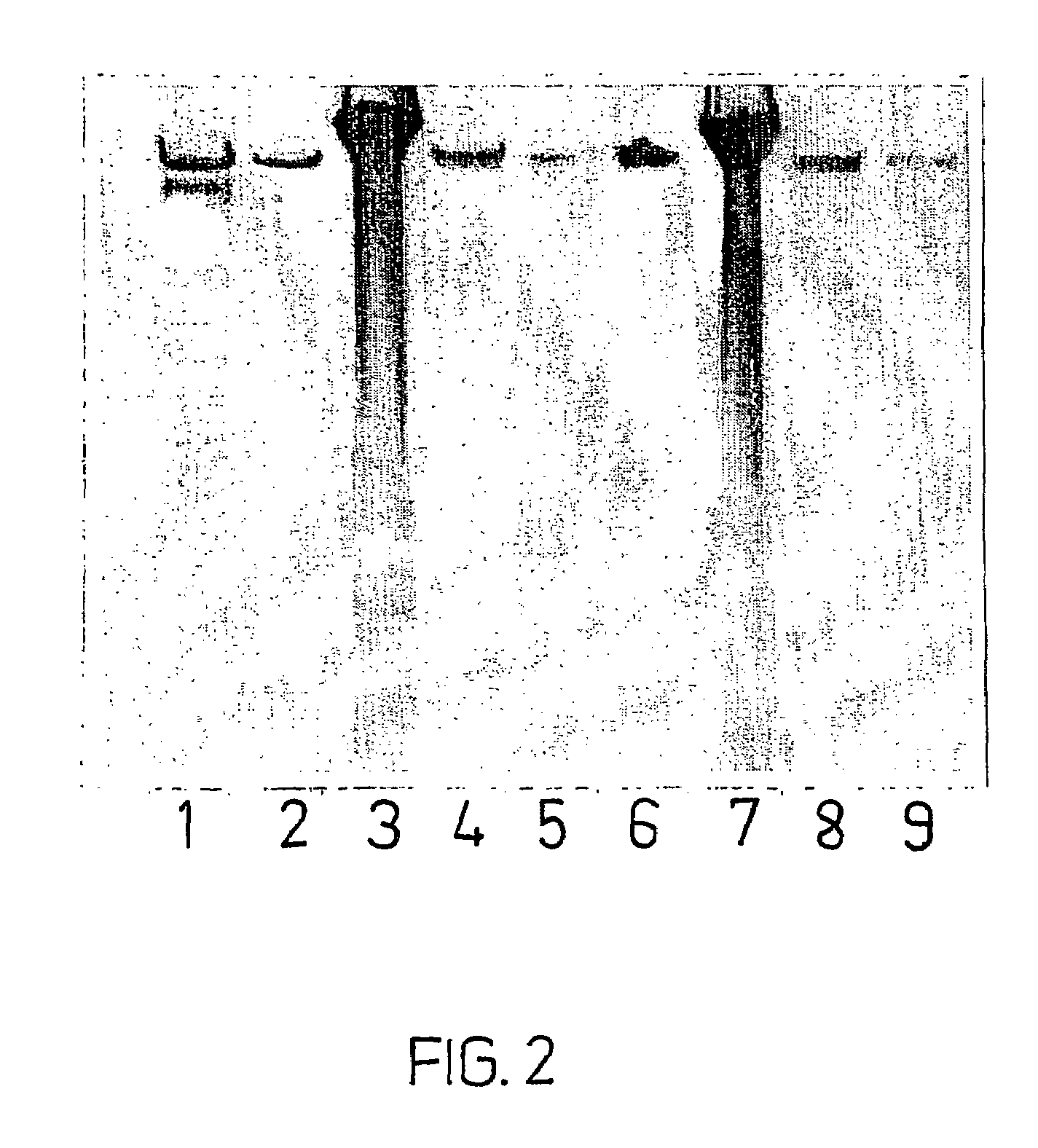
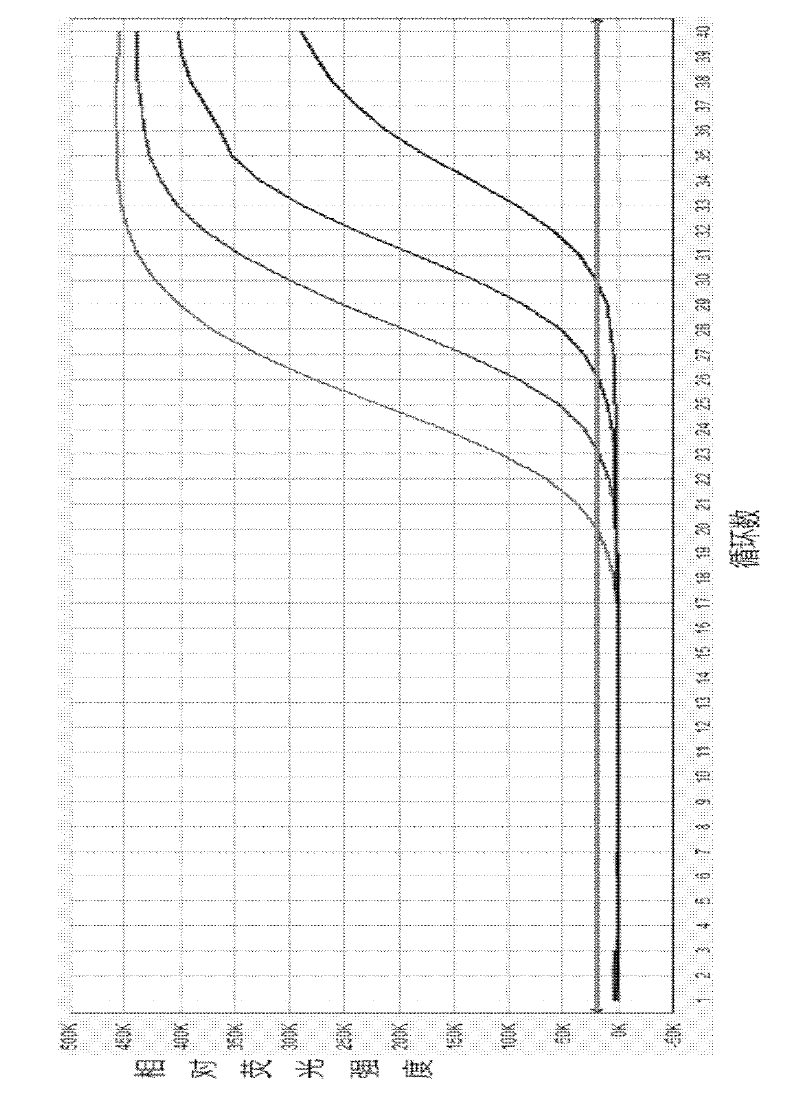
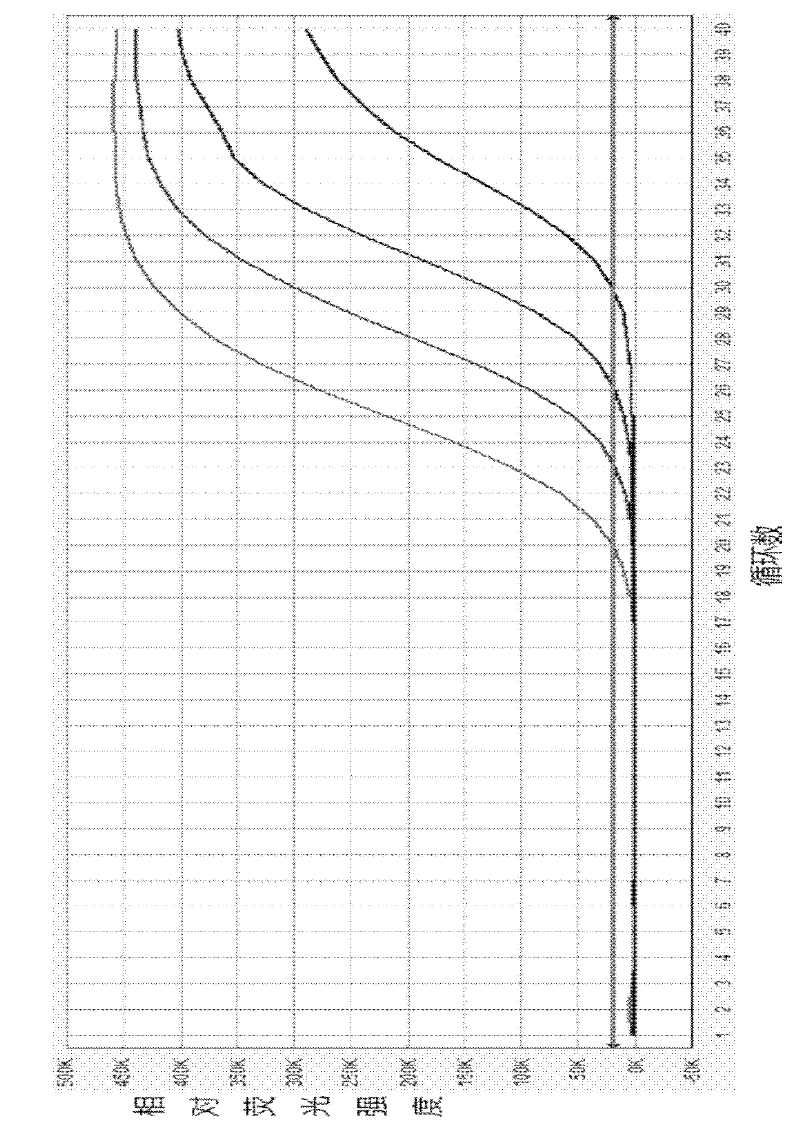
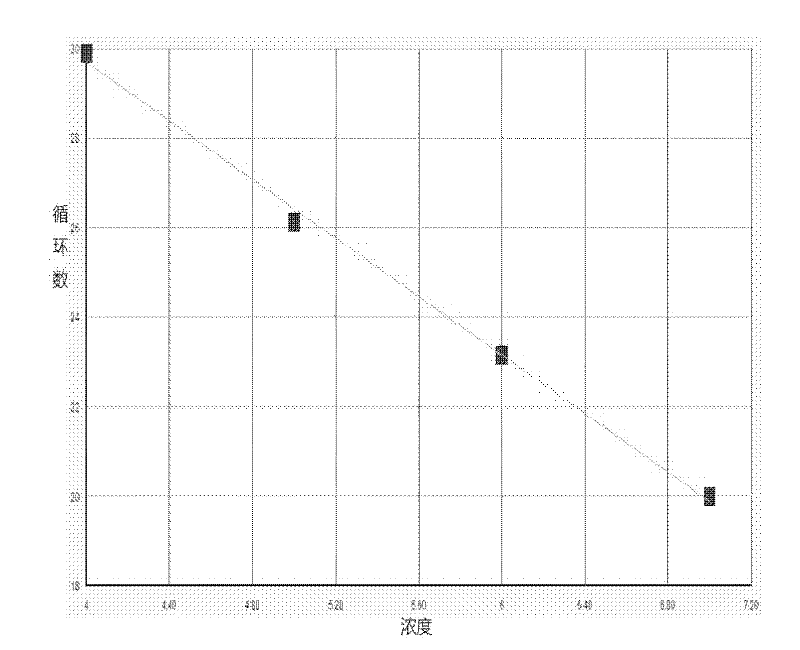
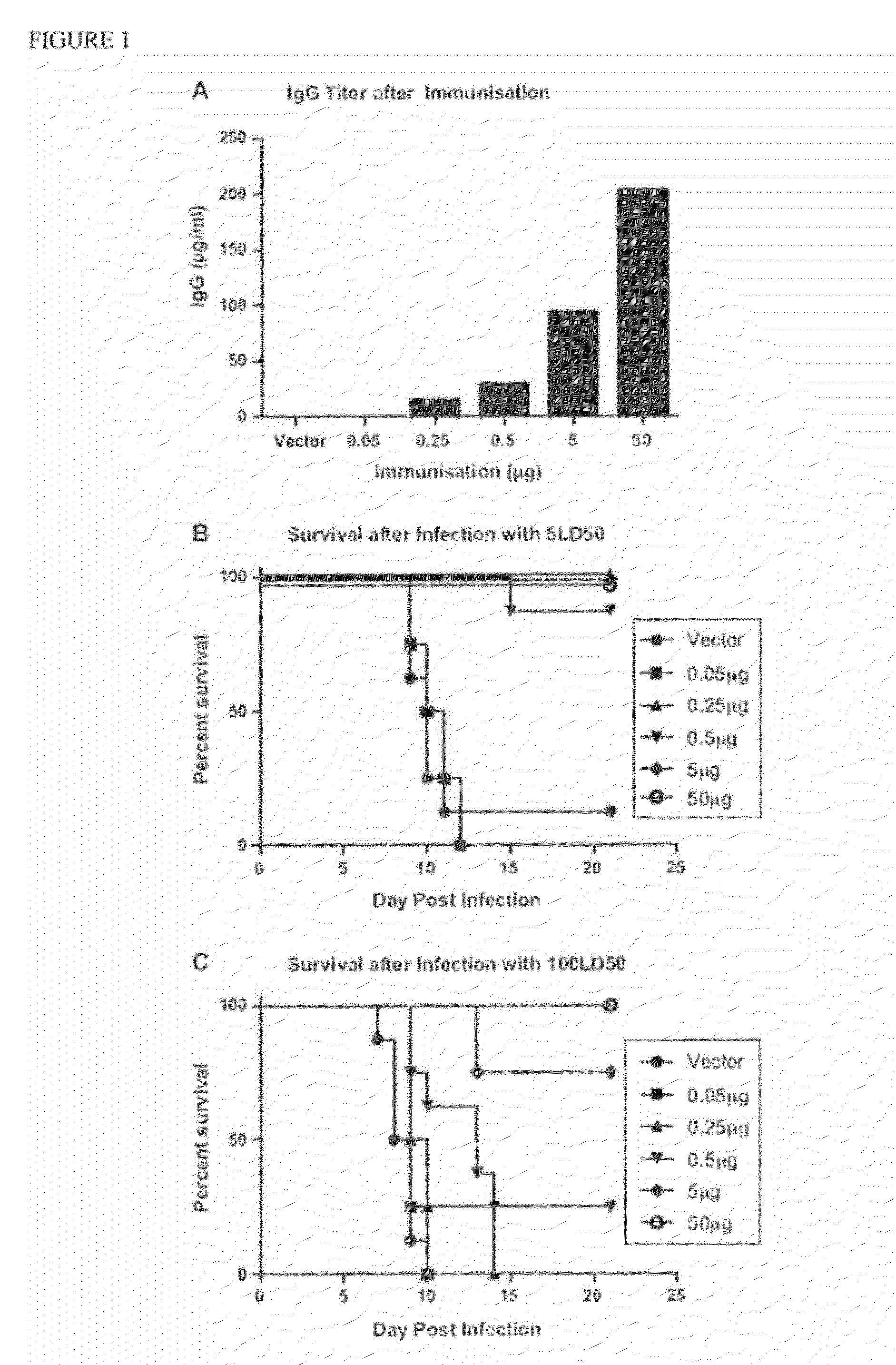
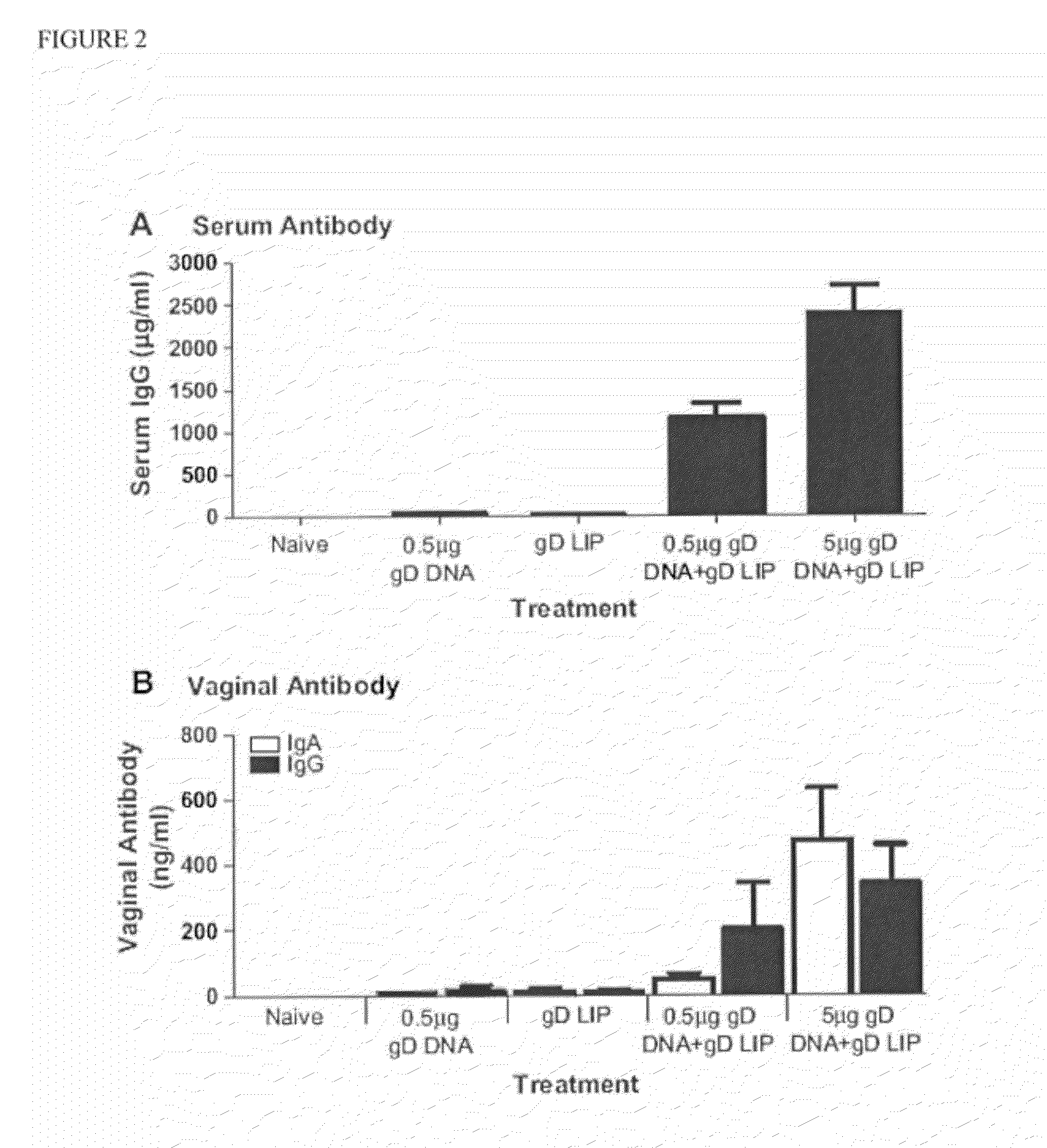
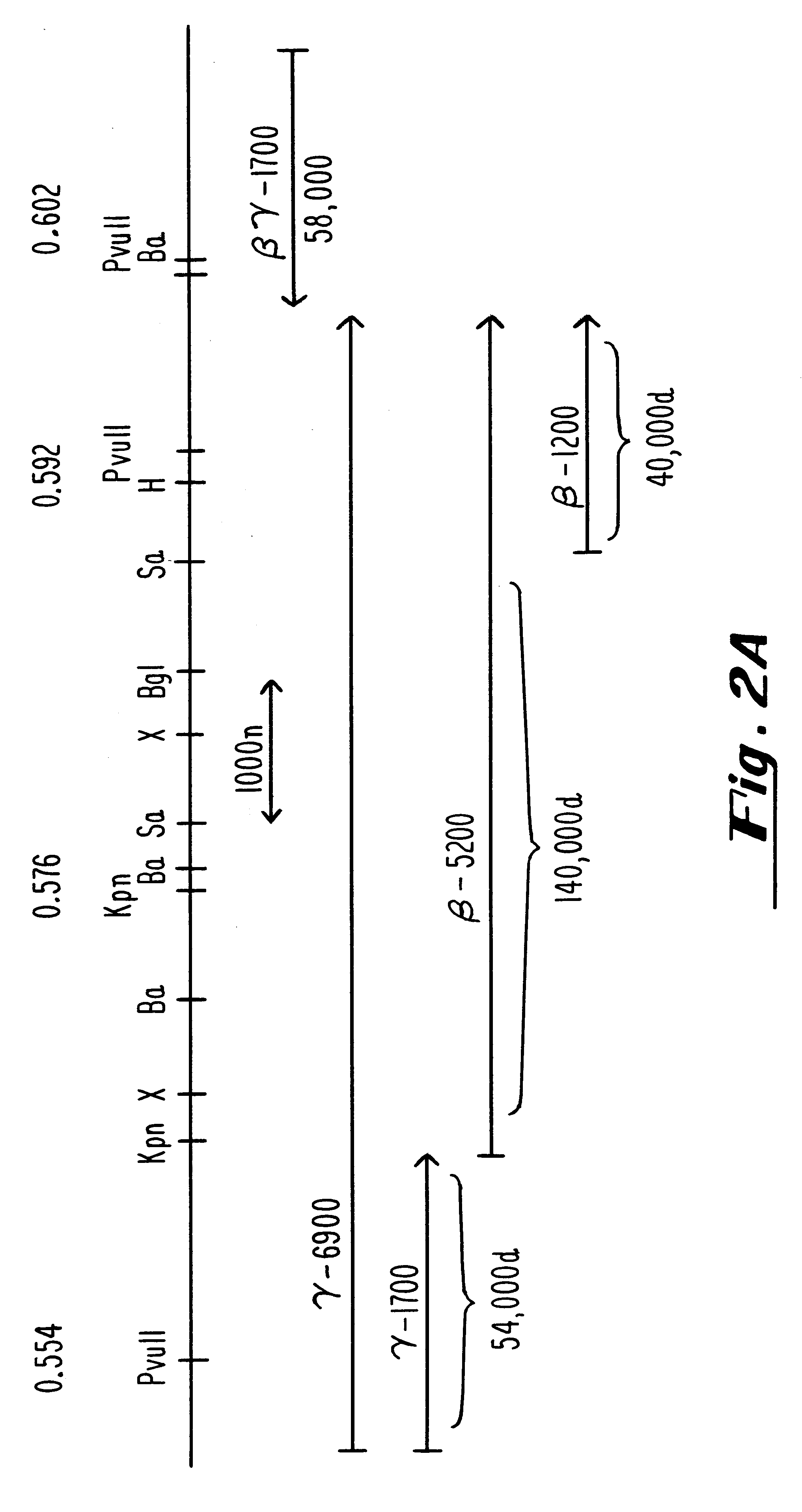
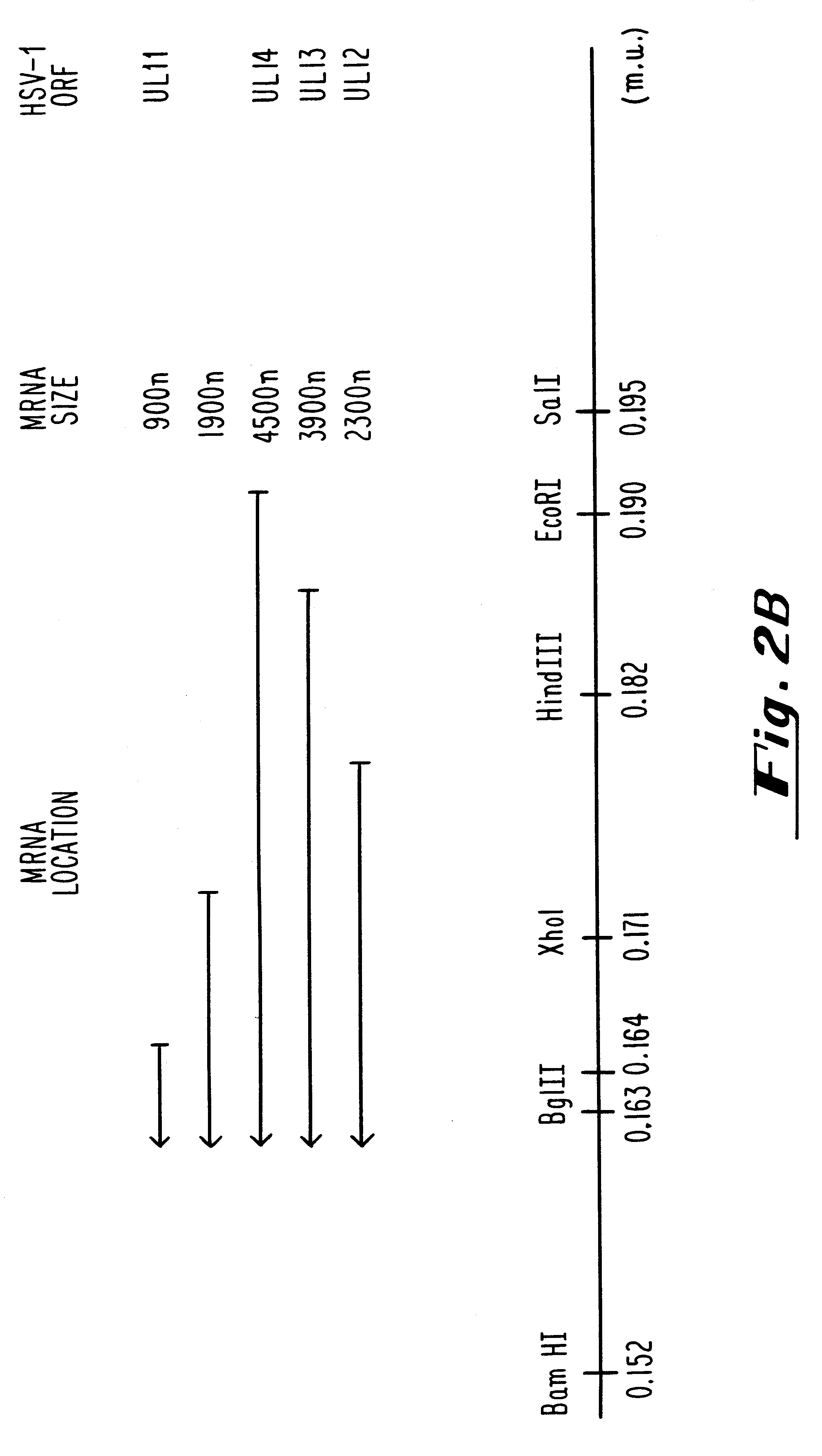
Patents
Literature
169 results about "Herpes simplex disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infection caused by the herpes simplex virus; affects the skin and nervous system; produces small temporary (but sometimes painful) blisters on the skin and mucous membranes.
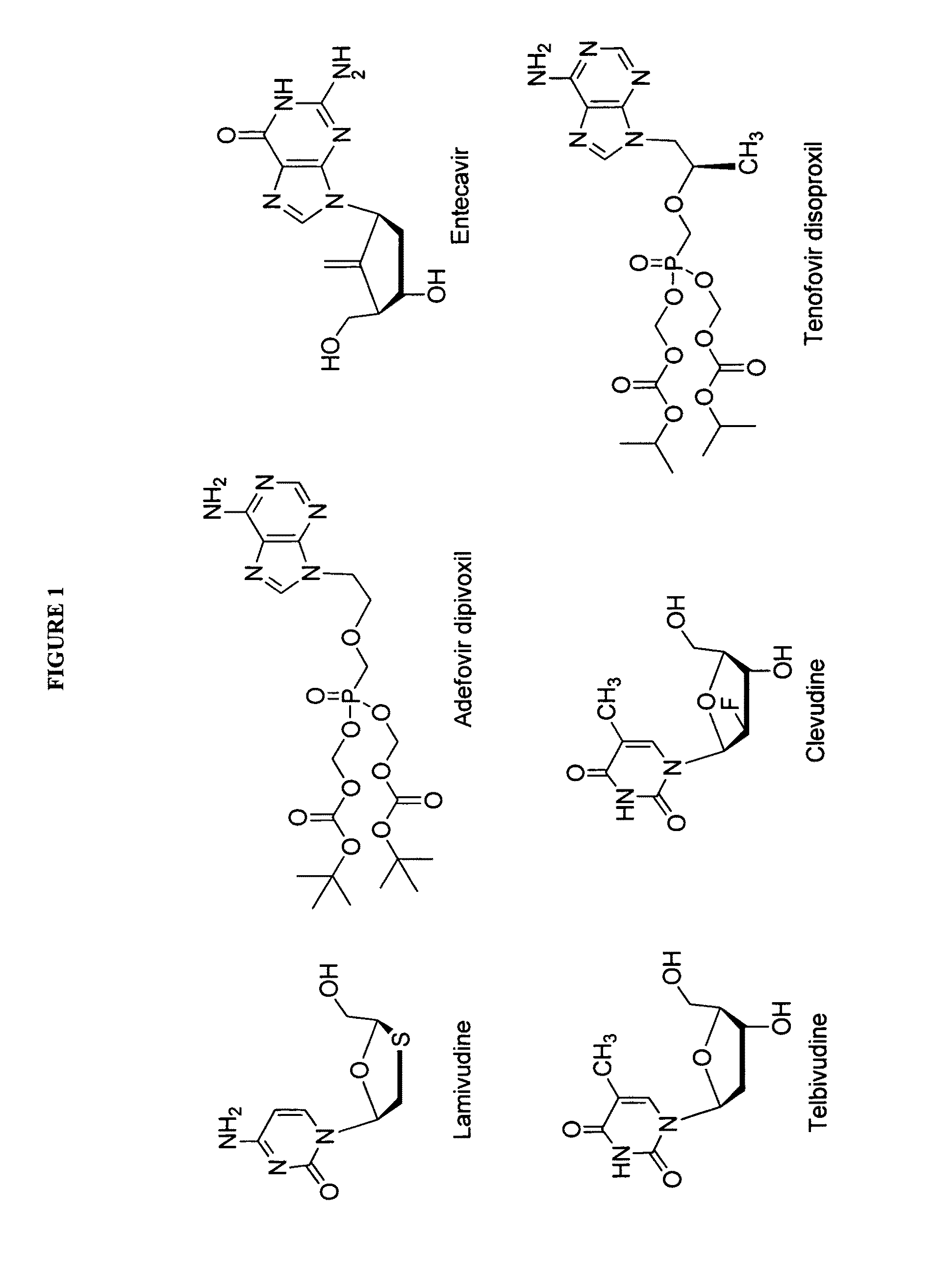
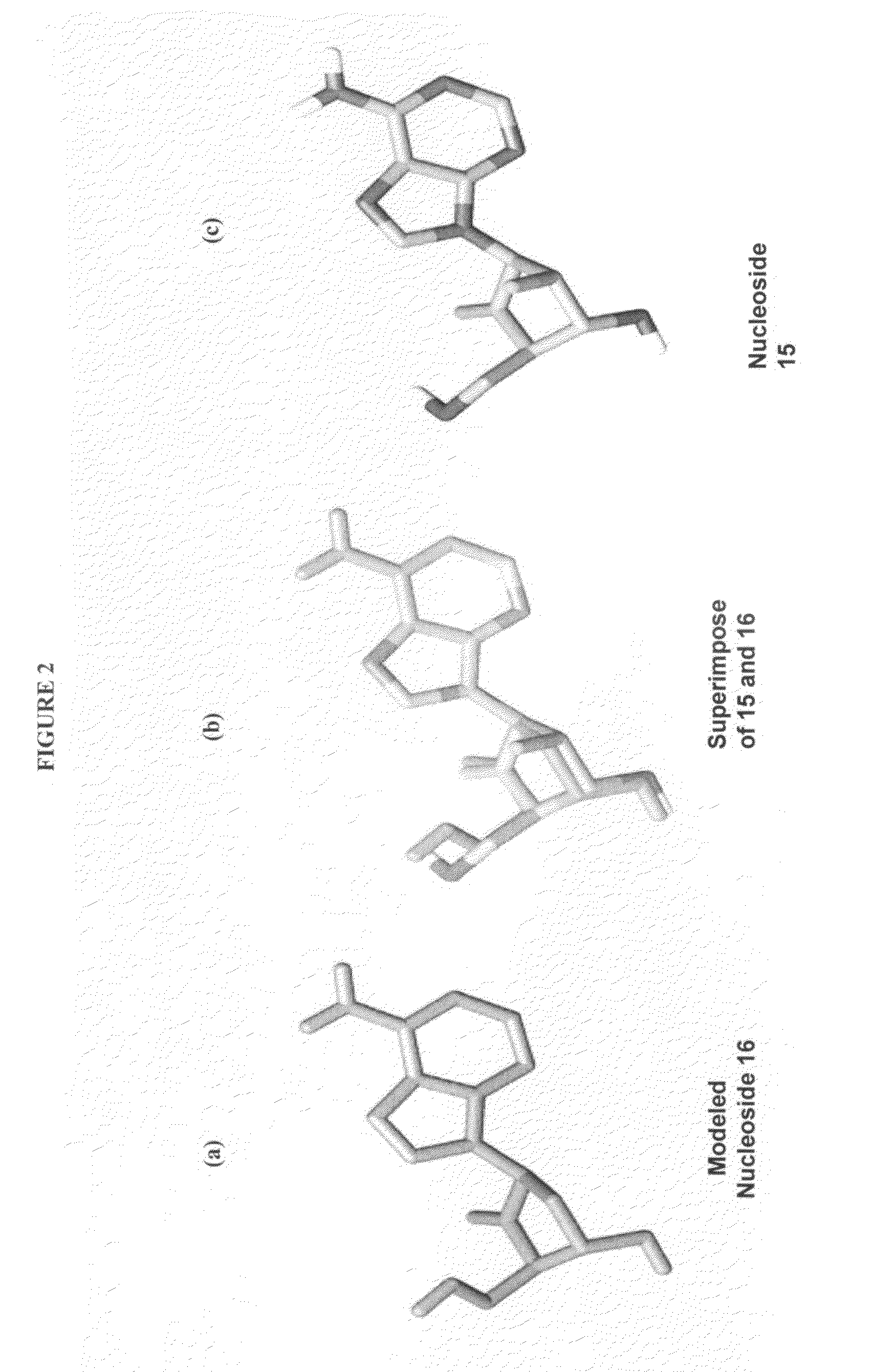
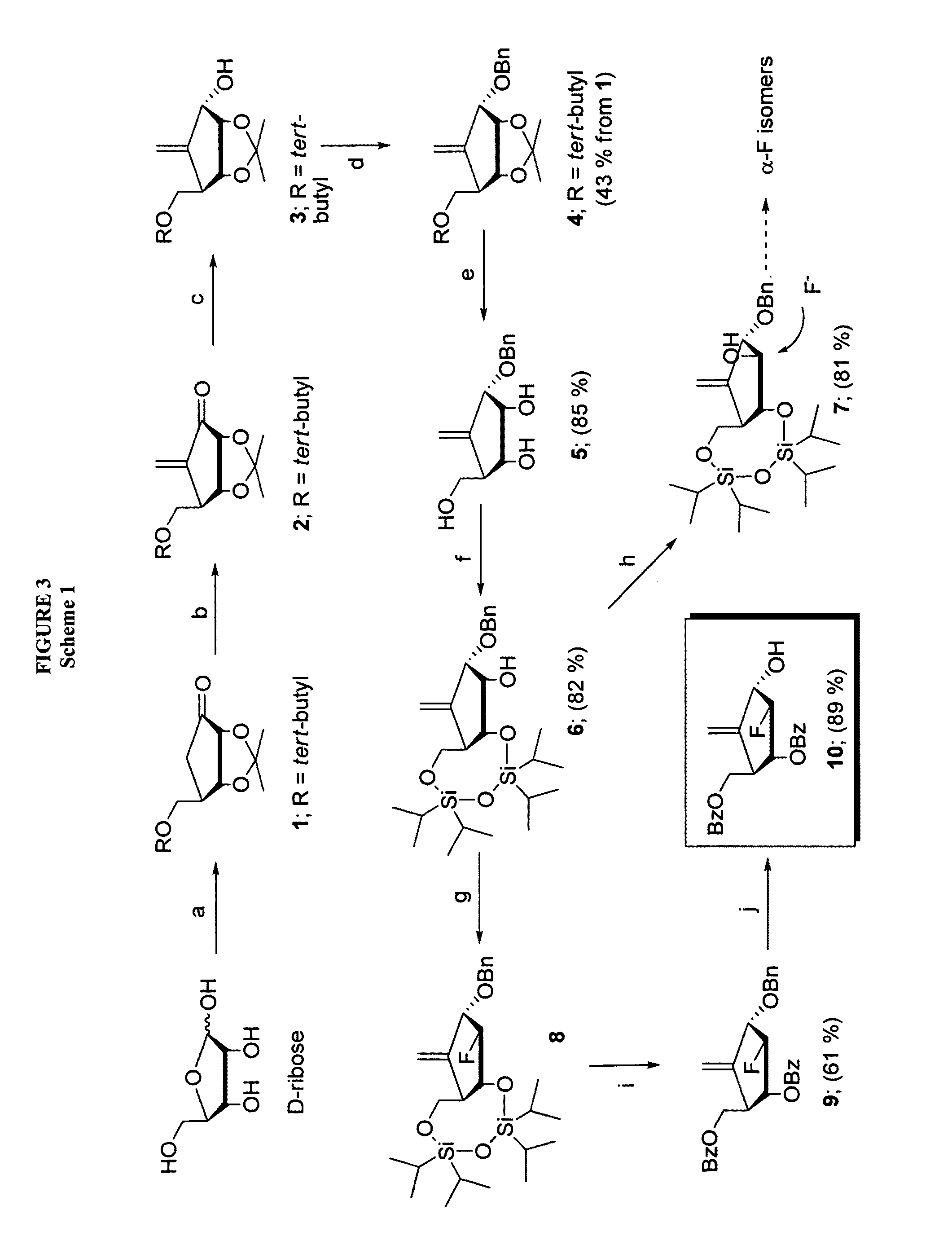
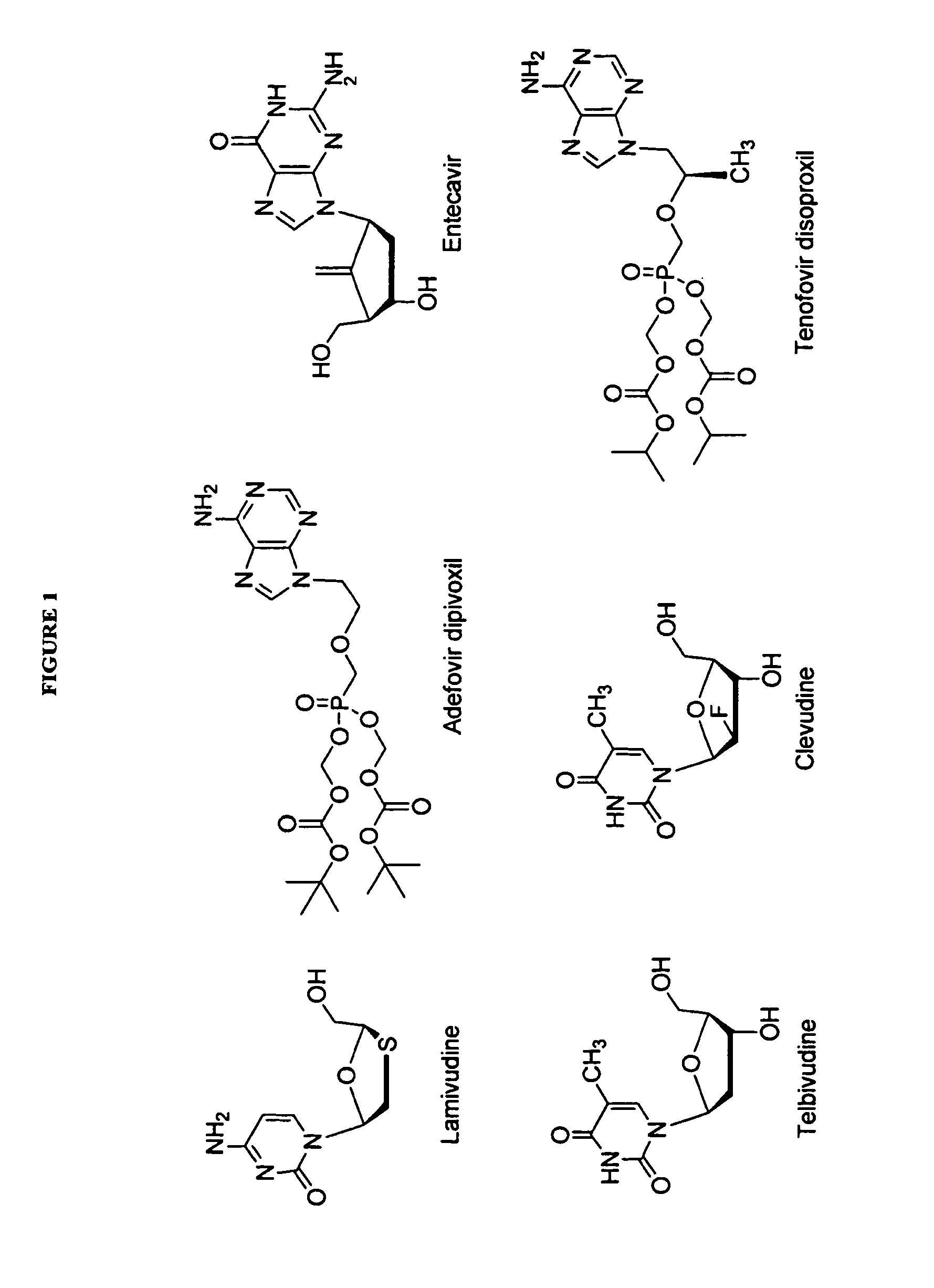
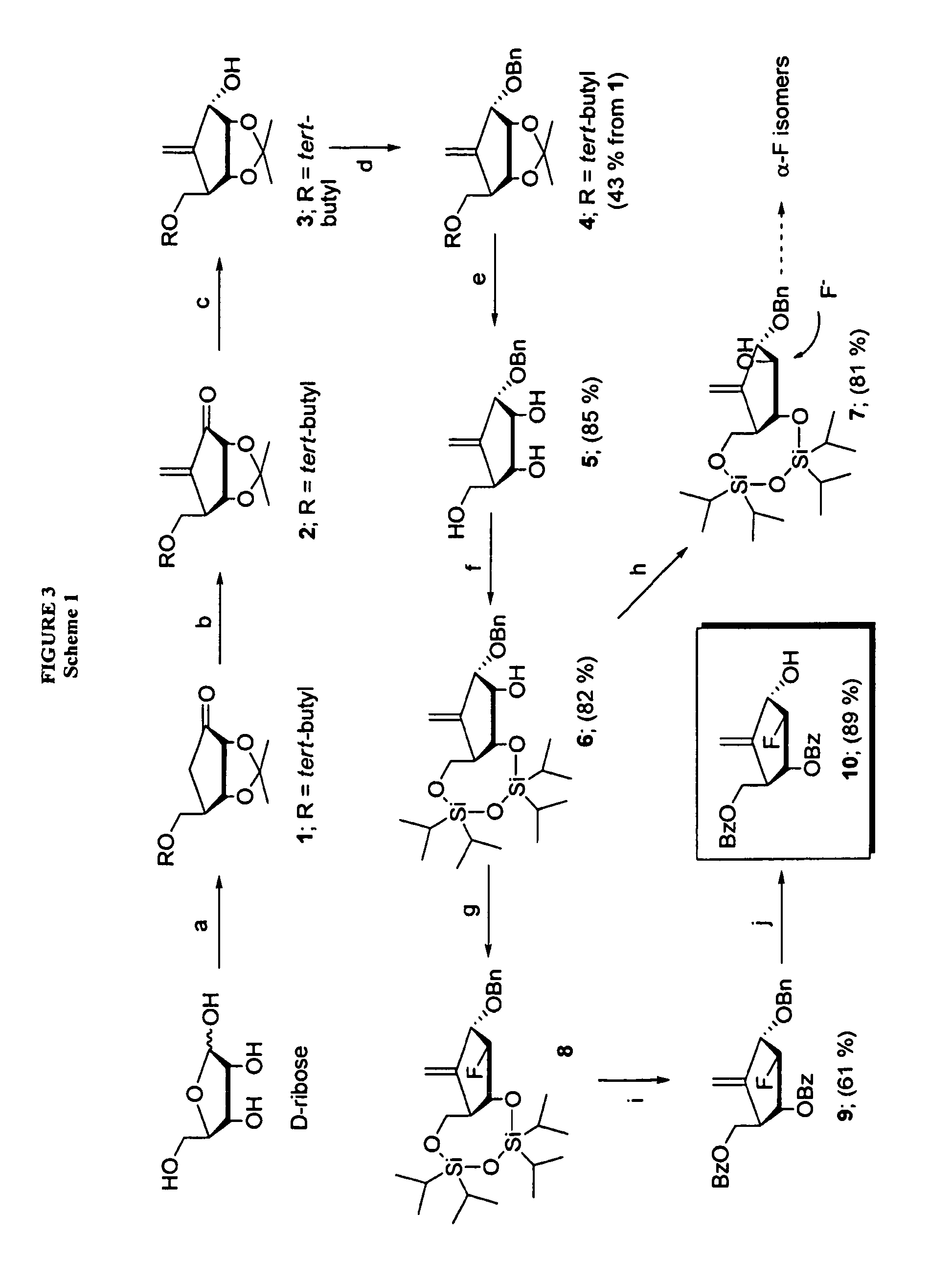
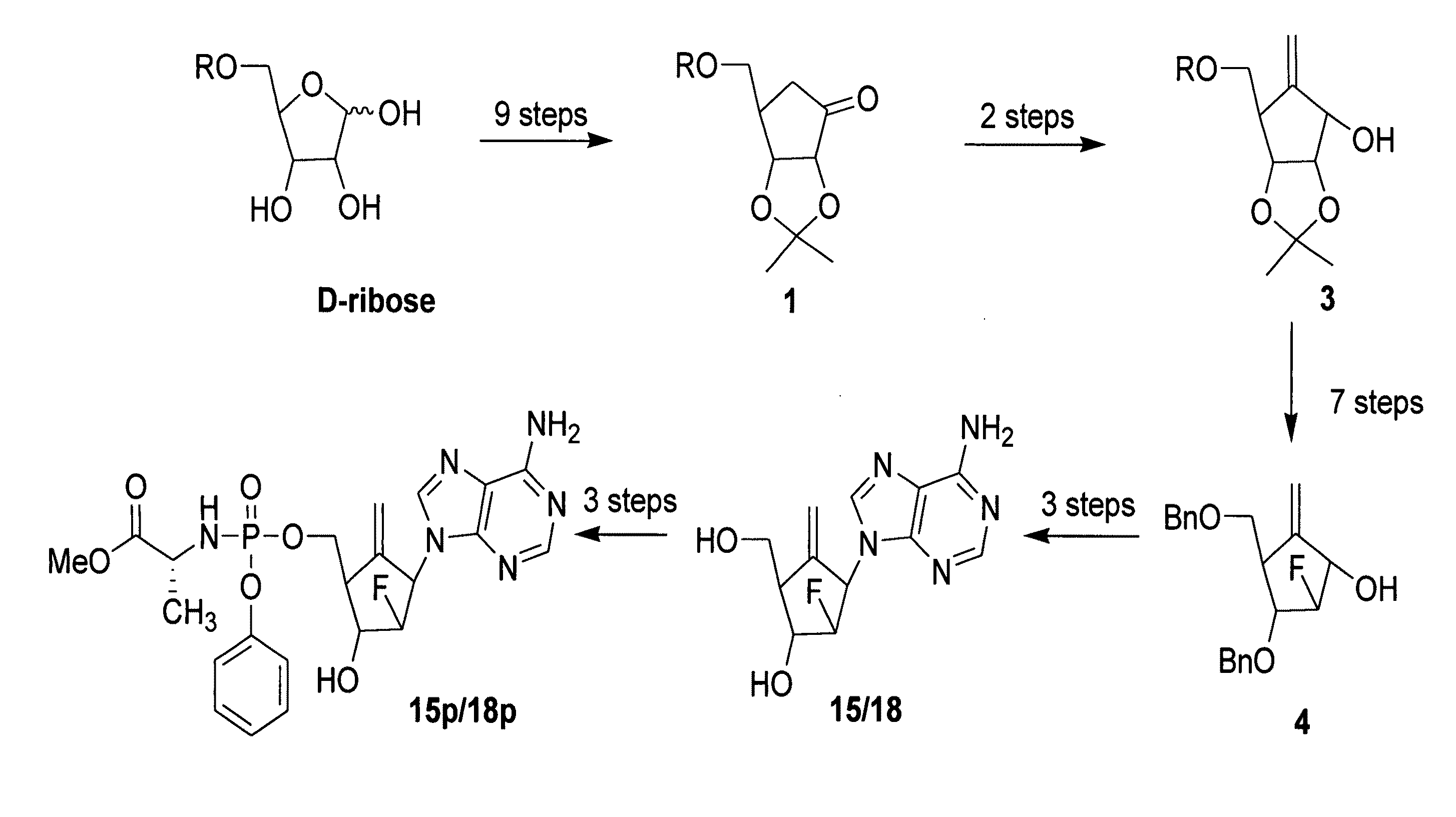
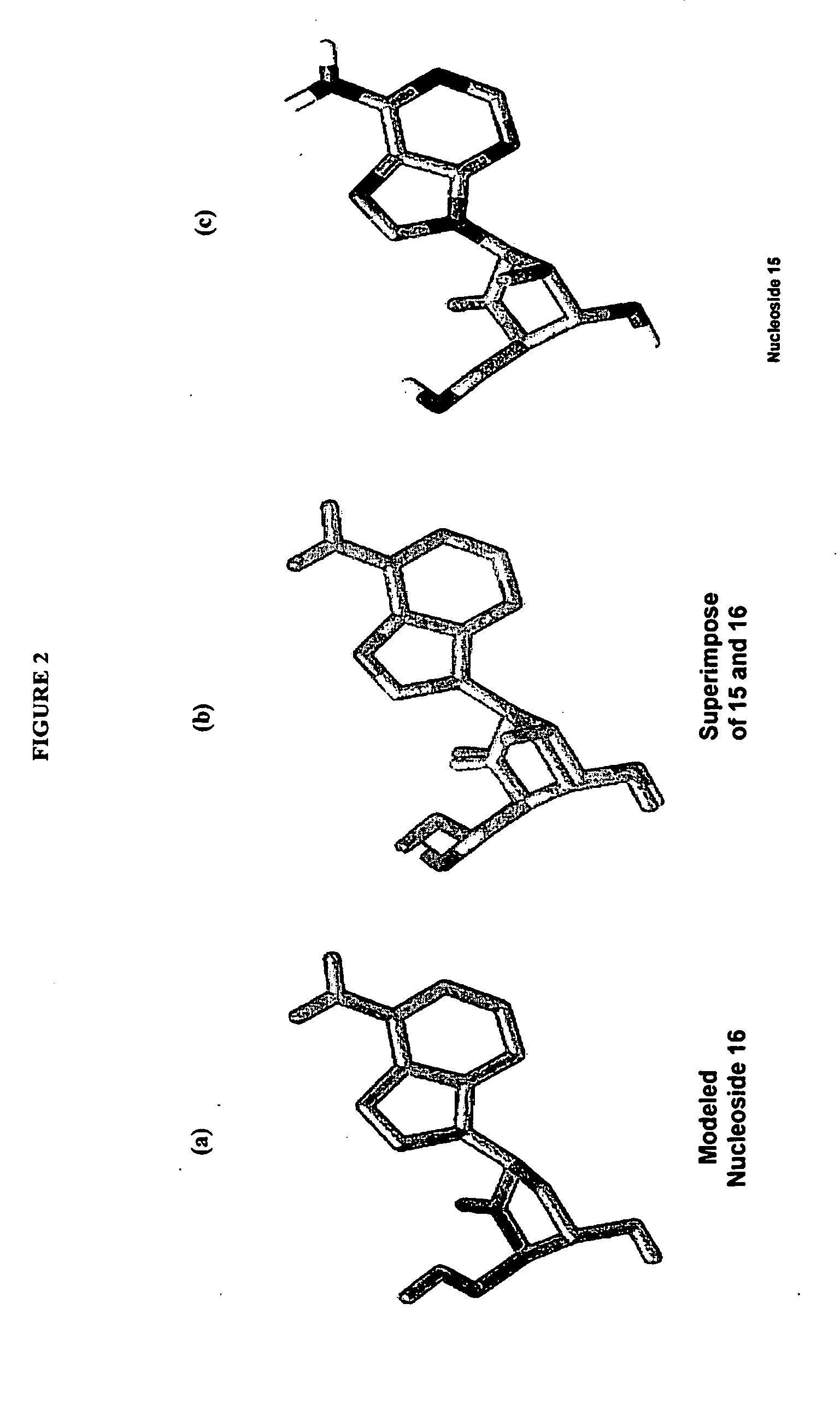
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
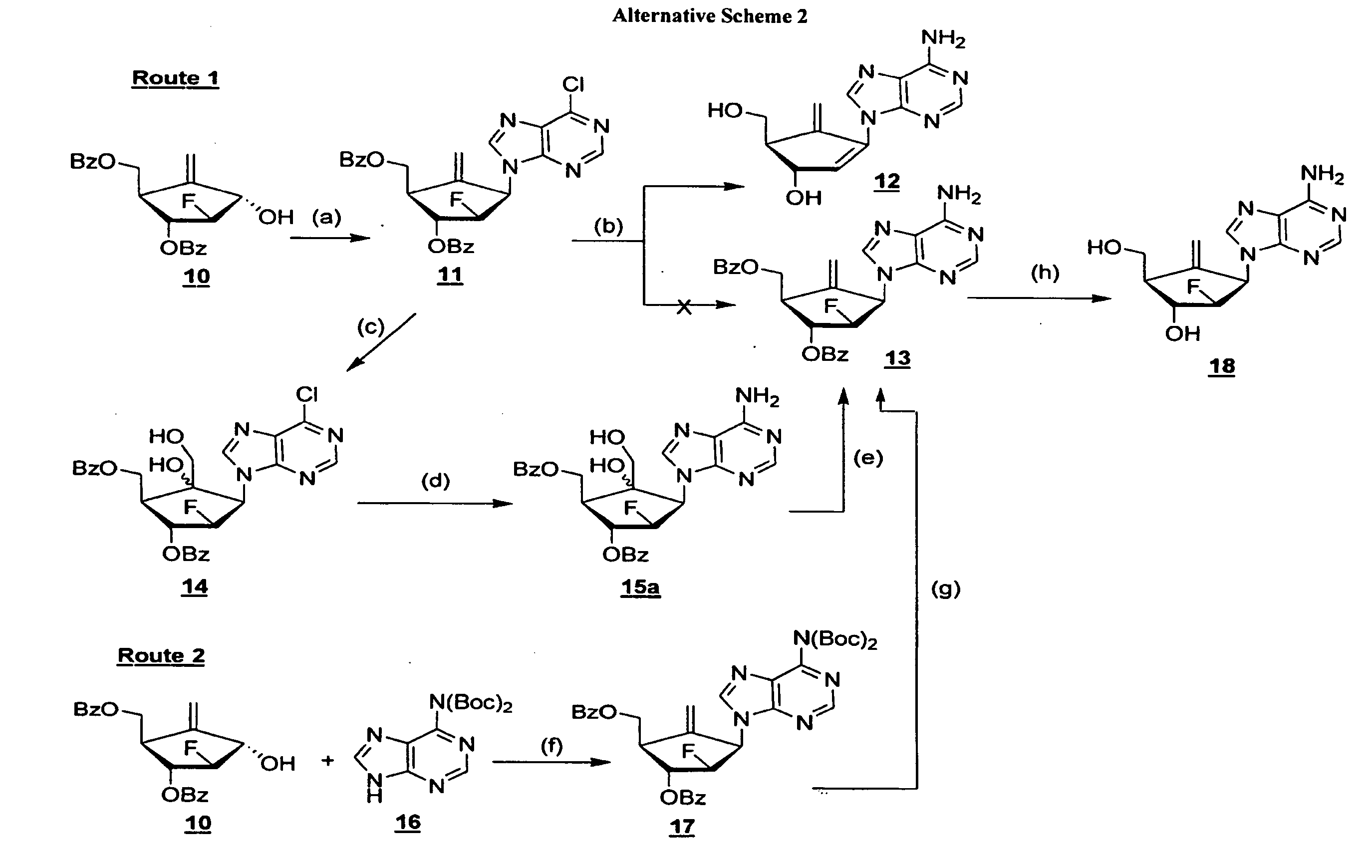
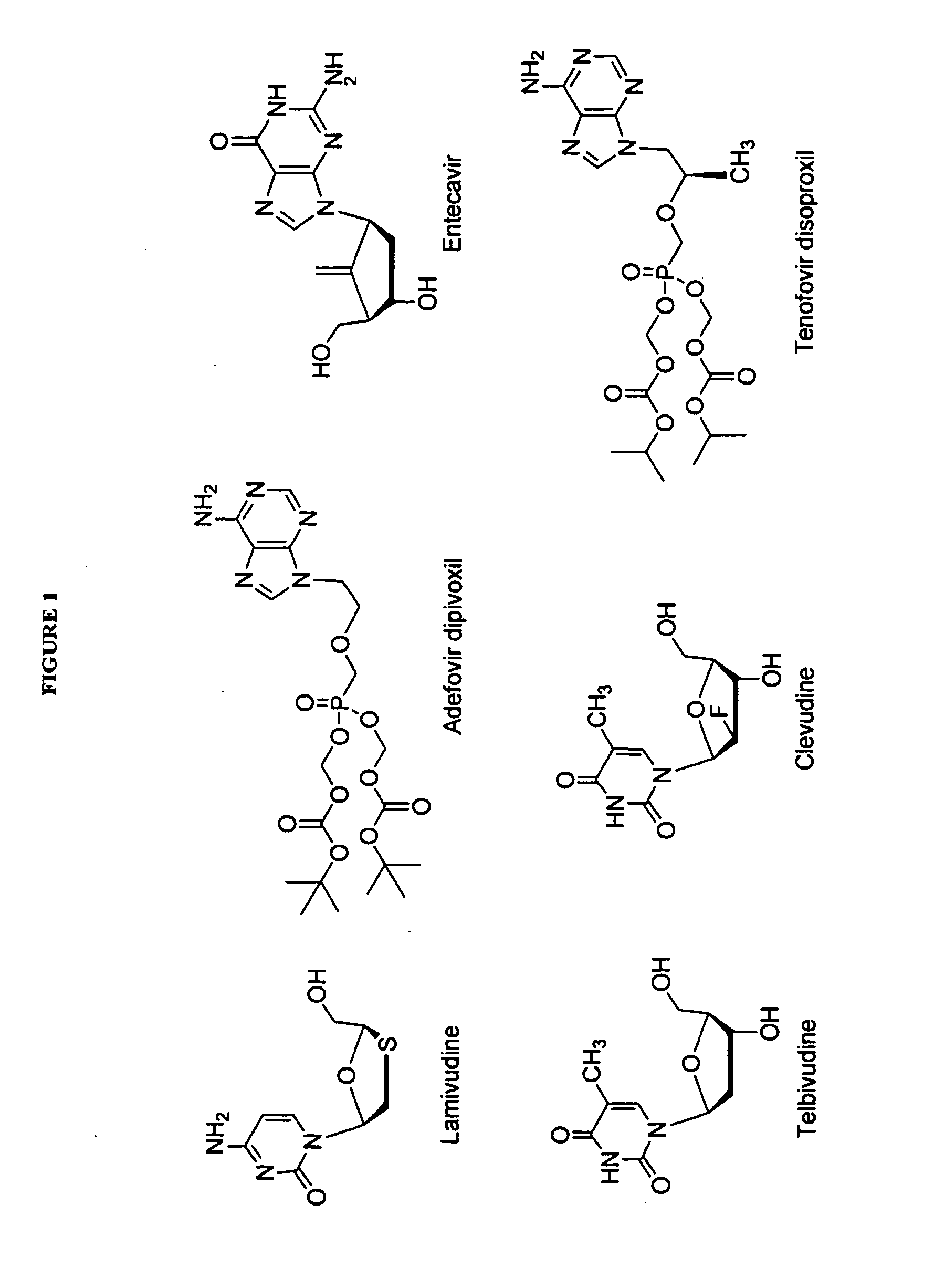
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
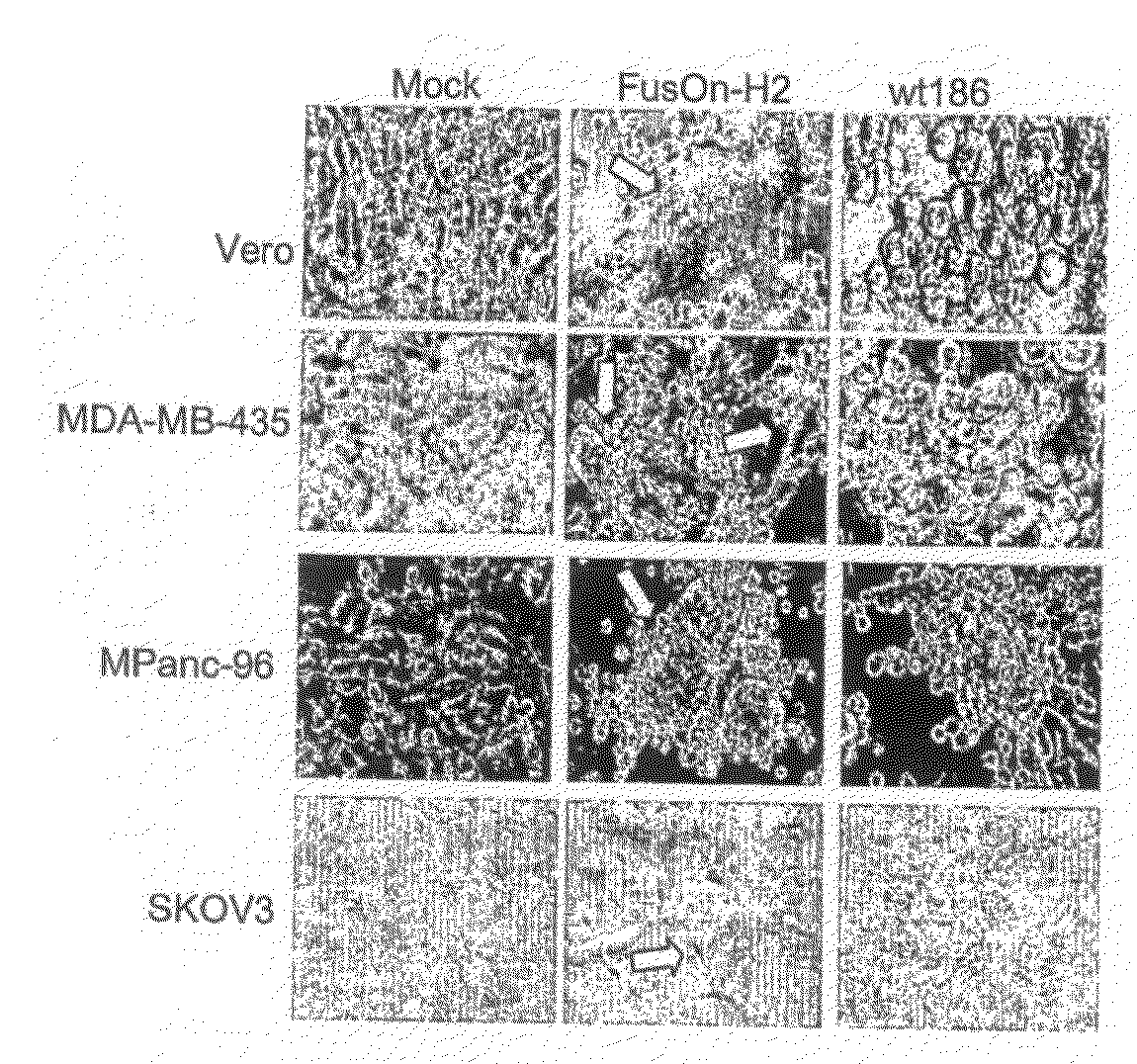
Use of Mutant Herpes Simplex Virus-2 for Cancer Therapy
ActiveUS20090215147A1Strong anti-tumor immune responseUseful in therapyPolypeptide with localisation/targeting motifVectorsNucleotideHerpes simplex virus DNA
Owner:UNIV HOUSTON SYST
2′-fluoro-6′methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS8946244B2Reduce the possibilityBiocideSugar derivativesHerpes zoster virusNasopharyngeal cancer
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Vaccines against herpes simplex virus type 2: compositions and methods for eliciting an immune response
ActiveUS20100330112A1Avoid symptomsReduce in quantityViral antigen ingredientsDsDNA virusesImmunogenicityHerpes simplex disease
Herpes Simplex Virus-2 (HSV-2) infection is a major health concern. The present disclosure provides, inter alia, certain highly effective vaccines and immunogenic compositions against HSV-2. The antigens can be used therapeutically or prophylactically.
Owner:RUNWAY ADVISORS LLC
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
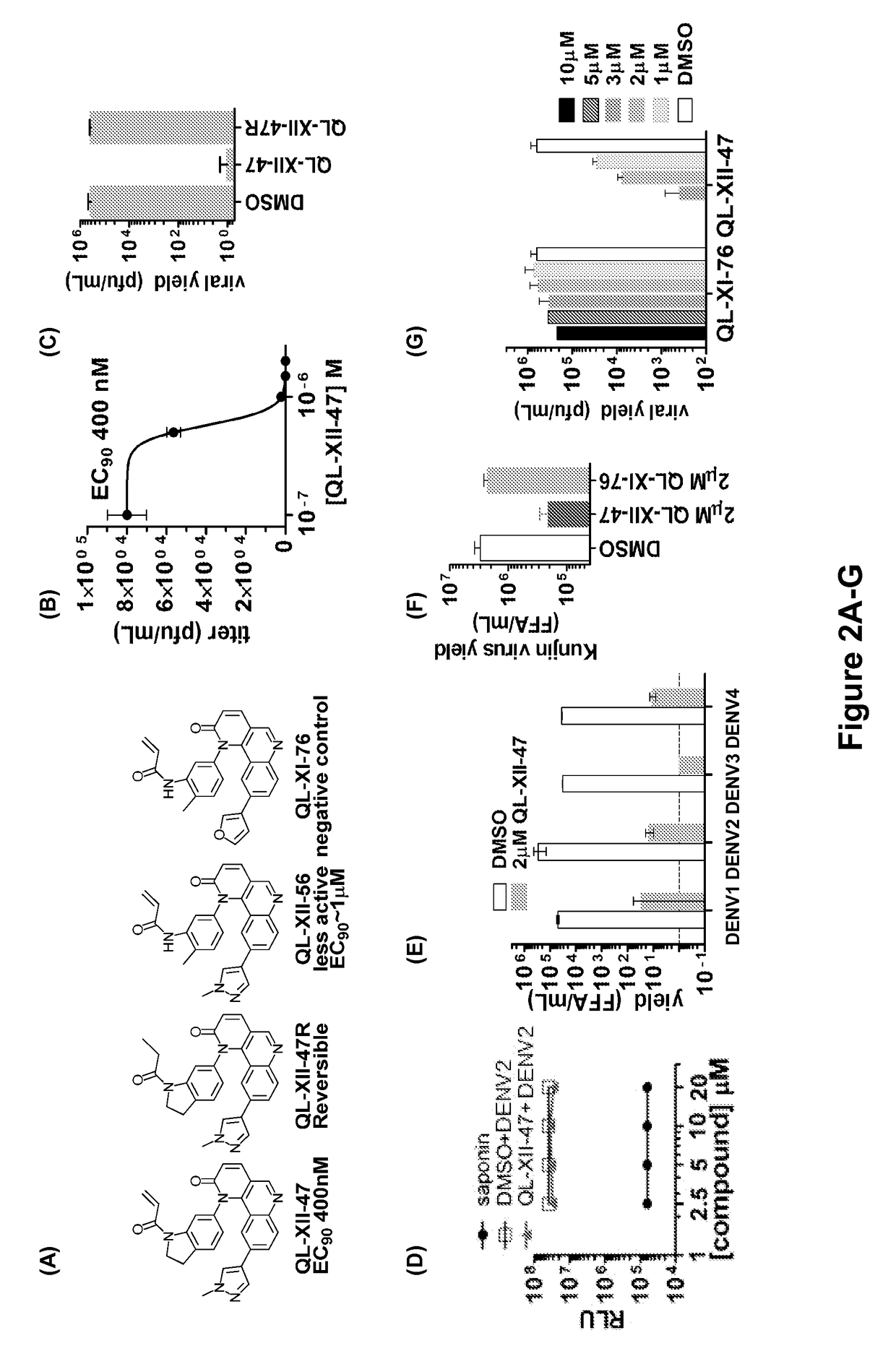
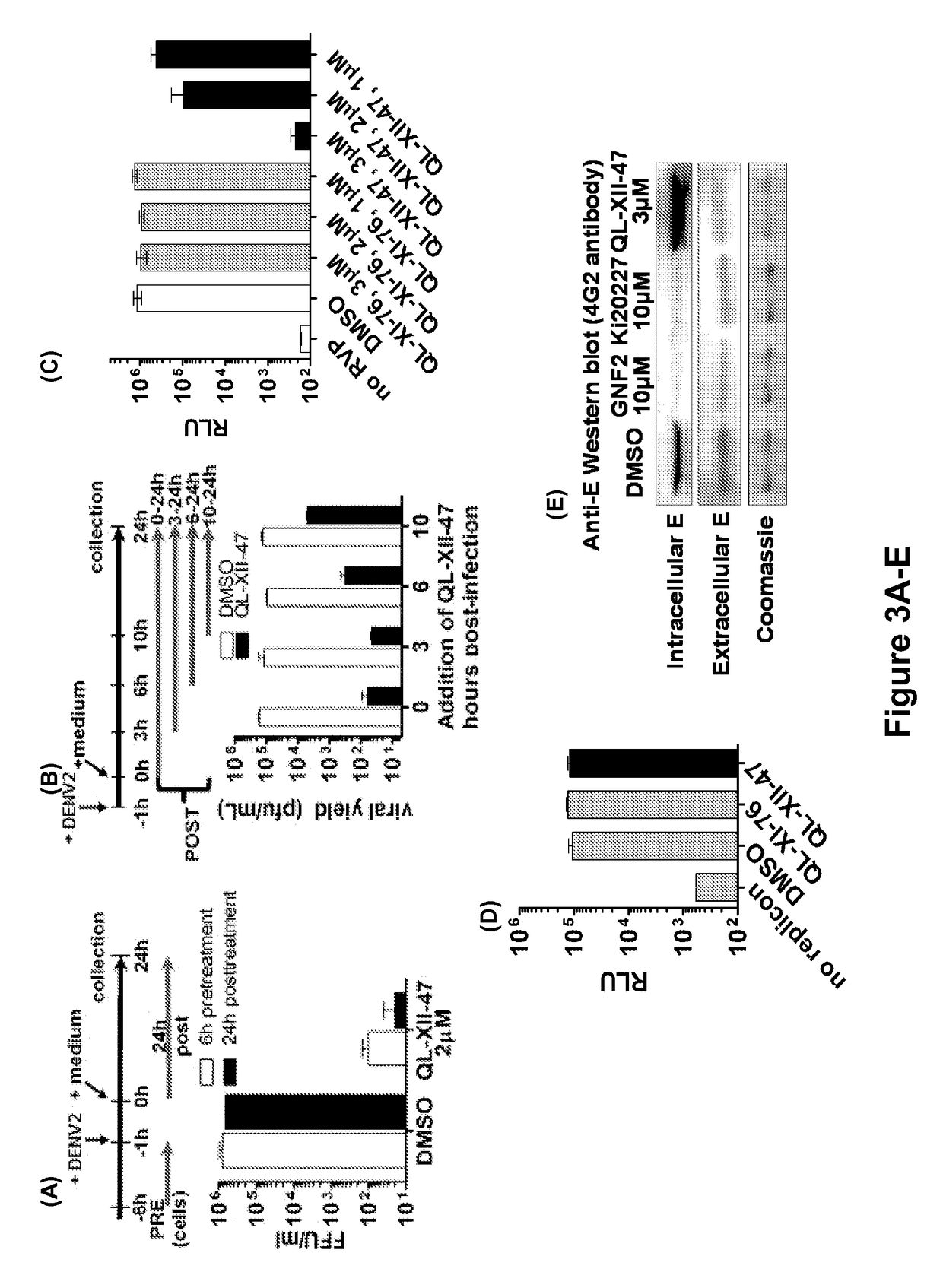
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
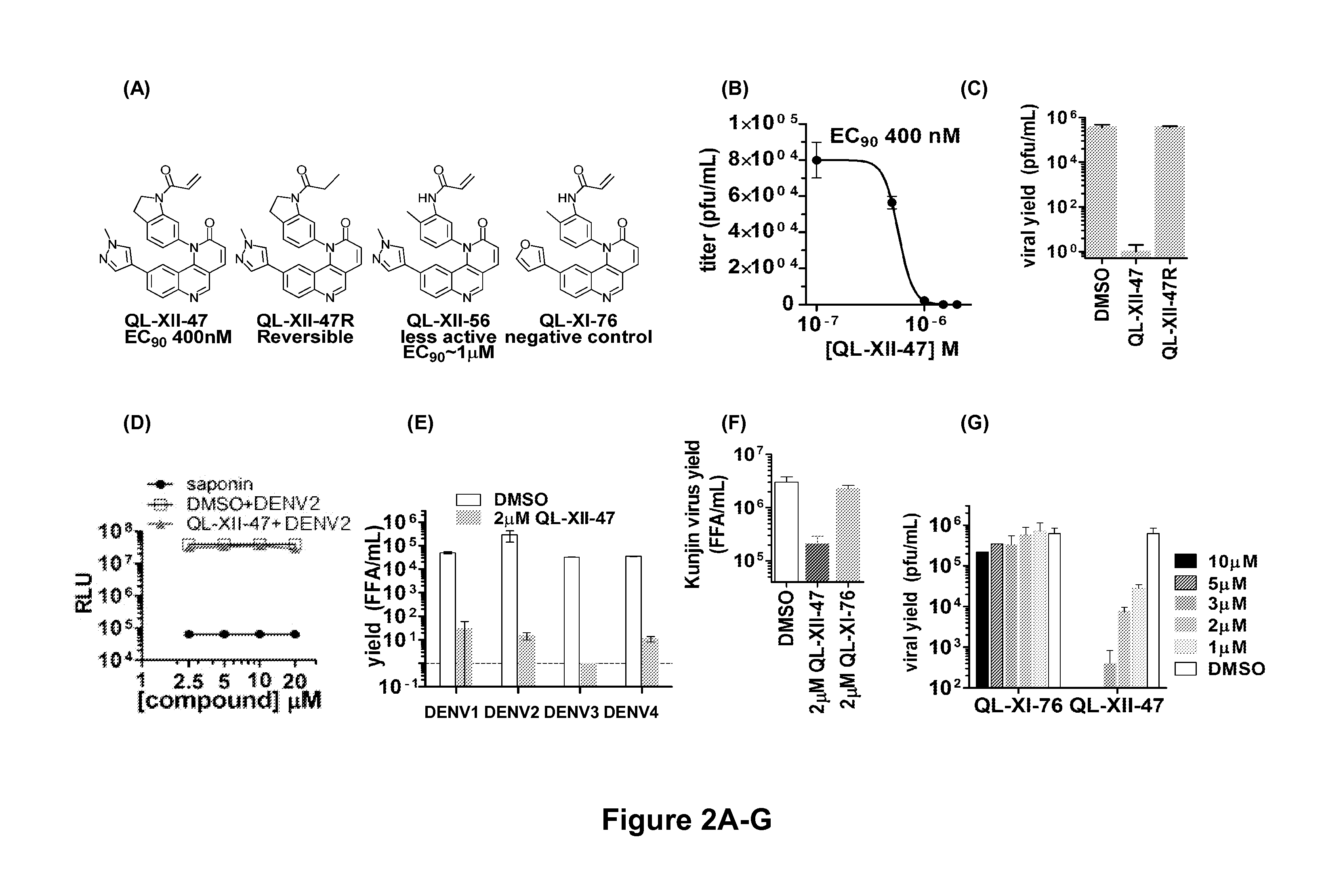
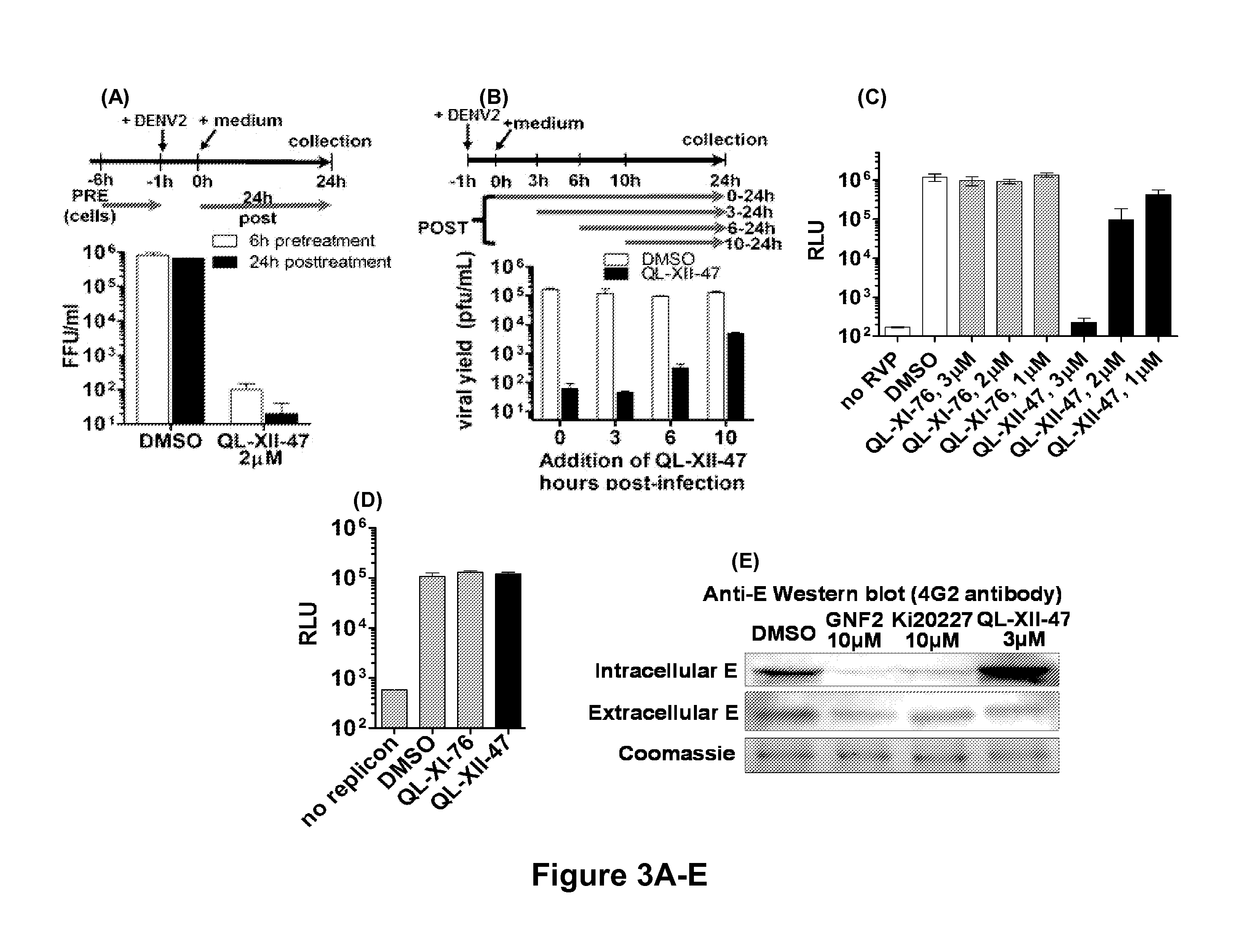
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
Preparation method for blattodea polypeptide substance, and medical use of blattodea polypeptide substance in anti-herpesvirus
InactiveCN102743739AHigh yieldThe source is easy to getAnthropod material medical ingredientsPeptide/protein ingredientsDiseaseChemical products
The present invention relates to a preparation method for a blattodea polypeptide substance, and a medical use of the blattodea polypeptide substance in anti-herpesvirus. Specifically the present invention relates to a blattodea extract effective fraction having a function of prevention and treatment of herpes simplex virus infection diseases, a preparation method and a medical use thereof. The Periplaneta Americana extract effective fraction of the present invention is a polypeptide substance with a molecular weight less than 5000 dalton, and is prepared by the following steps: carrying out extraction on fresh polypide or dried polypide of Periplaneta Americana with water or an organic solvent and a buffer, and then carrying out a membrane separation method to refine to obtain the product of the present invention, wherein the polypeptide active substance of the effective fraction has significant anti-herpes simplex virus (HSV-1 and HSV-2) activities, can be prepared into forms of a hydrogel, a cataplasm, lyophilized powder, a water agent, an aerosol, a suppository, a film agent, an external application liniment, and an ointment, and can be used for preparations of drugs for prevention and treatment of herpes simplex virus infections, daily chemical products or medical devices.
Owner:DALI UNIV
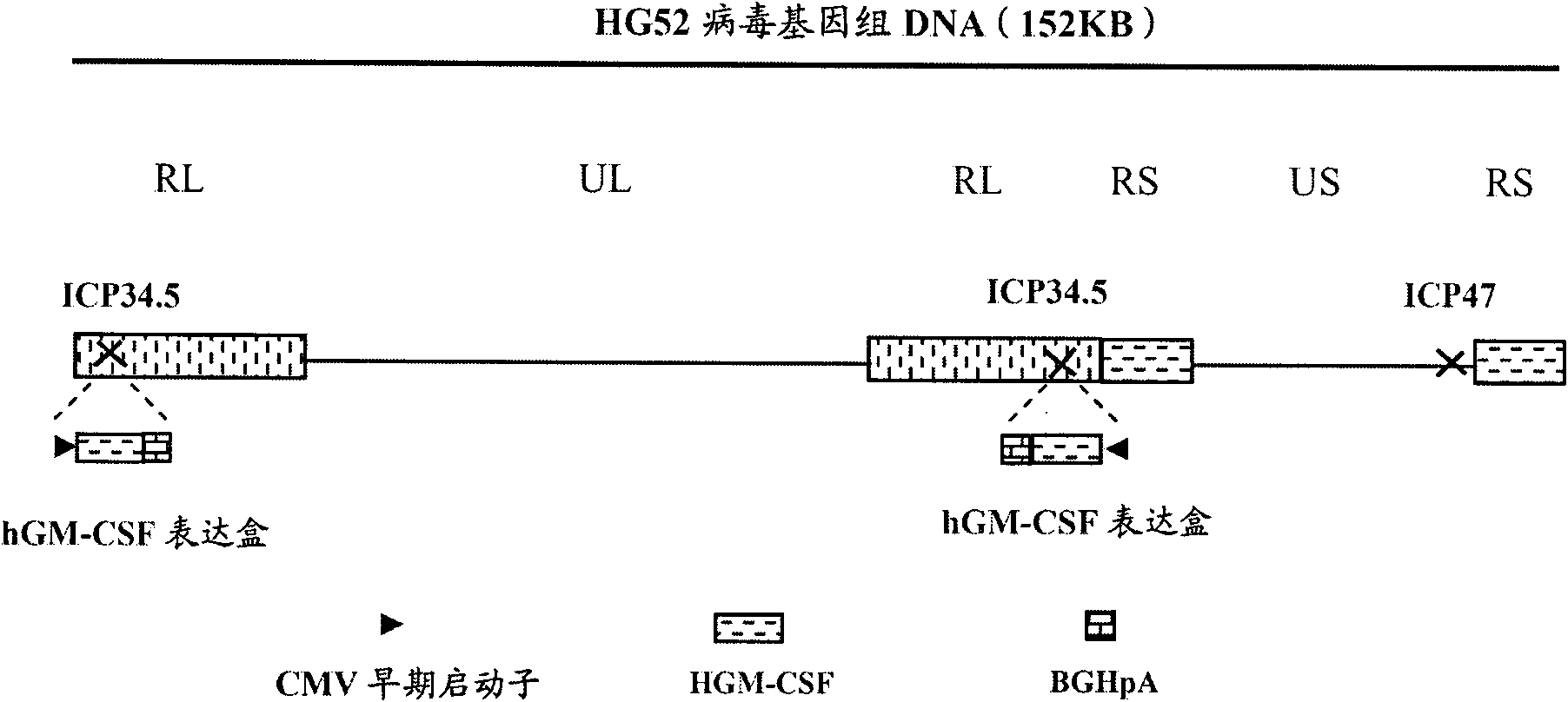
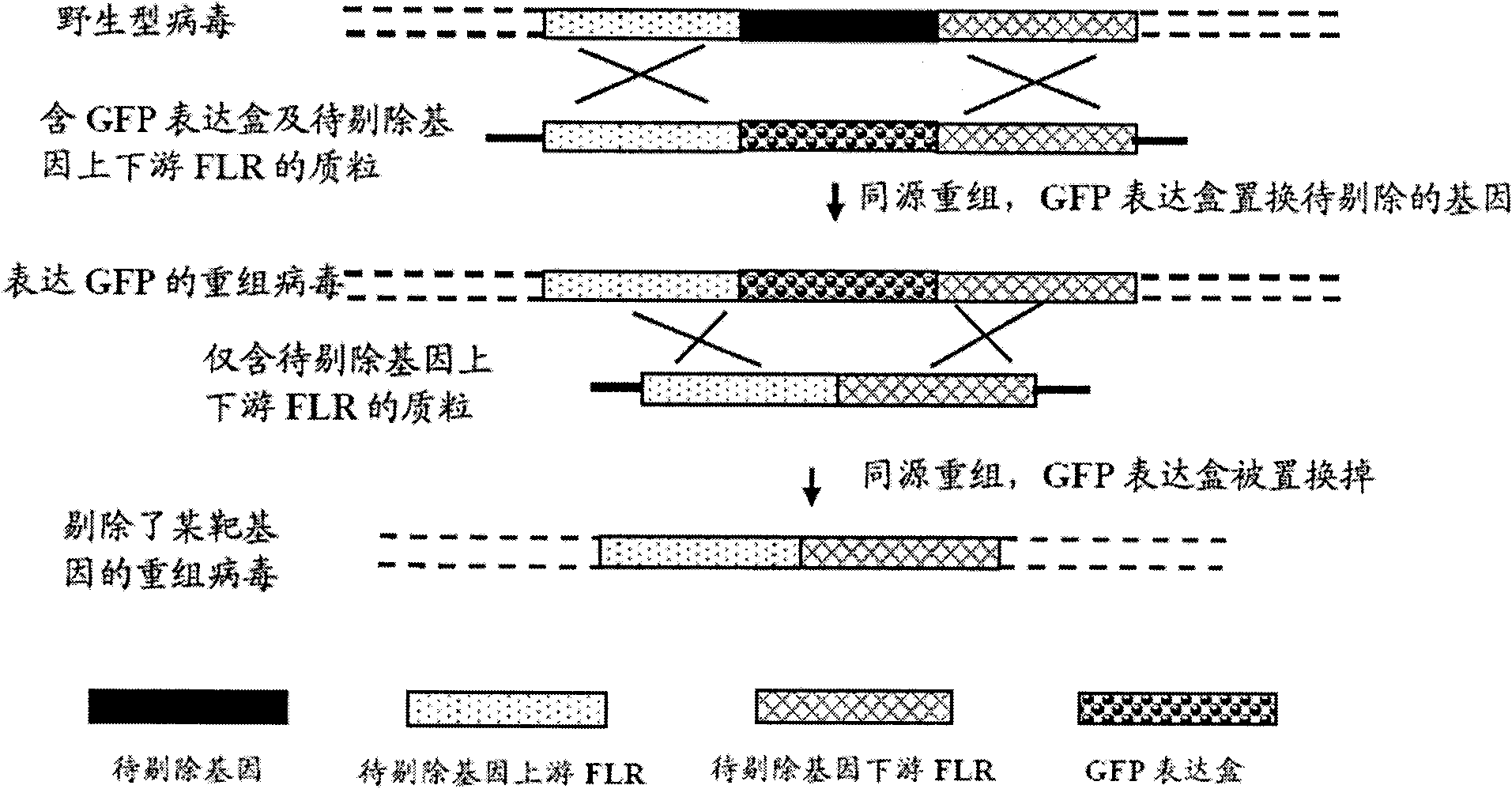
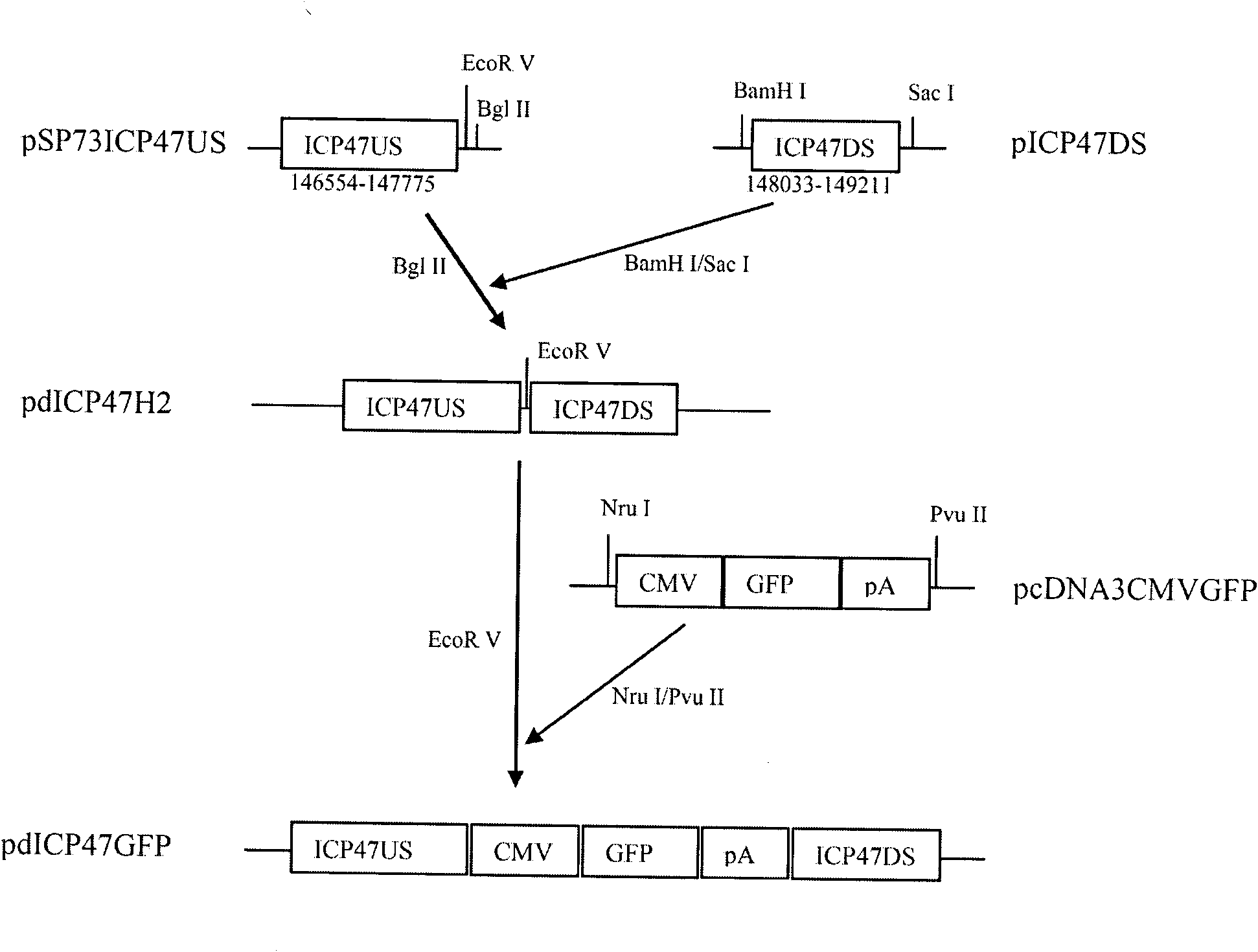
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
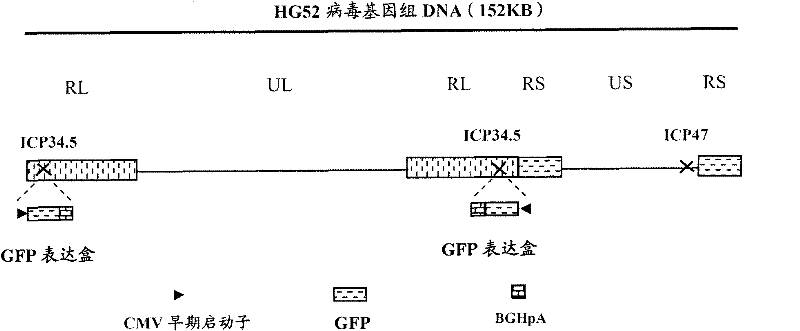
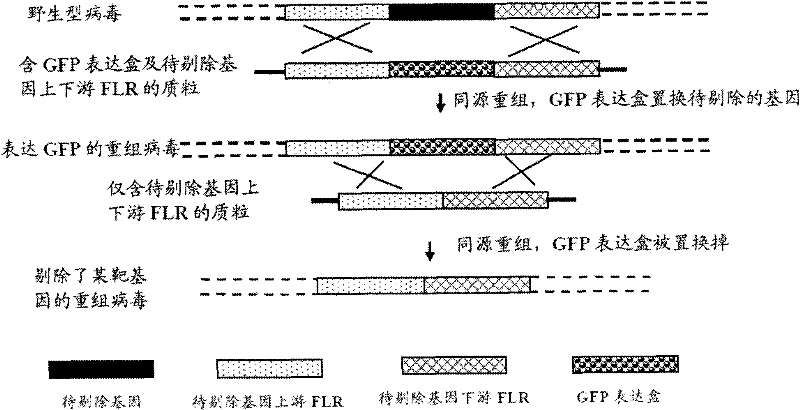
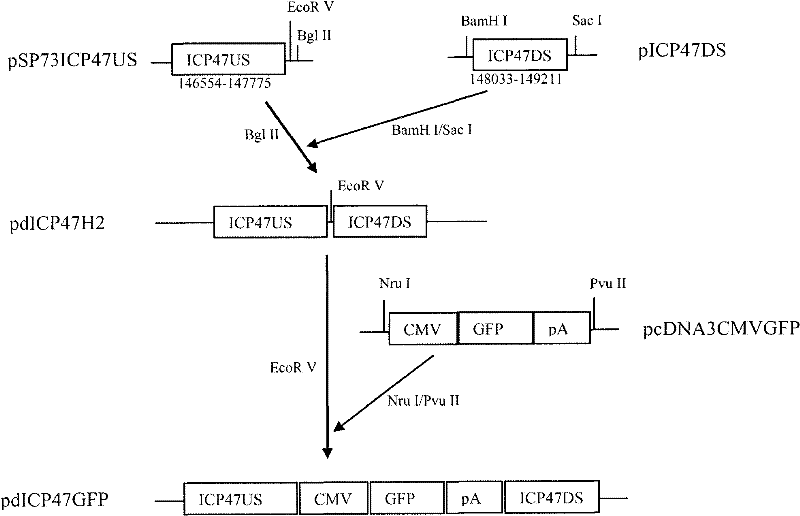
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
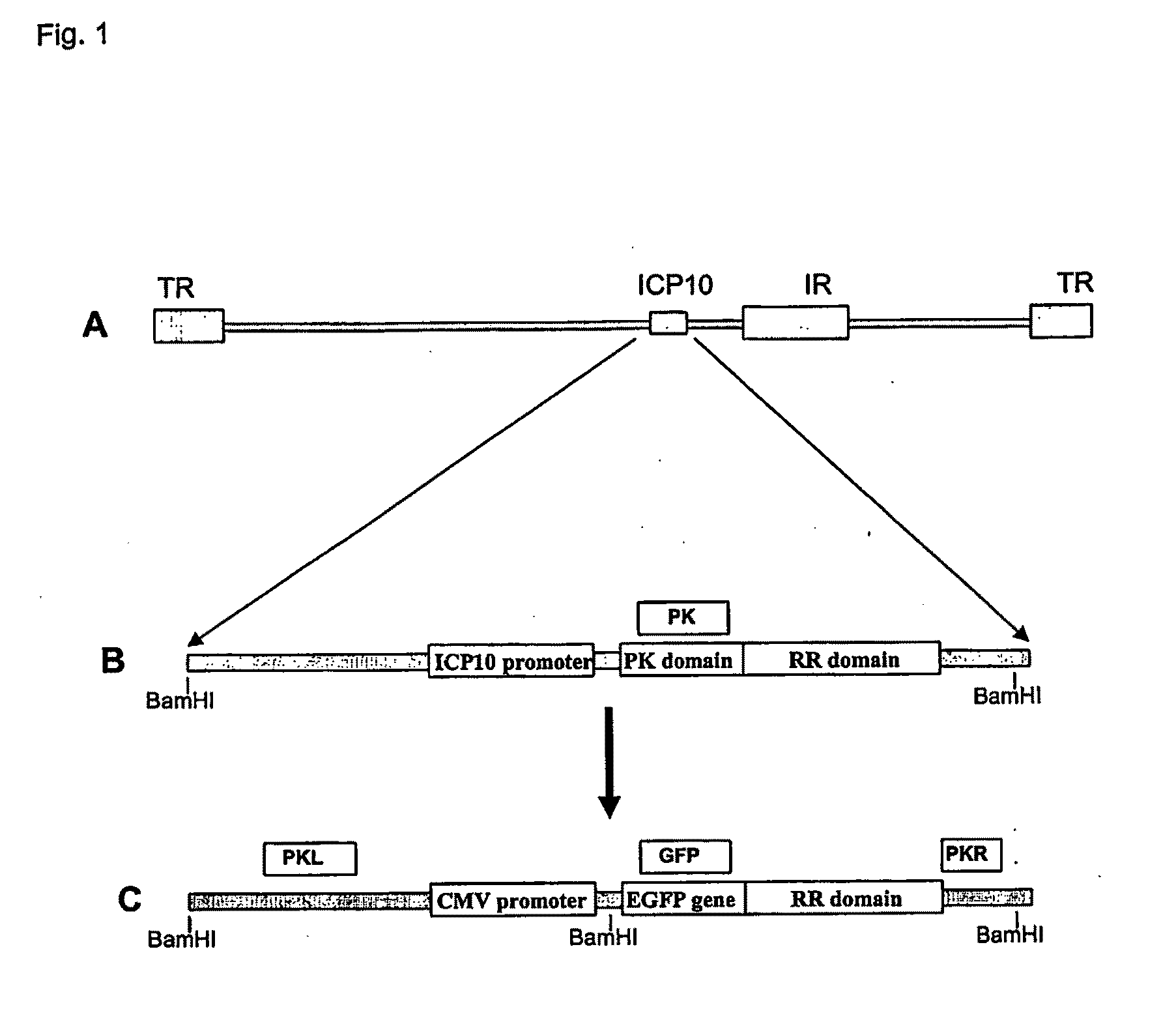
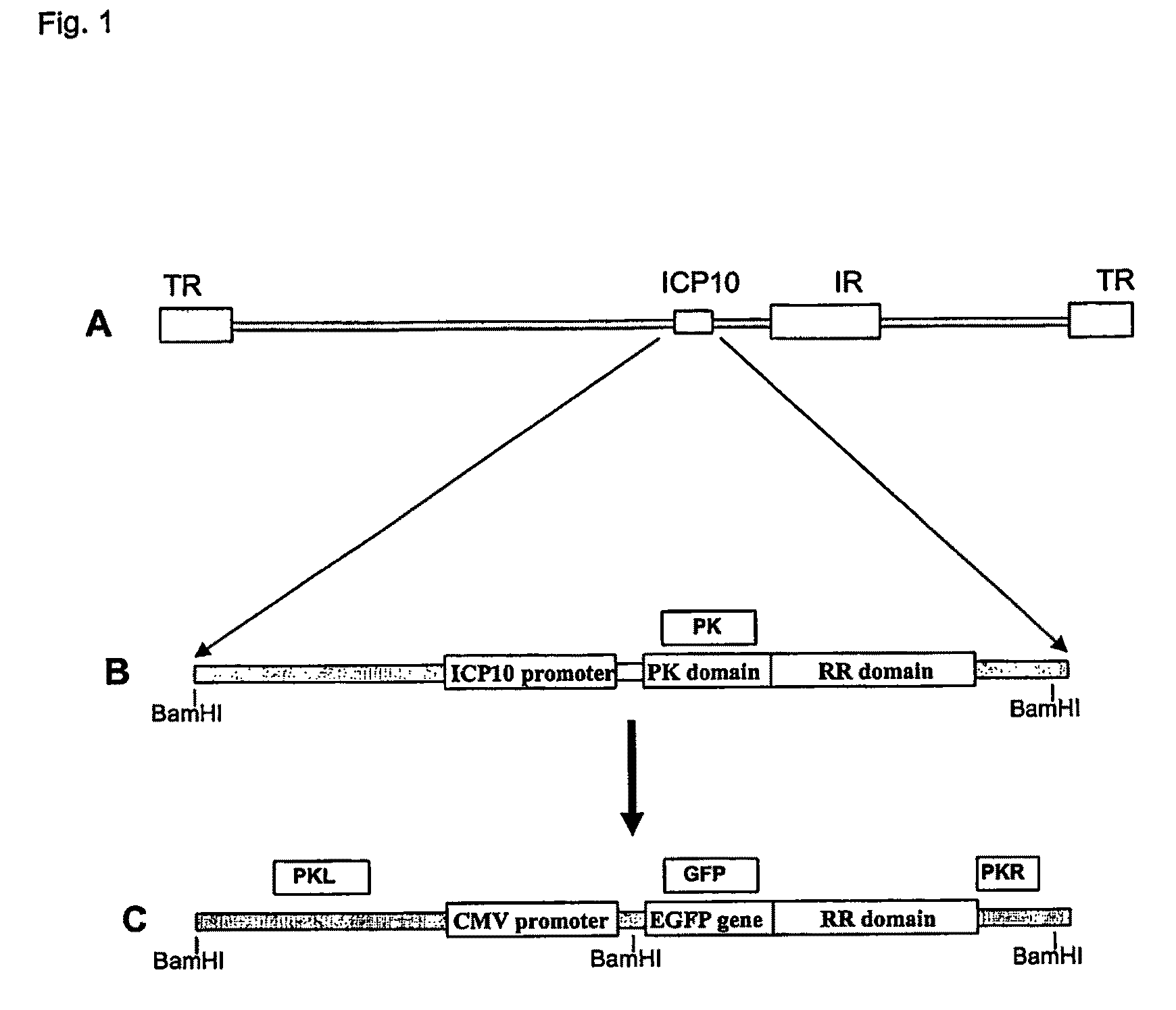
Protein kinase domain of the large subunit of herpes simplex type 2 ribonucleotide reductase (icp 10pk) has anti-apoptotic activity
The invention relates to a method of treating neuronal apoptosis in a mammal using nucleic acid encoding HSV-2 ICP10PK, or a polypeptide encoded thereby. The invention further relates to a method of treating neuronal apoptosis in a mammal using ICP10PK in combination with a nucleic acid encoding bcl-2, or the polypeptide encoded thereby. The invention also relates to the use of ICP10PK and ICP10PK in combination with bcl-2 to treat non-neuronal diseases characterized by apoptosis.
Owner:UNIV OF MARYLAND BALTIMORE +1
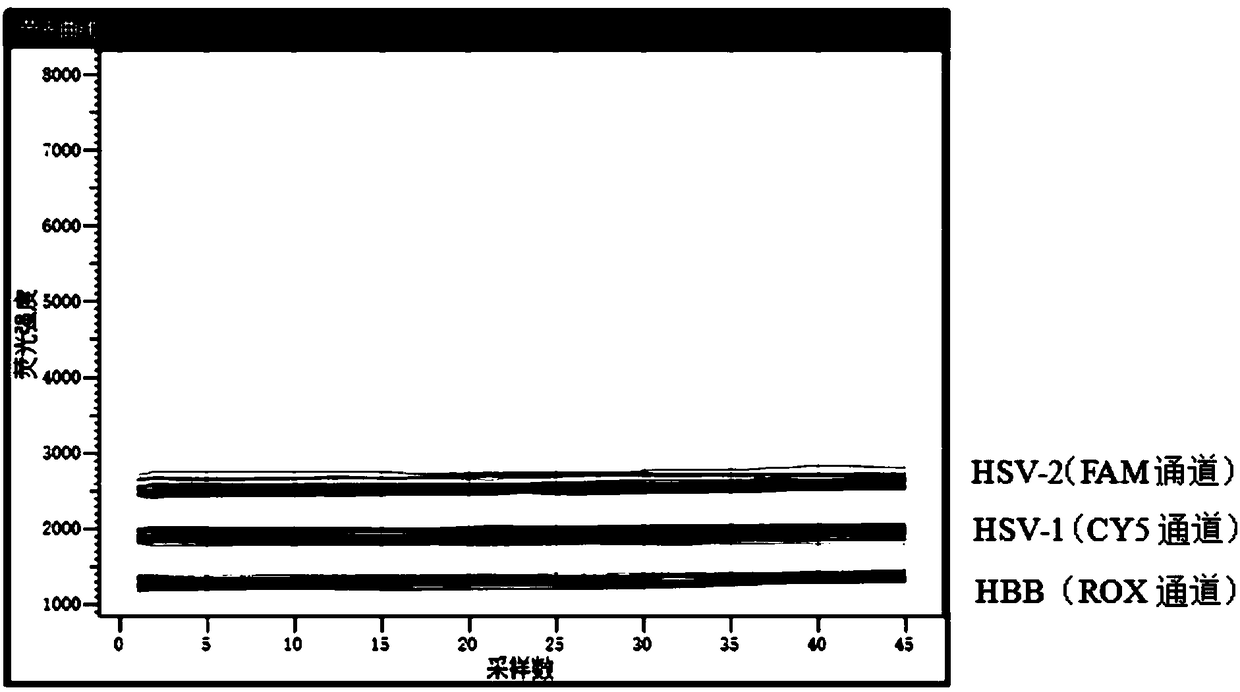
Herpes virus type I PCR (polymerase chain reaction) fluorescence quantitative rapid test kit and method
ActiveCN102337355AGuaranteed credibilityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesReference productFluorescence
The invention aims at providing a herpes virus type I PCR (polymerase chain reaction) fluorescence quantitative rapid test kit which can detect the specific nucleic acid sequence of pure herpes virus type I in a clinical sample and further achieve the purpose of rapidly judging the existence of the pure herpes virus type I. In order to realize the purpose, the technical scheme of the invention isas follows: the herpes virus type I PCR fluorescence quantitative rapid test kit provided by the invention comprises a DNA (deoxyribonucleic acid) extraction solution, a PCR reaction solution, a DNA polymerase, a positive quality control product, a weak positive quality control product, a negative quality control product and a quantitative reference product, wherein the PCR reaction solution comprises primers and a fluorescent probe, and the primers are divided into an upstream primer and a downstream primer. The herpes virus type I PCR fluorescence quantitative rapid test kit has the beneficial effect of filling in the blank of a fluorescence PCR kit for clinically detecting the pure herpes virus type I (HSV I) in China. Furthermore, a Taqman core technology platform and an arabidopsis thaliana internal control system are utilized for detecting the pure herpes virus type I (HSV I), so that the herpes virus type I PCR fluorescence quantitative rapid test kit has the advantages of highsensitivity, high specificity, stability, timeliness, convenience in operation and the like.
Owner:泰普生物科学(中国)有限公司
Vaccines against herpes simplex virus type 2: compositions and methods for eliciting an immune response
ActiveUS8617564B2Avoid symptomsReduce in quantityViral antigen ingredientsDsDNA virusesImmunogenicityHerpes simplex disease
Herpes Simplex Virus-2 (HSV-2) infection is a major health concern. The present disclosure provides, inter alia, certain highly effective vaccines and immunogenic compositions against HSV-2. The antigens can be used therapeutically or prophylactically.
Owner:RUNWAY ADVISORS LLC
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
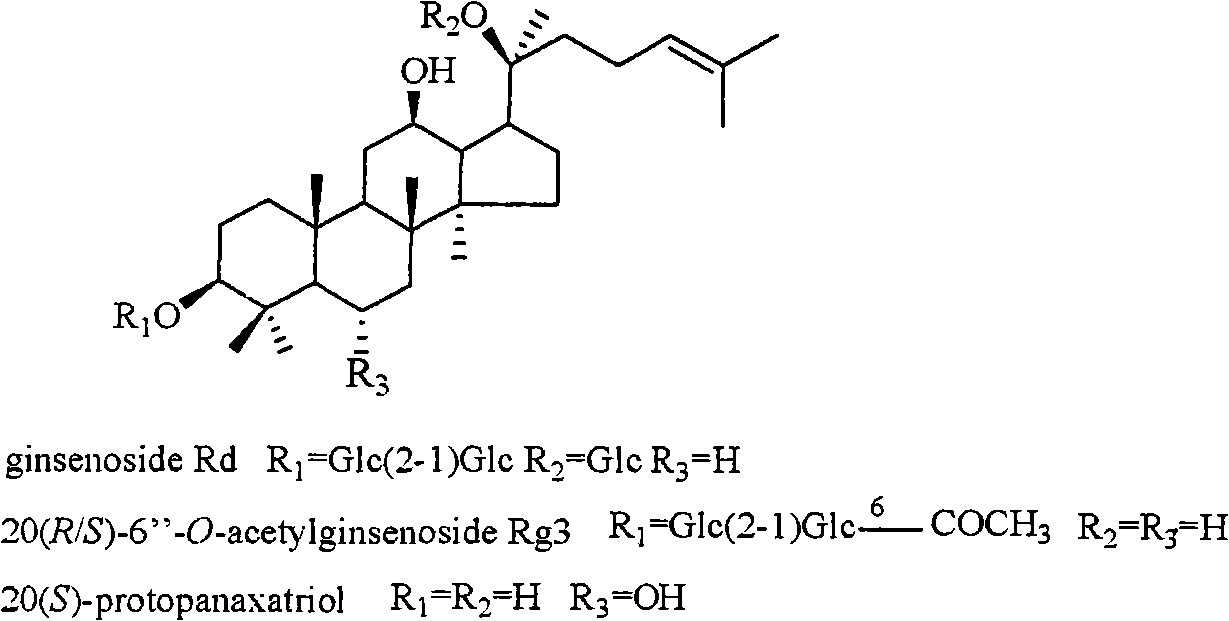
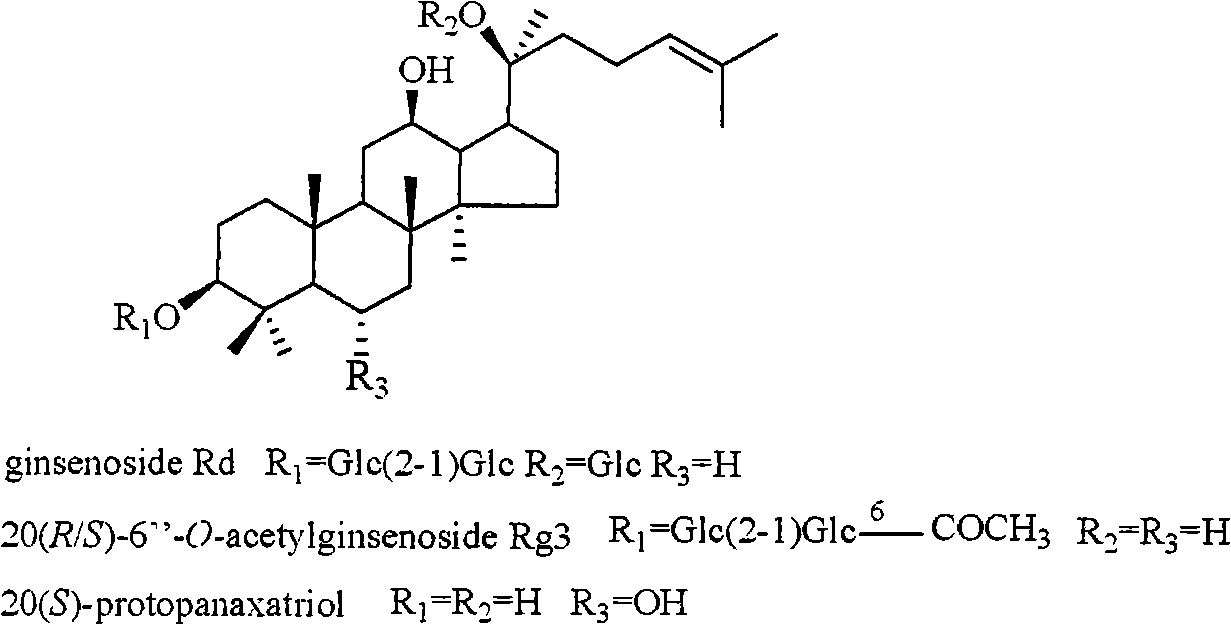
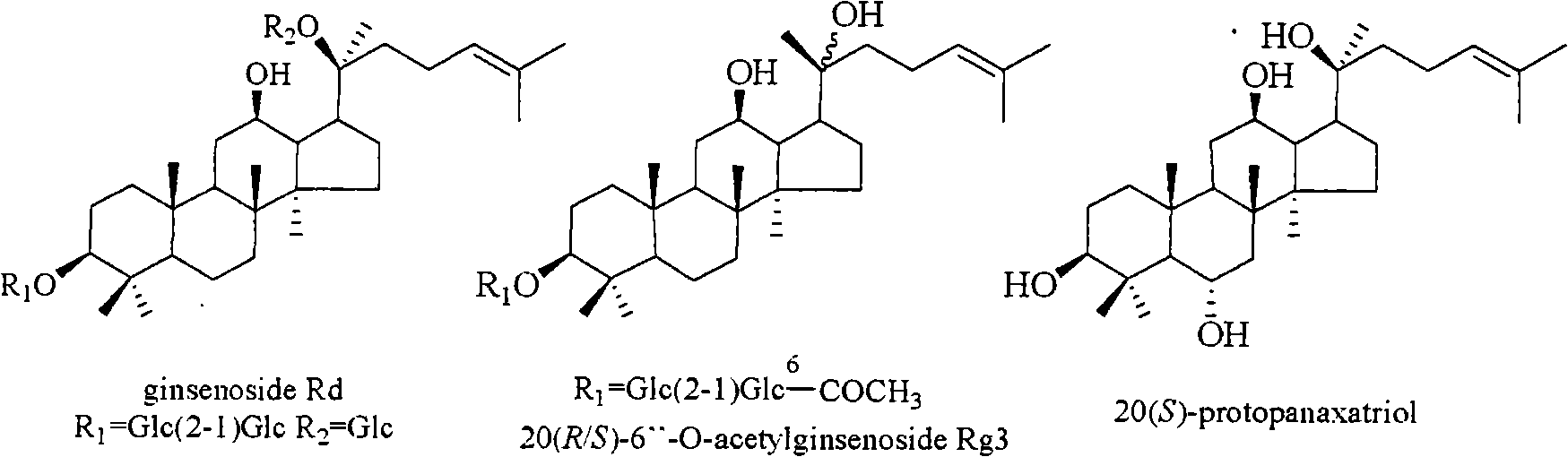
Panax notoginseng saponins ST-4, and medicinal composition, preparation and use thereof
ActiveCN101323635AWith preventionTherapeuticOrganic active ingredientsAntiviralsPANAX NOTOGINSENG ROOTMedicine
The invention discloses a notoginsenoside ST-4 which is a new dammarane type triterpenoid saponin compound shown in the structural formula (I), and a preparation method thereof. The invention also provides applications of the notoginsenoside ST-4 in the preparation of drugs against HSV-1. An HSV-1 strain and HELF (MRC-5) are used as cell experimental subjects to carry out the experiment of antiviral activity of the new compound which is the notoginsenoside ST-4. The results show that the notoginsenoside ST-4 has therapeutical effect for cells infected by HSV-1 and can reduce the infectivity of HSV-1 for MRC-5 cells and be applied to the preparation of drugs against HSV-1.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Composition and method for treating cancer using herpes virus
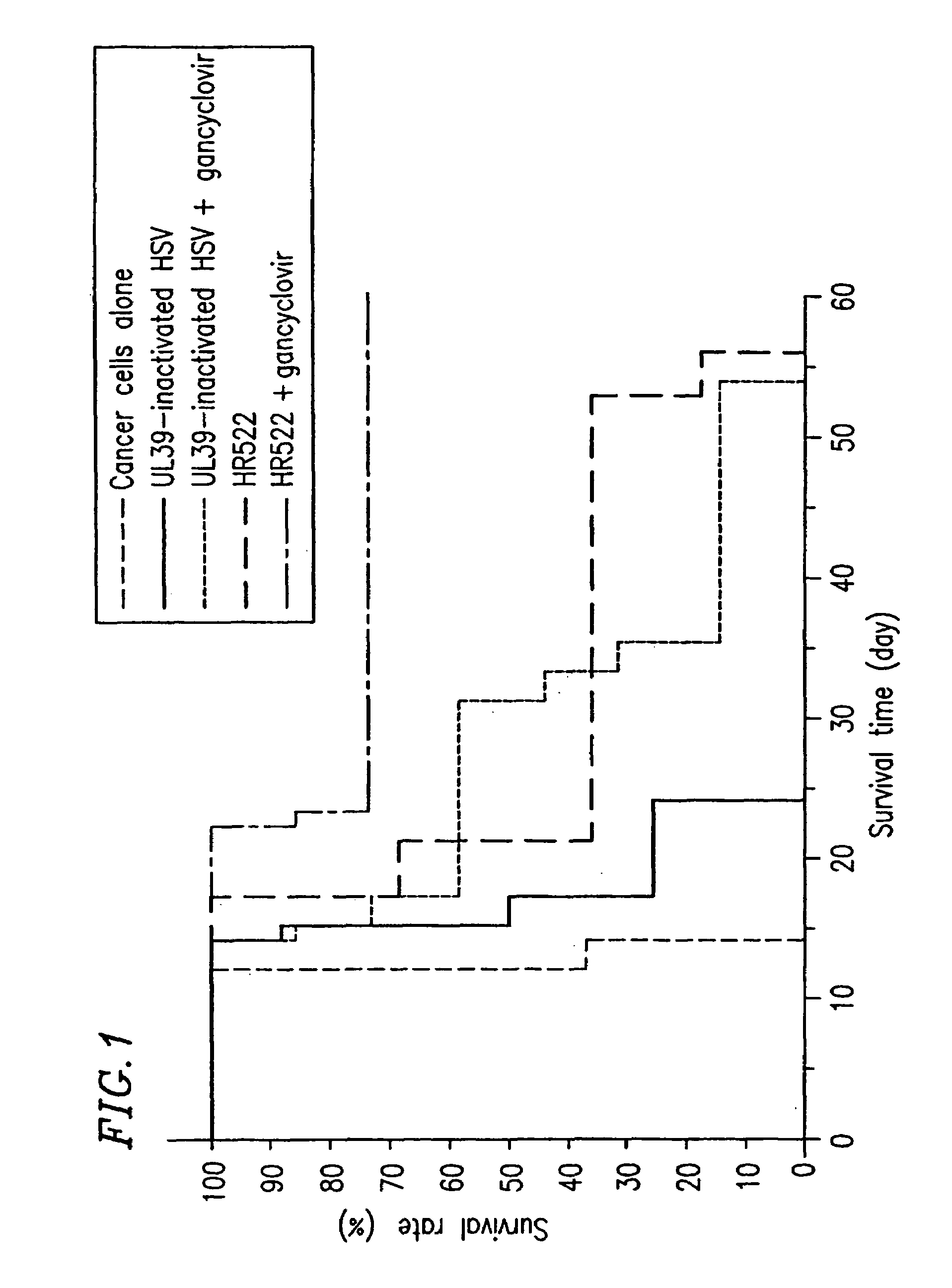
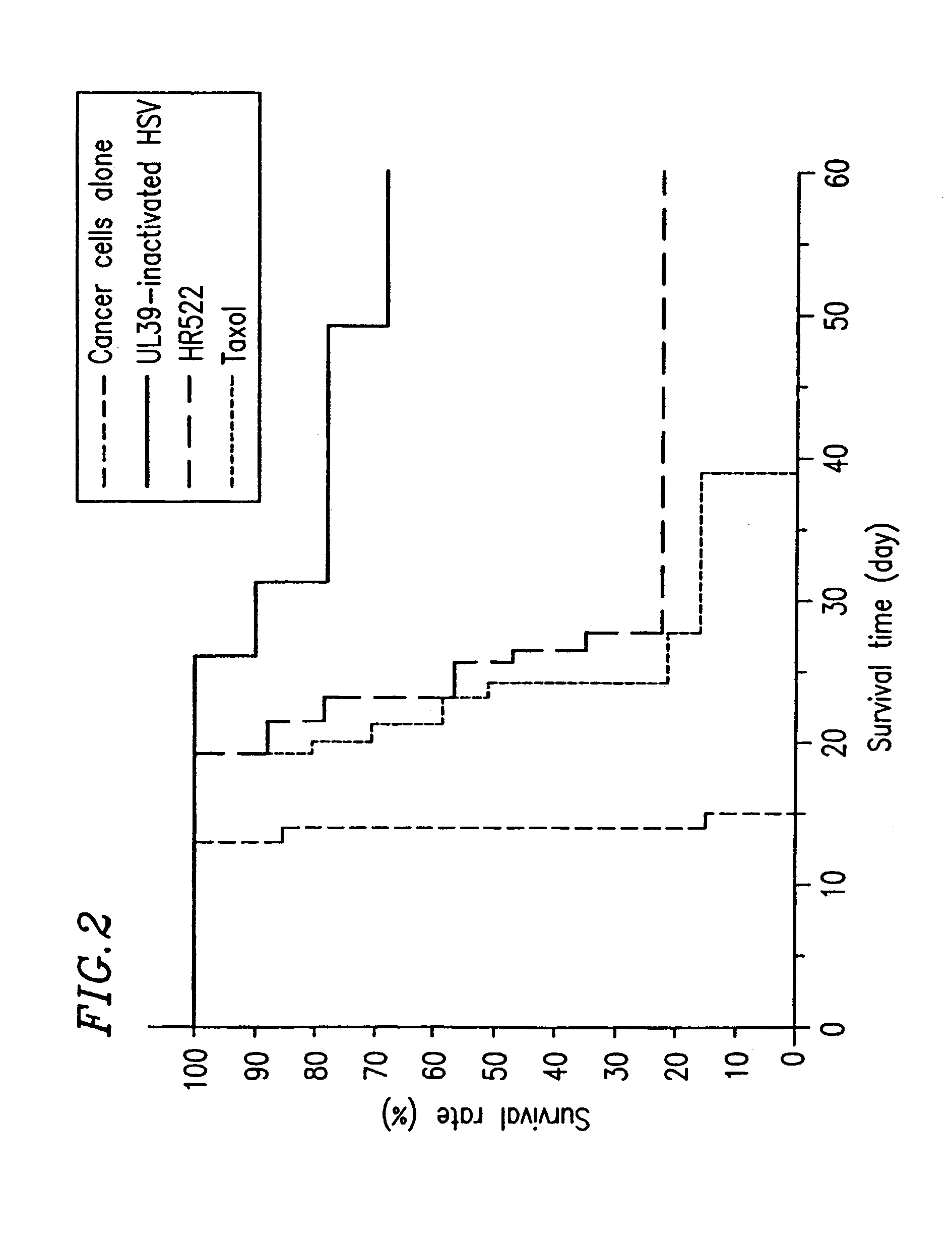
InactiveUS7264814B2Reduce rateReduce severityAntibacterial agentsViral antigen ingredientsHerpes simplex virus DNAInfective disorder
The present invention provides a herpes virus in which a non-essential gene for replication is inactivated More particularly, the present invention provides a herpes virus in which a non-essential gene for replication present in a UL or US region is inactivated. More preferably, the non-essential gene for replication contains US3 or UL56. The herpes virus may be preferably a herpes simplex virus, and more preferably herpes simplex virus 1 or herpes simplex virus 2. The present invention provides a method, composition and use for treating various diseases or disorders including tumor and infectious diseases. The present invention also provides a method, composition and use for activating a prodrug.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
Detection marker, primer probe pair, kit and detection method for herpesvirus hominis I type and II type
ActiveCN109487013ALow similarityHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesPathogenic microorganismHerpesvirus hominis
The invention relates to the technical field of detection of pathogenic microorganisms, in particular to a detection marker, primer probe pair, kit and detection method for herpesvirus hominis I typeand II type. By taking reverse repeated sequences RS1 and RS2 in an HSV genome as a target area, the detecting sensitivity can be further improved as two copies are available in the genome on the onehand and cross reactions are unlikely to be caused as the inter-nucleic acid similarity is low on the other hand.
Owner:中生方政生物技术股份有限公司
I, II herpes simplex virus fluorescence quantitative PCR detection method and kit thereof
InactiveCN101676409AHigh sensitivityNo amplification signalMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceType ii herpes simplex
The invention relates to an I, II herpes simplex virus fluorescence quantitative PCR detection method and kit thereof. Primers are designed direct towards DNA enzyme highly conservative region and then processed by PCR and a fluorescent probe is designed in the middle of the amplification sequence and then the annealing temperature of the reaction and the concentrations of the primers, the probe,the magnesium ion are optimized to establish the I, II herpes simplex virus real-time fluorescence PCR detection method. The kit ensures the sensitivity and accuracy and has features of simple and quick operation, reduced labor intensity of clinical detection, reduced cost of clinical PCR diagnosis, quick and simple performance of fluorescence quantitative PCR detection method. The method is usedfor quantitative detection of I, II herpes simplex virus with practical clinical application value.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Fluorescent PCR kit for detecting herpes simplex virus type II
InactiveCN102102131AIncreased sensitivityReduce the risk of contaminationMicrobiological testing/measurementFluorescencePolyethylene glycol
The invention discloses a fluorescent polymerase chain reaction (PCR) kit for detecting herpes simplex virus type II, and belongs to the field of in-vitro diagnostic kit for nucleic acid. The kit comprises a positive reference, a negative reference, fluorescent polymerase chain reaction liquid, PCR primers and a specific fluorescent probe, polyethylene glycol (PEG) precipitation solution and lysis solution. The invention comprises a PCR system based on a fluorescent PCR technology, contains forward and reverse primers and the fluorescent probe for detecting the gene sequence of the herpes simplex virus type II, can detect the nucleotide sequence of the gene of the herpes simplex virus type II under proper PCR condition, can easily and quickly detect the infection of the HSV II in clinical samples and is high in specificity.
Owner:上海裕隆医学检验所股份有限公司
Recombined II-type herpes simplex virus, preparation method and application and tumour diagnostic reagent kit
ActiveCN102220292AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesNeoplasm diagnosisFluorescence
The invention provides recombined II-type herpes simplex virus. In the virus, ICP34.5 gene of a wild II-type herpes simplex virus HG52 strain is excluded, and a fluorescent protein expression box is inserted in the gene group. The invention also provides a preparation method of the recombined II-type herpes simplex virus, the application of the recombined II-type herpes simplex virus in preparation of the drug for diagnosing tumours, and a tumour diagnostic reagent kit with the recombined II-type herpes simplex virus. In the recombined II-type herpes simplex virus disclosed by the invention, the ICP34.5 gene is excluded, so that the recombined II-type herpes simplex virus can selectively grow and breed in tumour cells, the recombined II-type herpes simplex virus is inserted in the fluorescent protein expression box and can give out fluorescence in tumour cells, so that the tumour diagnostic reagent kit with the recombined II-type herpes simplex virus can quickly, accurately, sensitively and broadly detect whether a sample to be detected contains tumour cells or not.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
Probe used for TORCH detection, gene chip and kit thereof
ActiveCN104846074AMeet the needs of on-site testingImprove detection efficiencyNucleotide librariesMicrobiological testing/measurementShootFluorescence
The invention discloses a set of probes used for TORCH detection, and comprises a toxoplasma TOX probe which is shown in a SEQ ID NO. 1; a rubella virus RV probe which is shown in a SEQ ID NO.2; a cytomegalovirus CMV probe which is shown in a SEQ ID NO.3; a herpes simplex virus HSV I type probe which is shown in a SEQ ID NO.4; and a herpes simplex virus HSV II type probe which is shown in a SEQ ID NO.5. The invention also comprises a gene chip containing the probe and a kit thereof, visible light passing through a biochip can be amplified, signal resolution is high, and a signal can be shoot by a common digital camera or directly read by naked eyes. Compared with a traditional fluorescent label, the gene chip has good effect. Through specific primer and probe selection, by combining a gene chip technology, detection efficiency and accuracy degree are increased, and requirement of on-site detection for hospital and antenatal testing.
Owner:ZHUHAI SINOCHIPS BIOSCIENCE CO LTD
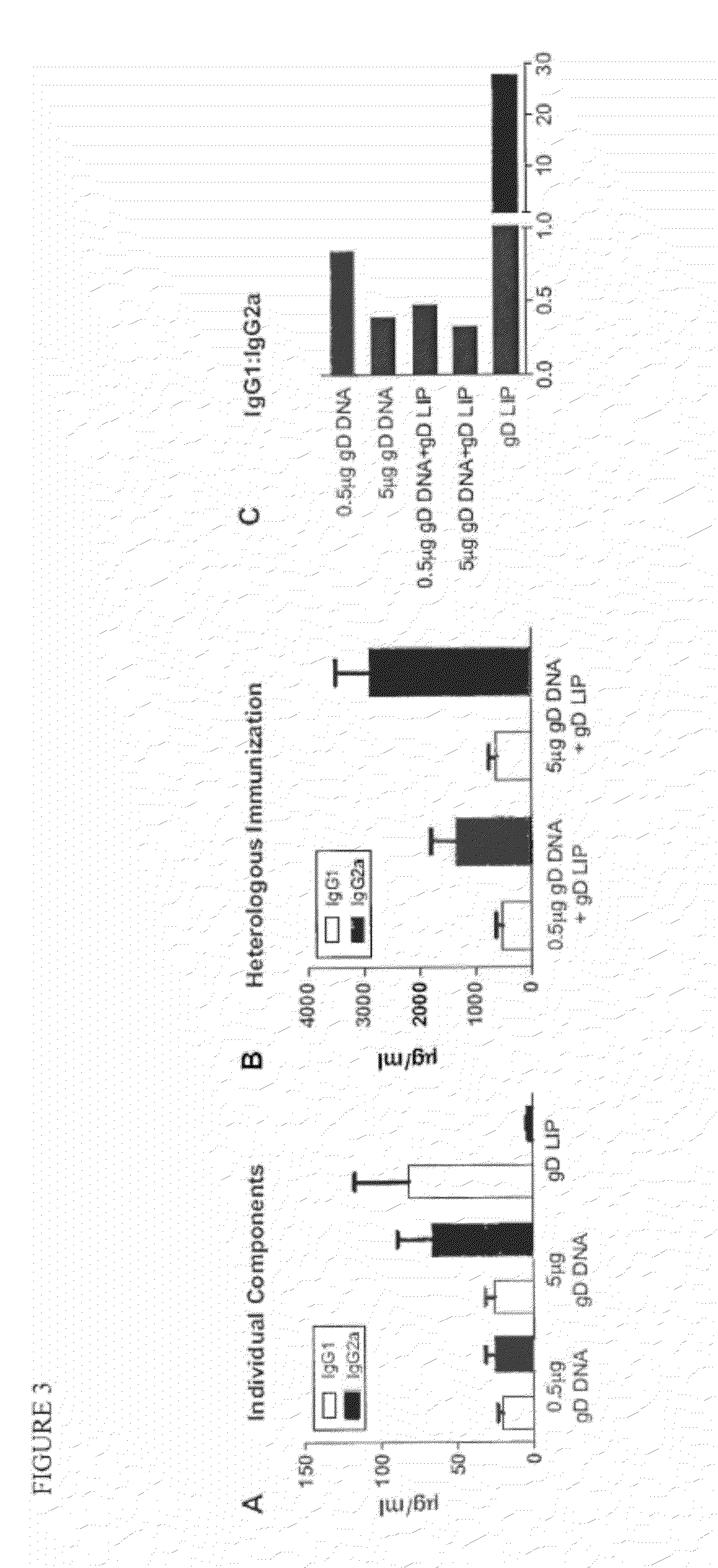
Novel mucosal vaccination approach for herpes simplex virus type-2
InactiveUS20120027841A1High potencyPrevent relapseOrganic active ingredientsViral antigen ingredientsHeterologousLiposome
The invention provides methods and kits for immunizing animals (e.g. mammals) against viral antigens, including herpes-simplex virus type 2. The protective immune response elicited by the methods and kits of the invention is characterized by robust humoral, cellular, and mucosal immunity. In particular, the invention provides a heterologous immunization method comprising a priming DNA vaccine encoding an antigen and a boosting protein vaccine, in which the protein form of the antigen is encapsulated in liposomes. Methods of preventing primary acute, latent and recurrent viral infections, such as that caused by HSV-2 virus, and methods of providing passive protective immunity against a viral pathogen such as HSV-2 virus to a mammal are also disclosed.
Owner:MUCOSAL VACCINE TECH LLC
Fluorescence quantitative polymerase chain reaction (PCR) kit for quickly detecting herpes simplex viruses 2
ActiveCN101979666AAccurate determination of starting copy numberMeet the requirements for rapid diagnosis of herpes simplex virus type IIMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceDNA extraction
Owner:WUHAN BIOTECH GENE ENG
Anti-herpes simplex virus I-form medicament composition and uses thereof
InactiveCN101322714AOrganic active ingredientsDigestive systemStructural formulaHerpes simplex disease
The invention relates to a pharmaceutical composition with dammarane-type tetracyclic triterpene compound as an active component and an application thereof to the pharmaceutical field. Panax notoginseng is separated to obtain three dammarane-type tetracyclic triterpene compounds as shown in the following structural formula, in vitro pharmacological experiments show that the pharmaceutical composition has good in vitro inhibitory activity for herpes simplex virus type I and can be applied to the preparation of medicines for resisting herpes simplex virus type I and is used for treating herpetic keratitis, encephalitis, pneumonia, ulcerative stomatitis, blister and the like caused by herpes simplex virus type I.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Use of mutant Herpes Simplex Virus-2 for cancer therapy
ActiveUS8986672B2Strong responseUseful in therapyBiocidePeptide/protein ingredientsNucleotideDeficiency protein
Owner:UNIV HOUSTON SYST
Topical treatment of skin disease and eye afflictions
A method of treating patients having viral infections including herpes simplex virus 1 and 2 infections and human papillomavirus infections by topically administering Product R, a peptide-nucleic acid preparation, by itself or in a composition comprising Product R and other pharmaceutically acceptable carriers for topical administration, is disclosed.
Owner:BBM HLDG
Tetracycline Repressor Regulated Oncolytic Viruses
ActiveUS20100015687A1Minimize damageMinimize spreadingVectorsSugar derivativesSolid tumorTetracycline
The present invention is directed oncolytic Herpes simplex-1 viruses whose replication is controlled using a tetracycline operator / repressor system. The invention also includes DNA sequences used in making the viruses and methods in which these viruses are used in the treatment of cancer patients with solid tumors.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Oligonucleotide therapies for modulating the effects of herpesviruses
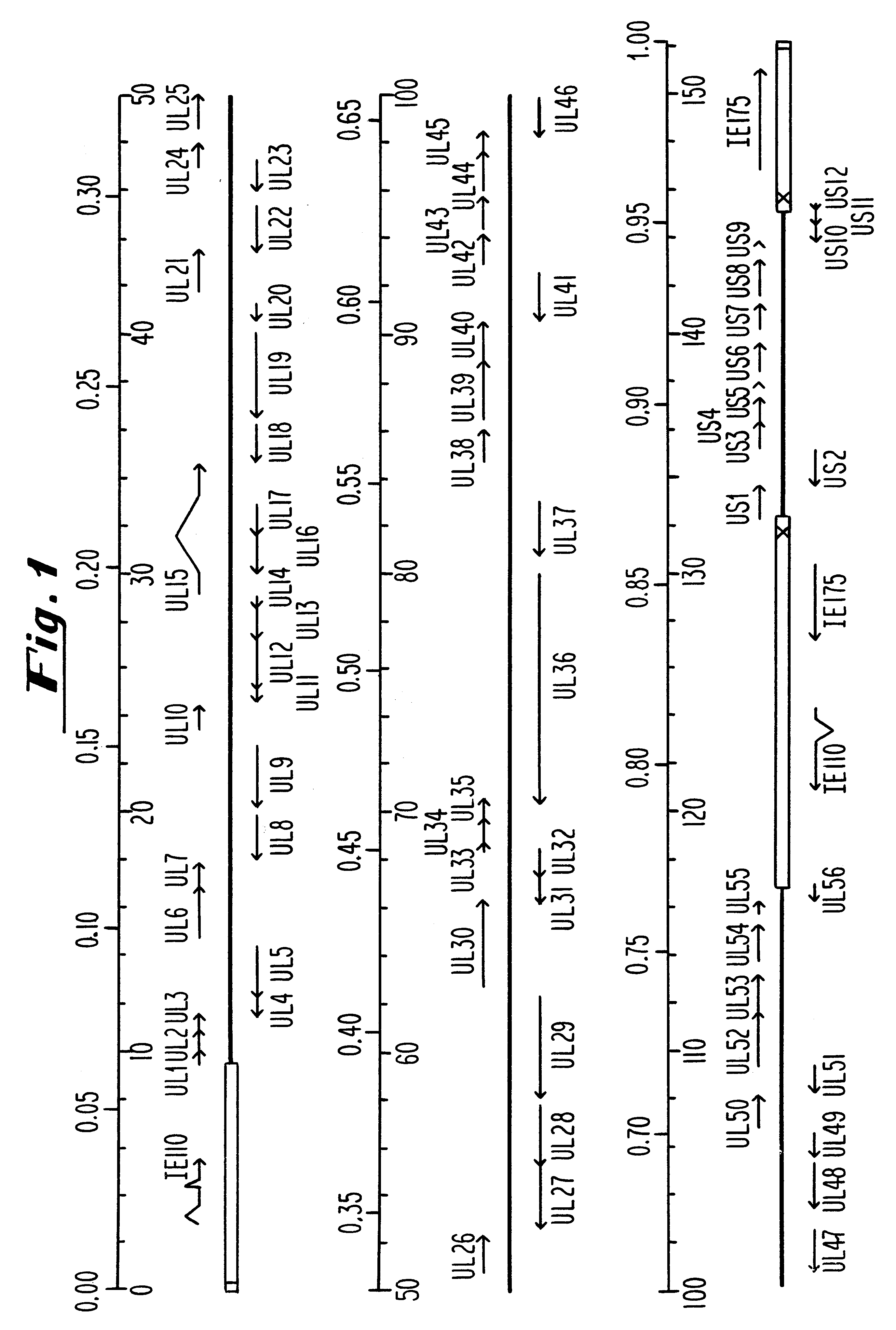
InactiveUS6310044B1Conveniently and desirably presentedFaster replicationPeptide/protein ingredientsGenetic material ingredientsOpen reading frameHerpesvirus infection
Compositions and methods are provided for the treatment and diagnosis of herpesvirus infections. In accordance with preferred embodiments, oligonucleotides are provided which are specifically hybridizable with RNA or DNA deriving from a gene corresponding to one of the open reading frames UL5, UL8, UL9, UL13, UL29, UL30, UL39, UL40, UL42 AND UL52 of herpes simplex virus type 1. The oligonucleotide comprises nucleotide units sufficient in identity and number to effect said specific hybridization. In other preferred embodiments, the oligonucleotides are specifically hybridizable with a translation initiation site; it is also preferred that they comprise the sequence CAT. Methods of treating animals suspected of being infected with herpesvirus comprising contacting the animal with an oligonucleotide specifically hybridizable with RNA or DNA deriving from one of the foregoing genes of the herpesvirus are disclosed. Methods for treatment of infections caused by herpes simplex virus type 1, herpes simplex virus type 2, cytomegalovirus, human herpes virus 6, Epstein Barr virus or varicella zoster virus are disclosed.
Owner:IONIS PHARMA INC
Protein chip for detecting blood and cerebro spinal fluid pathogen antibody, and its preparing method and use
InactiveCN1641355ASmall sample sizeSolve the detection speed is slowMaterial analysisAntigenChickenpox
The invention discloses a protein chip for detecting pathogen antibodies of blood and cerebrospinal fluid and its preparing method, and the protein chip includes substrate and peculiar-culture or shape protein or polypeptide antigen of array distributed pathogen and contrasted dot coating, where the substrate is a glass substrate; and the antigen and contrasted dot coating refers to 13 antigens with ten indexes including HSV-I, HSV-II, VZV, CMV, EBV, RV, JEV, MV, MT and LP, uniformly distributed on the glass substrate in a dot matrix form, and positive contrast, negative contrast and blank contrast. The protein chip of the invention can obtain multiple-index reacting result only by one reaction, judges infection of different pathogens and has the characters of quickness, high efficiency, accuracy and parallel diagnosis.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
Use of extract of Fructus Pithecellobii clypeoriae conrmon Apes Ear-ring Fruit
The present invention relates to an application of pithecellobium clypearia extract in medicine and food. It adopts pithecellobium clypearia aqueous extract or ethyl alcohol extract as effective component and adds conventional medicinal auxiliary or food additive, and can make them into tablet, capsul, granule preparation, pills preparation and oral liquor preparation. Said pithecellobium clypearia extract has strong inhibition effect for influenza virus, parainfluenza virus, enteric virus, rhinovirus and mumps virus and has strong antioxidation activity.
Owner:SHANHE PHARMA GUANGZHOU CITY
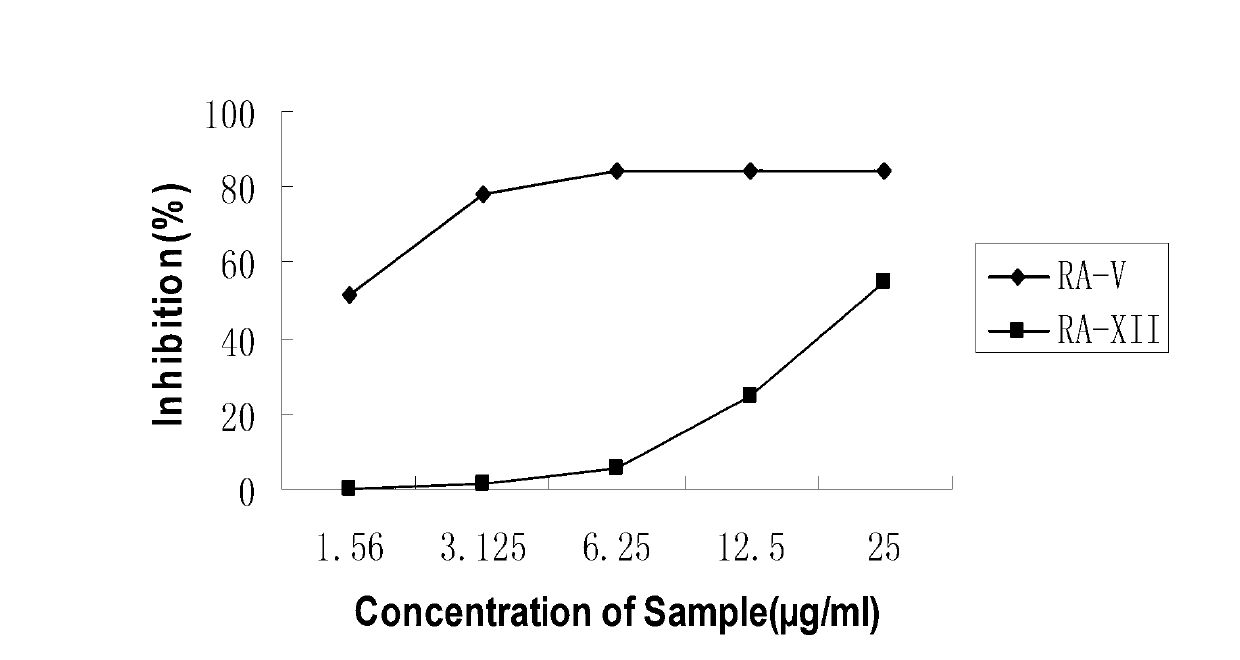
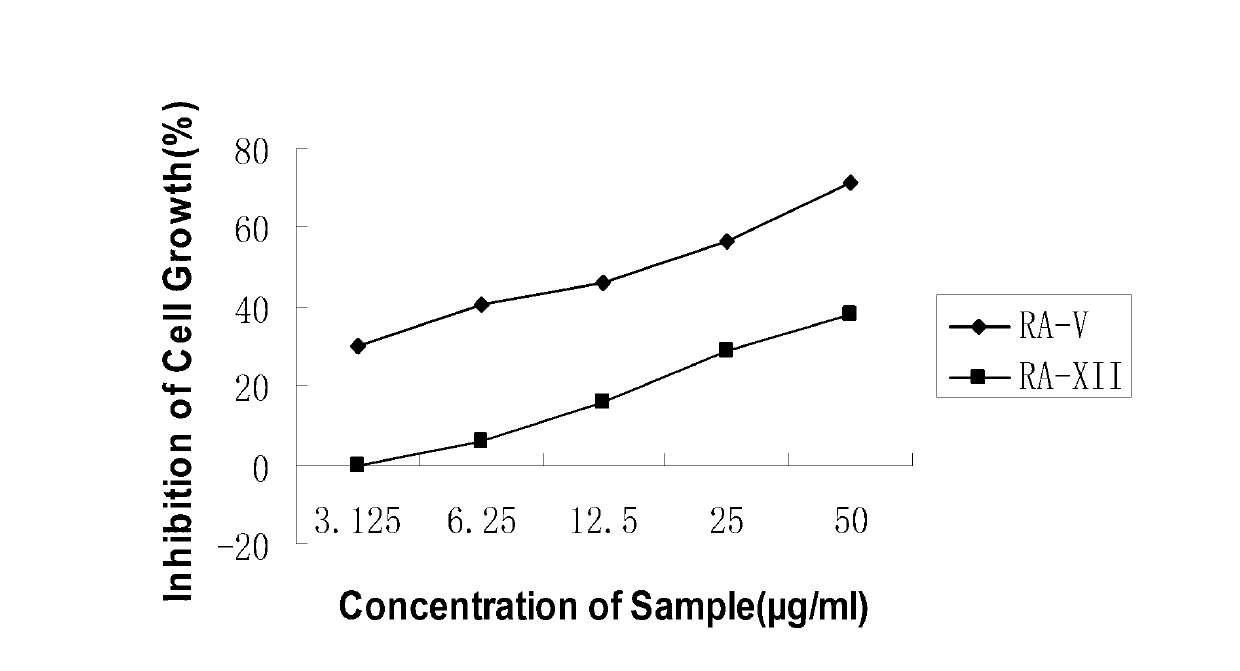
Rubiaceae-type cyclopeptide, its pharmaceutical composition and application
The invention provides a rubiaceae-type cyclopeptide compound rubiyunnanin F(8), a pharmaceutical composition with the compound as an active component, as well as a preparation method and application of the compound. The invention provides the preparation method of the compound and application of the compound in preparation of anti-herpes simplex virus type I (HSV-1) medicines.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com