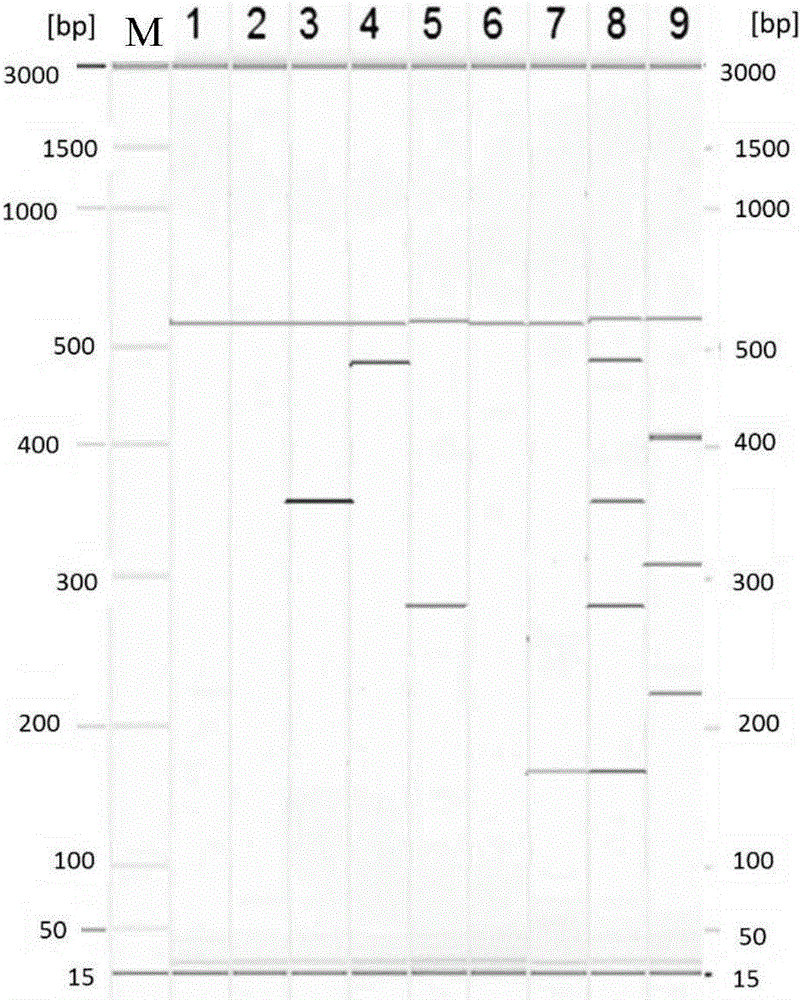
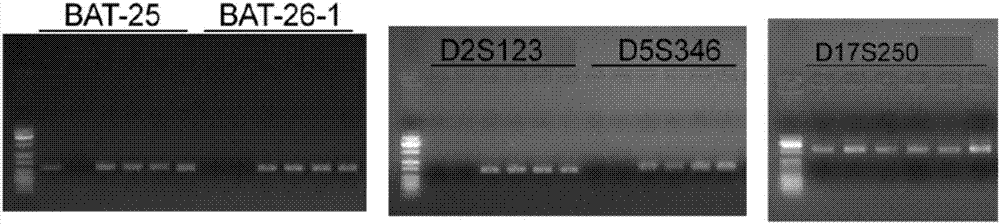
ActiveCN107217103AHigh sensitivityImprove singlenessMicrobiological testing/measurementDNA/RNA fragmentationElectrophoresisMicrosatellite
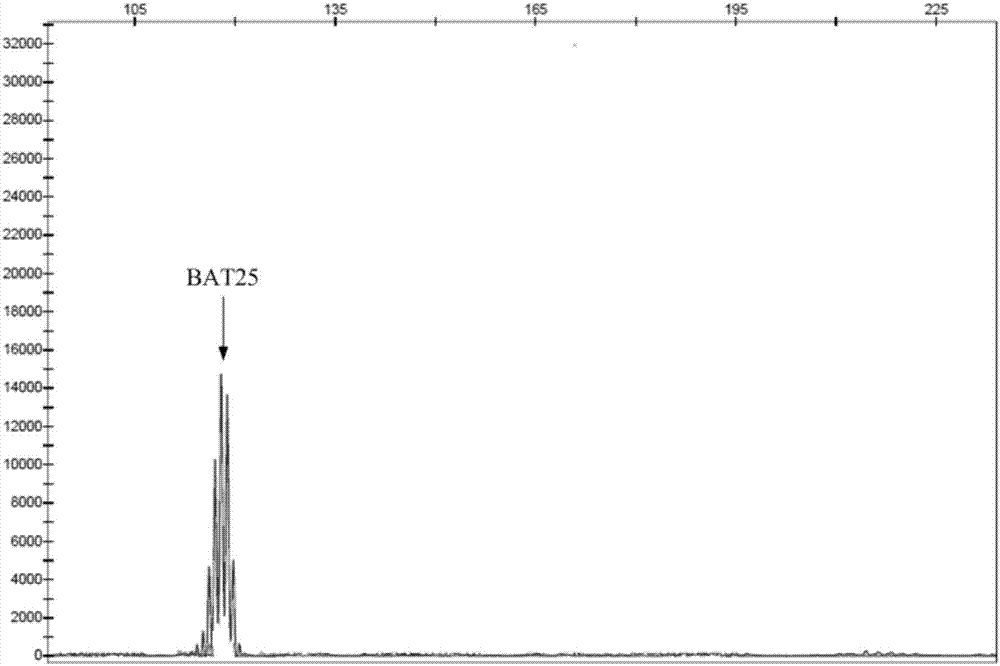
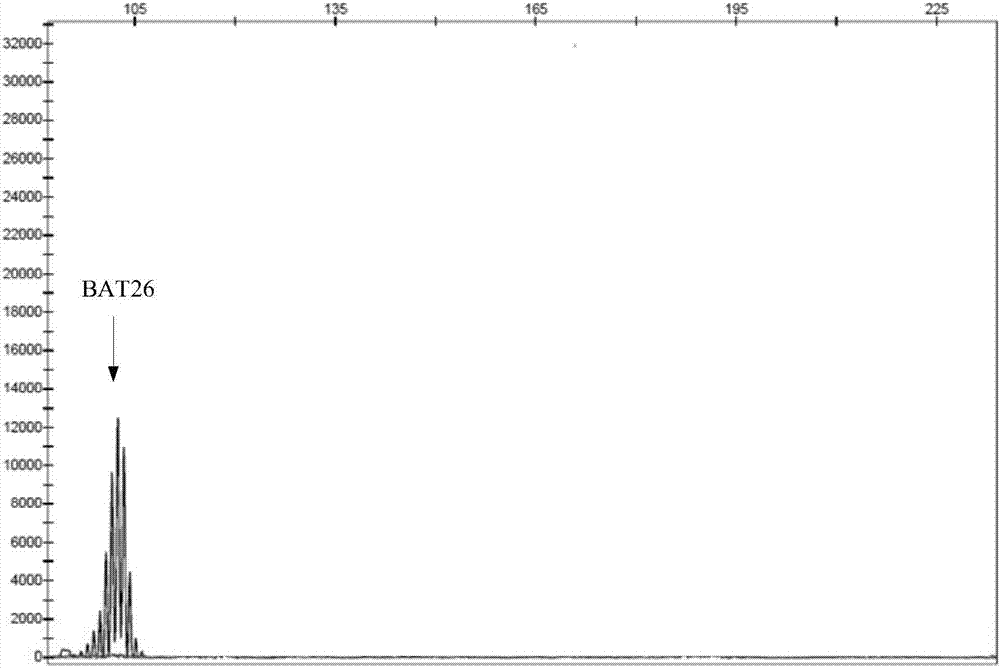
The invention discloses a multiple-fluorescent PCR (polymerase chain reaction) amplification reagent and kit for detecting instability of a microsatellite. The PCR amplification reagent comprises upstream primers and downstream primers which are designed for microsatellite sites BAT25, BAT26, D2S123, D5S346 and D17S250, so that multiple microsatellite sites can be simultaneously amplified in a same PCR system, and the instability of the microsatellite is detected by the multiple-fluorescent PCR. The PCR amplification reagent has the advantages that after electrophoresis detection, the fluorescent peaks produced by the amplified products of the microsatellite sites are not crossed or superposed; the detection efficiency is high, and the sensitivity is good.
Owner:常州桐树生物科技有限公司
Multiple PCR (polymerase chain reaction) primer combination and detection method used for human paternity test
InactiveCN103173557ASignificant father-son relationshipMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceInverse polymerase chain reaction
The invention relates to a multiple PCR (polymerase chain reaction) primer combination used for a human paternity test. Eighty SNP (single nucleotide polymorphellosm) genetic markers can be detected by virtue of the primer combination. Nucleotide sequences of primers at middle and upper reaches in the primer combination are respectively shown in SEQ ID NO.1-80; nucleotide sequences of primers at a lower reach in the primer combination are respectively shown in SEQ ID NO.81-160; and nucleotide sequences of single-base extension primers are sequentially shown in SEQ ID NO.161-240. According to the multiple PCR primer combination, the human paternity test is carried out by preferably utilizing a flight time spectral method, a result is accurate, a method is rapid and simple, and flux is high, so that the multiple PCR primer combination has a good application prospect.
Owner:上海邃志生物科技股份有限公司
Avian influenza H7N9 virus RT-PCR (reverse transcription-polymerase chain reaction) detecting kit and detecting method
InactiveCN103276109AStrong specificityMeet the needs of prevention and control in a timely mannerMicrobiological testing/measurementMicroorganism based processesFluorescenceReverse transcriptase
The invention relates to an avian influenza H7N9 virus RT-PCR (reverse transcription-polymerase chain reaction) detecting kit and a detecting method, and aims at providing the detecting kit which has the characteristics of convenience in use and accuracy in detection, and the detecting method which has the characteristics of high accuracy, simplicity and convenience in detection. The technical scheme is as follows: the avian influenza H7N9 virus fluorescence-quantitative RT-PCR detecting kit comprises deoxynucleotide triphosphate, MgCl2, an RT-PCR buffer solution, an avian influenza H7N9 virogene standard product, an RNA (Ribonucleic Acid) enzyme inhibitor, an MMLV (Moloney Murine Leukemia Virus) reverse transcriptase and a DNA (Deoxyribonucleic Acid) polymerase, and is characterized in that the detecting kit also comprises an upstream primer, a downstream primer and a specific probe. The avian influenza H7N9 virus fluorescence-quantitative RT-PCR detecting method comprises the following steps of: (1) extracting RNA of a sample to be detected; (2) carrying out RT-PCR reaction; and (3) carrying out fluorescence detection on the RT-PCR reaction product.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method
InactiveCN106520984AQuick destructionStrong specificityMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention discloses a Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method. The detection kit comprises a PCR reaction solution, an enzyme mixed solution, a positive control, a negative control and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, primers for target polynucleotide amplification and probes for target polynucleotide detection; the primers comprise a forward primer and a reverse primer. Or, the detection kit comprises a PCR reaction solution, an enzyme mixed solution and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, a forward primer for target polynucleotide amplification, a reverse primer for target polynucleotide amplification and probes for target polynucleotide detection. The Pseudomonas aeruginosa nucleic acid fluorescent PCR detection kit shown in the embodiment of the invention with the advantages of no need of nucleic acid extraction, high detection sensitivity, wide detection range and high detection accuracy can quickly and accurately detect Pseudomonas aeruginosa DNA (deoxyribonucleic acid) in plasma, urine and other samples.
Owner:SANSURE BIOTECH INC
PCR (polymerase chain reaction) synchronous detection kit for staphylococcus aureus enterotoxin A and B genes
InactiveCN102181547ASensitive and accurate detectionReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationNucleotideInverse polymerase chain reaction
The invention discloses a PCR (polymerase chain reaction) synchronous detection primer for staphylococcus aureus enterotoxin A and B genes, a detection kit thereof and a detection method. The nucleotide sequence of the synchronous detection primer is as shown in SEQ (sequence) ID (identity) No. 1 and 2. The invention further provides the PCR detection kit containing the primer. By adopting the detection kit, the staphylococcus aureus enterotoxin A and B in a food can be accurately and sensitively detected, and the lowest detection concentration of DNA (deoxyribonucleic acid) is 3.58ng; furthermore, the detection kit has no cross reaction with other bacteria, and the specificity is good; simultaneously, the pretreatment process of samples is simple, the consumed time is short, a large number of the samples can be detected simultaneously, and the cost is low.
Owner:BEIJING SANYUAN FOOD
Real-time fluorescence polymerase chain reaction (PCR) detection kit for screening listeria monocytogenes and detection method thereof
InactiveCN102010913AHigh sensitivityAccurate identificationMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Primer probes and probe for real-time fluorescent polymerase chain reaction (PCR) detection of Mycobacterium Tuberculosis and using method of primer probes
InactiveCN102559905AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceBiology
The invention discloses a group of specific primers and a probe, which are designed and screened according to an IS6110 sequence of M. Tuberculosis (MTB), and a method for real-time fluorescent polymerase chain reaction (PCR) detection of DNA of the MTB by using the primers and the probe. A PCR reaction system comprises anti-pollution UNG enzyme, and belongs to the field of biotechnology. The real-time fluorescent PCR detection method established by the primers and the probe has high sensitivity and specificity, and particularly has higher tested positive rate for low MTB carried smear negative sputum specimens and blood samples than other similar detection methods.
Owner:GUANGZHOU SUPBIO BIO TECH & SCI
Multiplexed polymerase chain reaction for genetic sequence analysis
InactiveUS7695941B2Microbiological testing/measurementFermentationSequence analysisInverse polymerase chain reaction
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
PCR (polymerase chain reaction) primer group, probe set and kit for detecting multiple respiratory pathogens
ActiveCN105648115AAvoid pollutionEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesFluorescenceTyping
The invention discloses a PCR (polymerase chain reaction) primer group, a probe set and a kit for detecting multiple respiratory pathogens. Sequences of PCR primers are represented as SEQ ID NO.1-SEQ ID NO.12; probes comprise at least two of six molecular beacon probes for respiratory pathogens, a fluorescence group and a quenching group are formed at two ends of each molecular beacon probe respectively, and sequences are represented as SEQ ID NO.13-SEQ ID NO.18. The invention further discloses a PCR reaction liquid containing the PCR primers and the probes as well as a kit containing the PCR reaction liquid for at least one person. With the adoption of the PCR primer group, the probe set and the kit, the six respiratory pathogens can be rapidly and accurately detected in a typing manner.
Owner:新疆亿立方生物技术有限公司
Degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and kit for hantavirus group
InactiveCN102382907AStrong specificityIncreased sensitivityMicrobiological testing/measurementOligonucleotide primersInverse polymerase chain reaction
The invention discloses a degenerate reverse transcription-polymerase chain reaction (RT-PCR) detection reagent and a kit for hantavirus group. The detection reagent comprises a pair of degenerate heterozygous oligonucleotide primers, and the sequences of the degenerate heterozygous oligonucleotide primers are respectively G1: GCAACAGCAACATGGTTTcartaytayac and G2: CTTCTTCATTCATATTTCCATGCarnccyttytc; the non-merger consensus sequence of the 5' ends in the primers plays a role in stabilizing the combination of a 3' merger core area and a template under the condition that the degeneracy of the primers is not increased, so that the specificity of the degenerate PCR reaction is improved, various viruses in hantavirus can be amplified and detected, the homologous unknown viruses of the extendedgenes can also be detected and the amplified target fragments can be sequenced by the detection reagent; and the kit are relatively high in sensitivity and good in hantavirus group specificity, can be used for detecting domestic popular Hantaan and Seoul viruses, and can also be used for detecting other oversea epidemic strains. The degenerate RT-PCR detection reagent and the kit for the hantavirus group are high in sensitivity, good in hantavirus specificity and universal.
Owner:中华人民共和国大榭出入境检验检疫局
Polymerase chain reaction (PCR) primer pair for identifying or assisting in identifying duck tissues and/or organs and application of PCR primer pair
InactiveCN102242220AStrong specificityClear resultMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceInverse polymerase chain reaction
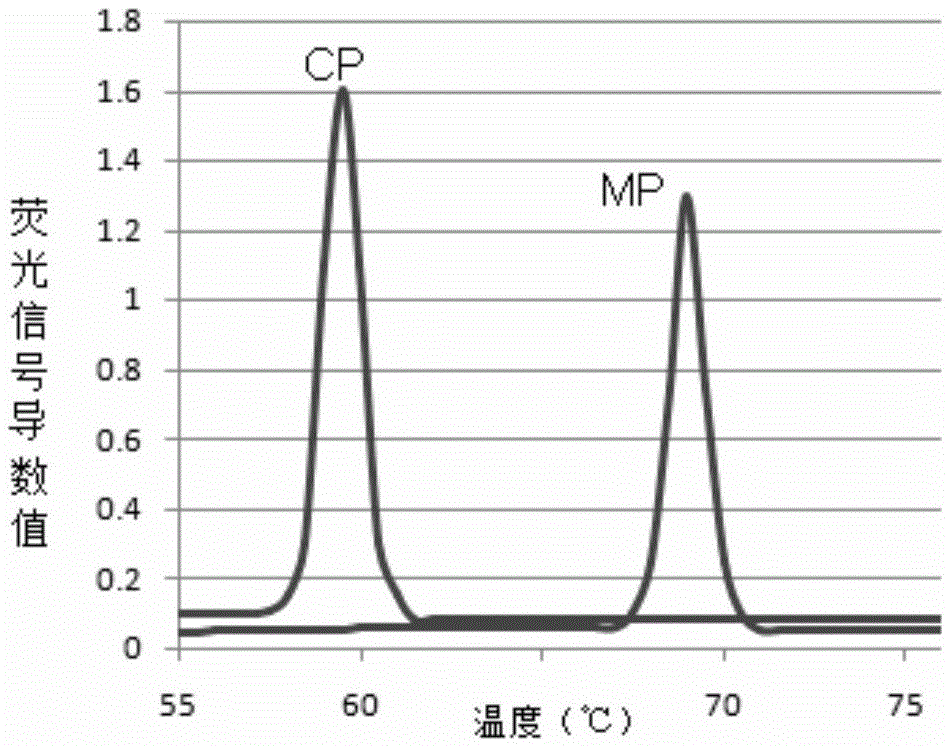
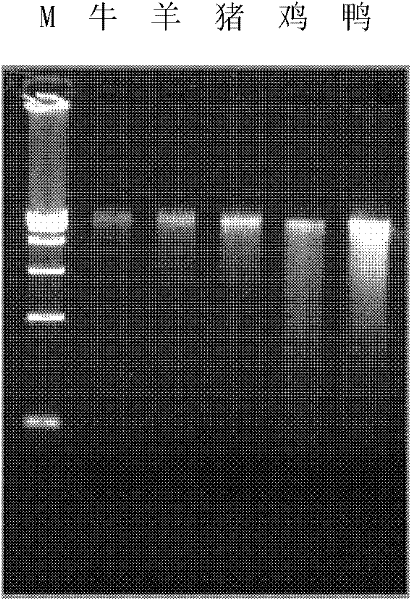
The invention discloses a polymerase chain reaction (PCR) primer pair for identifying or assisting in identifying duck tissues and / or organs and application of the PCR primer pair. The PCR primer pair for identifying or assisting in identifying the duck tissues and / or organs consists of two single-stranded deoxyribonucleic acids (DNAs), namely 1) a single-stranded DNA shown as a sequence 1 in a sequence table and a single-stranded DNA shown as a sequence 2 in the sequence table, and 2) a single-stranded DNA shown as a reverse complementary sequence of the sequence 1 in the sequence table and a single-stranded DNA shown as a reverse complementary sequence of the sequence 2 in the sequence table. The detection method is high in specificity, and the duck tissues and / or organs doped into cattle, sheep, pigs and chicken can be identified; the whole result is clear and has high credibility; and the method is short in detection time, and only 8 hours is needed from the extracting of genomes to the finishing of enzyme cutting.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
High-specificity multiplex-PCR (polymerase chain reaction) detection method for base mutation
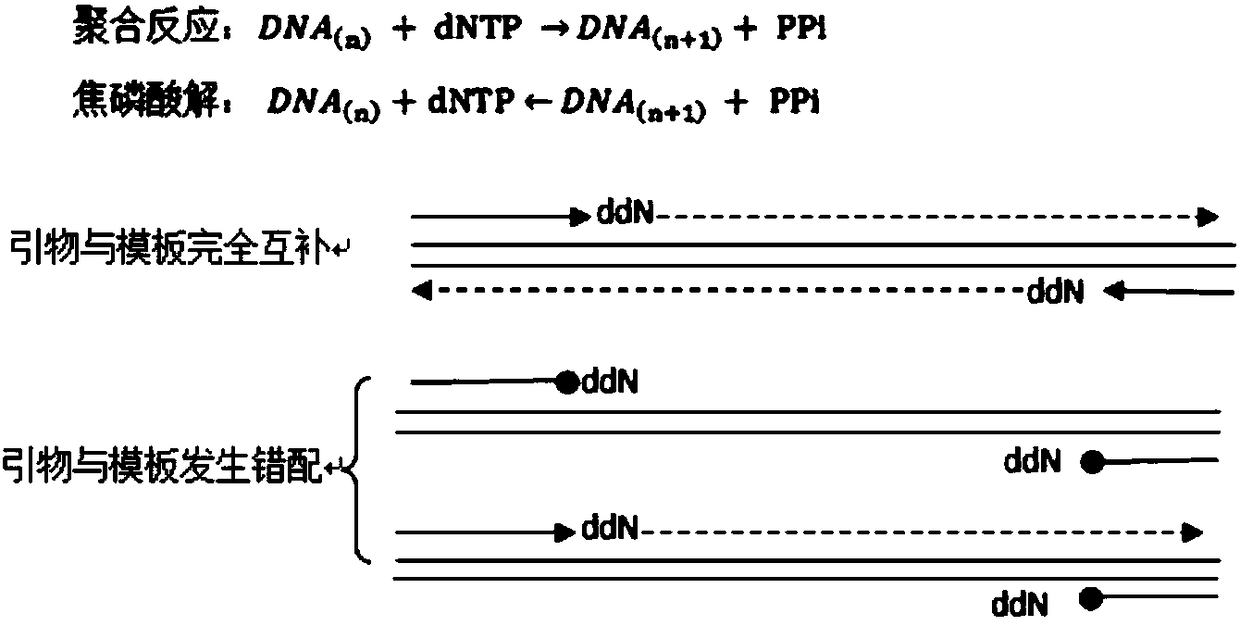
InactiveCN108103159ALarge capacityImprove the problem of heterogeneityMicrobiological testing/measurementNucleotideExonuclease I
The invention discloses a high-specificity multiplex-PCR (polymerase chain reaction) detection method for base mutation. The method comprises steps as follows: under the action of DNA polymerase without 3'-5' exonuclease activity and with pyrophosphorolysis activity, multiple pairs of modified primers are utilized for PCR amplification in the presence of pyrophosphate, dideoxy nucleotide is located at 3' ends of upstream and downstream primers of the modified primers, the 3' ends are complementary with upstream and downstream sequences of a to-be-detected mutant type template, the DNA polymerase relies on the template to perform pyrophosphorolysis on dideoxy nucleotide located at the 3' ends of the modified primers and complementary with the template, the multiple pairs of primers are specifically bonded with the template and extend normally, and multiplex-PCR amplification is realized. According to the method, the problems of non-specificity strip interference and primer dimer of multiplex-PCR are solved, detection specificity and sensitivity are improved, and capacity of a system for the primers is increased; the method can be used for detecting body fluid DNA collected from cancer patients or healthy persons.
Owner:TIANJIN MEDICAL LAB BGI +2
Duplex PCR (polymerase chain reaction) detection primer and kit for quickly distinguishing porcine circoviruses type 2 and type 3
InactiveCN108531656AEasy to operateQuick checkMicrobiological testing/measurementMicroorganism based processesDuplex pcrSterile water
The invention belongs to the technical field of animal virology and molecular biology and discloses a duplex PCR (polymerase chain reaction) detection primer and kit for quickly distinguishing porcinecircoviruses type 2 and type 3. The kit comprises primer sequences shown as SEQ ID1-4, 2*F8 Fastlong PCR MasterMix, a cDNA template and sterile water. The duplex PCR detection kit for quickly distinguishing the porcine circoviruses type 2 and type 3 has advantages that by duplex PCR amplification, two types of DNA viruses similar in lesion can be detected in one time, the total detection time iscontrolled to be about two hours, a detection method is easy in operation, convenient and quick, and quick detection can be realized.
Owner:GUANGXI VETERINARY RES INST
Multiplexed polymerase chain reaction for genetic sequence analysis
InactiveCN101273143AMicrobiological testing/measurementFermentationSequence analysisInverse polymerase chain reaction
A PCR method involving: providing a biological sample suspected of containing one or more pathogen nucleic acids; adding a plurality of PCR primers corresponding to genes found in the pathogens; and performing a polymerase chain reaction on the sample to amplify a subset of the nucleic acids that correspond to the genes. The primers include at least one primer pair for each pathogen, and the primers contain a tail sequence that is not homologous any pathogen DNA or to any background DNA in the sample. The concentration of at least one primer in the polymerase chain reaction is no more than about 100 nM.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
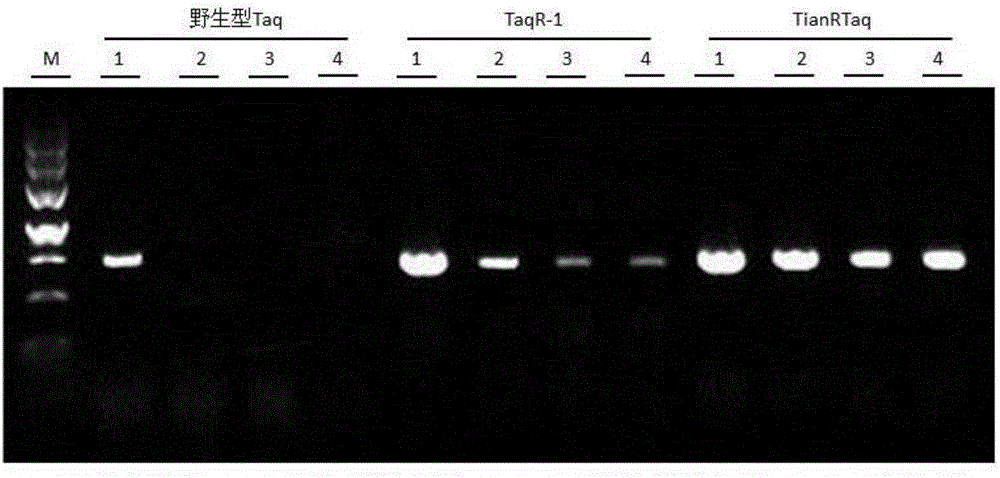
Taq DNA polymerase, and PCR (polymerase chain reaction) fluid and application thereof
The invention relates to a Taq DNA polymerase, and PCR (polymerase chain reaction) fluid and application thereof. Compared with SEQ ID NO:1, the amino acid sequence of the Taq DNA polymerase has following mutations: p.E641K, p.Q698E, and p.E708Q or p.E708D. By the Taq DNA polymerase, blood resistance and PCR inhibitor are improved obviously, and blood samples and soil samples can be directly used for PCR detection. Therefore, experimental time and cost can be greatly saved for experimenters, inter-sample cross contamination caused by multi-step operation can be avoided, and PCR detection results are more credible; an alternative way is provided for PCR detection of precious micro samples.
Owner:TIANGEN BIOTECH BEIJING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com