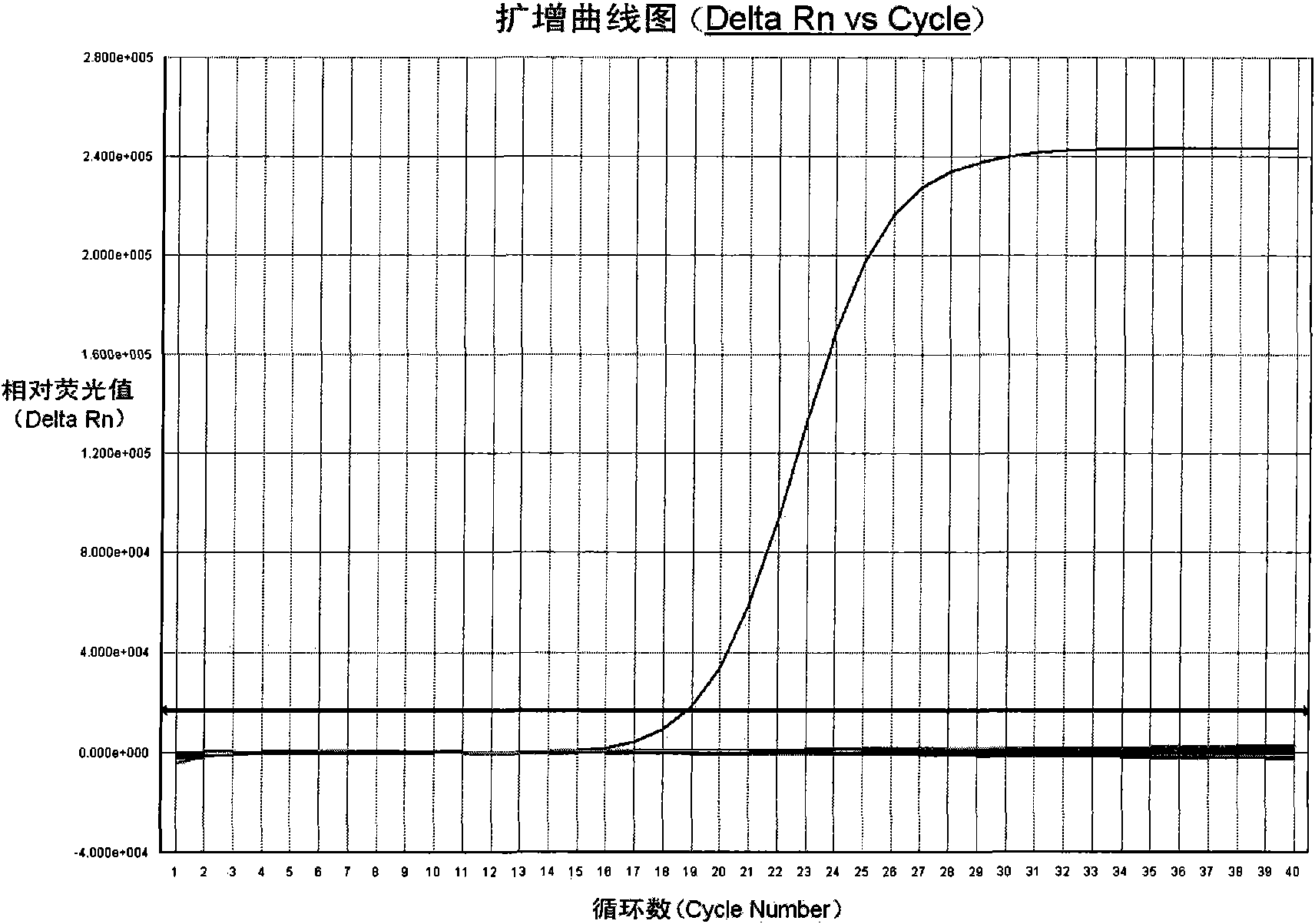
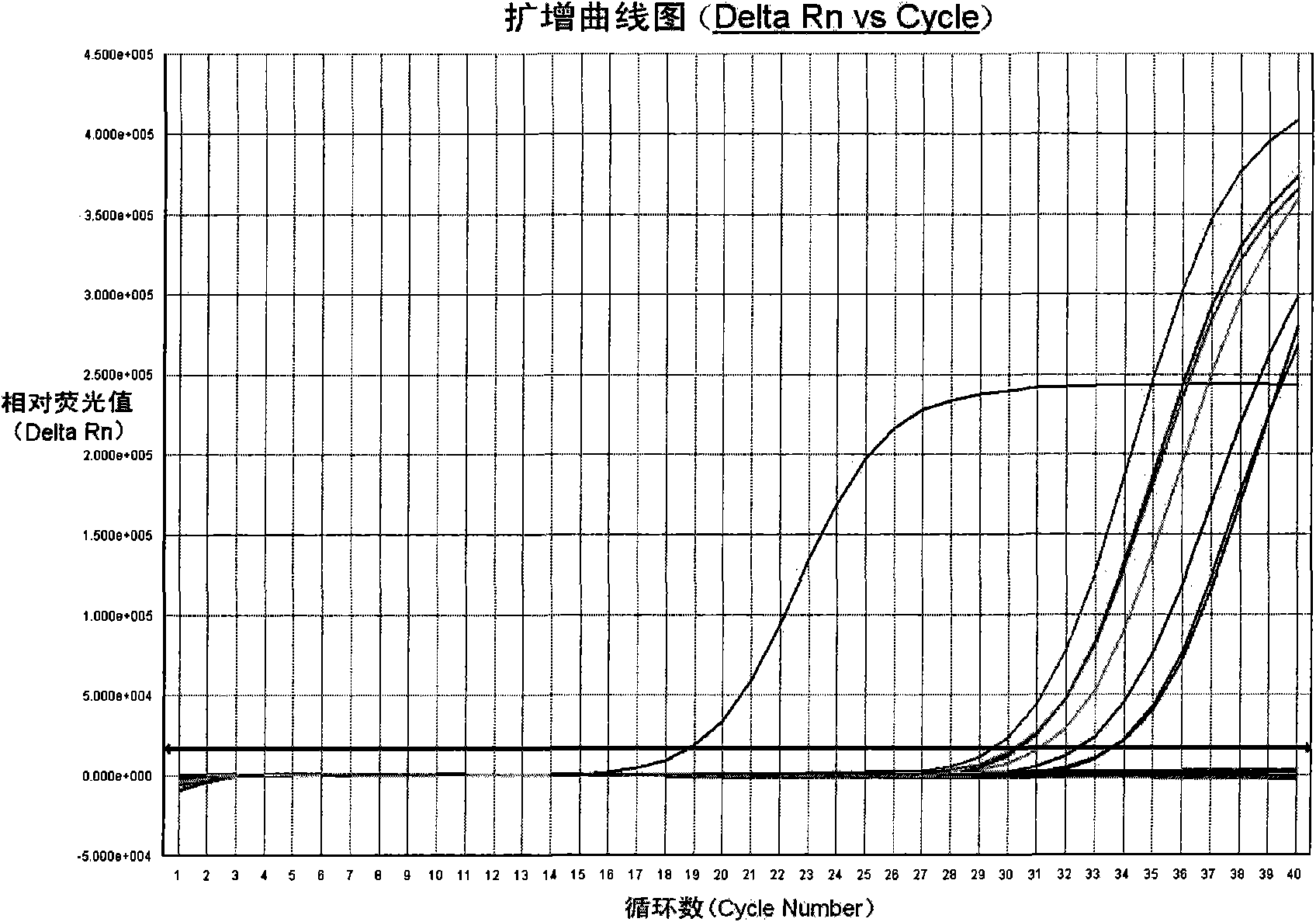
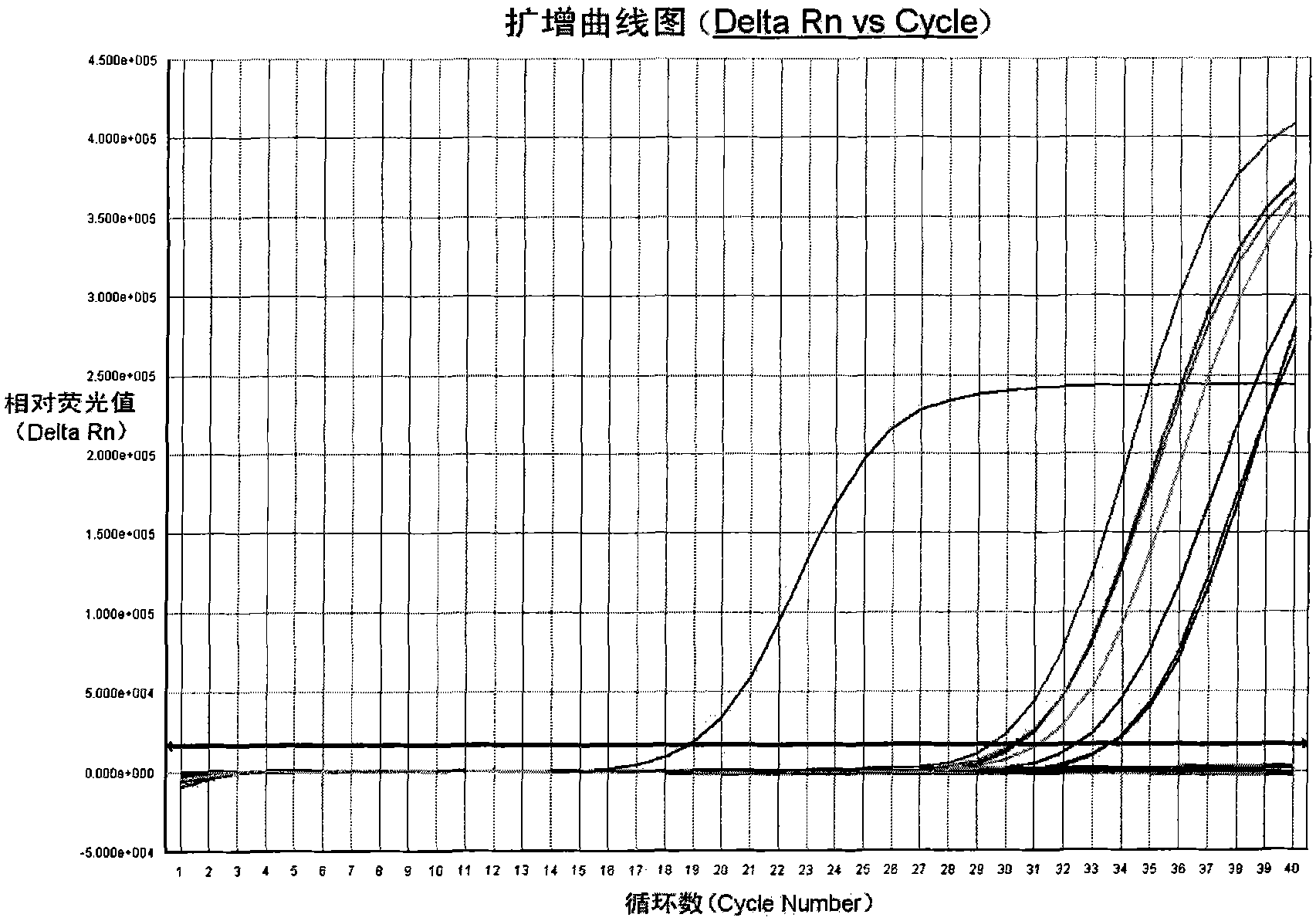
Fluorescent PCR kit for detecting herpes simplex virus type II
A herpes simplex virus and kit technology, which can be used in the determination/examination of microorganisms, biochemical equipment and methods, etc., can solve problems such as unfavorable HSV infection census and rapid diagnosis, unfavorable early diagnosis of HSV infection, and little clinical diagnostic value. , to reduce the risk of PCR product contamination, avoid reaction product contamination, and achieve the effect of fast detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of kit
[0032] 1. Primer and probe design and synthesis
[0033] Use Primer Express 3.0 to screen the primers in the conserved region for the complete HSV II gene, and design HSV II probes based on the selected primers. Both primers and probes were synthesized by a professional company (Shenggong), wherein the primers were purified by PAGE, and the probes were purified by HPLC. The 5' end of the probe was labeled with a FAM fluorescent group, and the 3' end was labeled with a TAMRA fluorescent group.
[0034] The amplified sequence is shown in Table 1:
[0035] Table 1. Specific probe and primer sequences
[0036] sequence name
Oligonucleotide sequence (5'-3')
Base length (bp)
HSV II probe
tgctcatcaagggcgtggatctggtgc
27
HSV II forward primer
acgttcaccaagctgctgc
19
HSV II reverse primer
atcaaccgcacctccagg
18
[0037] 2. Preparation of HSV II plasmid positive templ...
Embodiment 2
[0045] Embodiment 2: the use of kit
[0046] 1. Sample extraction
[0047] Use the DNA extraction solution to extract the HSV II nucleic acid in the sample to be tested
[0048] 1) Sample pretreatment: wipe off excess secretions from the cervix, use a cotton swab soaked in normal saline to cling to the cervical mucous membrane and rotate it for 2 weeks to obtain secretions and exfoliated cells, and put the cotton swab after sampling Rinse fully in an EP tube with 1ml of sterile saline, and squeeze dry by sticking to the wall. The HSV II gene was extracted by PEG precipitation and alkaline lysis.
[0049] PEG precipitation solution formula: 30% PEG8000.
[0050] Lysis solution formula: 60mmol / L TrisHCl, pH 8.0; 0.5% SDS; 200mmol / L NaCl; 1M / L NaOH.
[0051] 2) Operation steps: Take 500 μl of secretion and an equal volume of PEG precipitation solution, shake and mix, centrifuge at 13,000 rpm for 10 minutes, discard the supernatant; add 50 μl of lysate, incubate at 100°C for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com