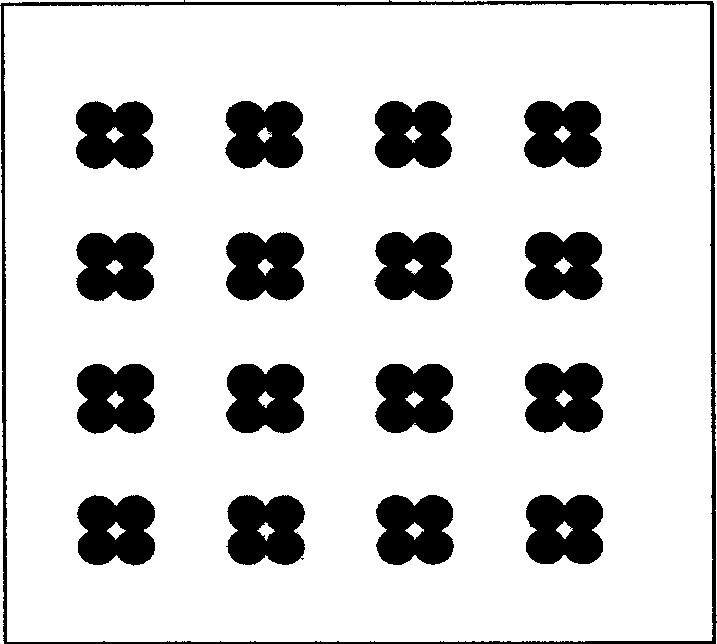Protein chip for detecting blood and cerebro spinal fluid pathogen antibody, and its preparing method and use
A pathogen antibody and protein chip technology, applied in the field of biochips and their preparation, can solve the problems of large differences in specificity, sensitivity, stability, threat to the health of experimenters, serious cross-reaction, etc., so as to retain a high degree of specificity, improve Detection speed and efficiency, the effect of low sample consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
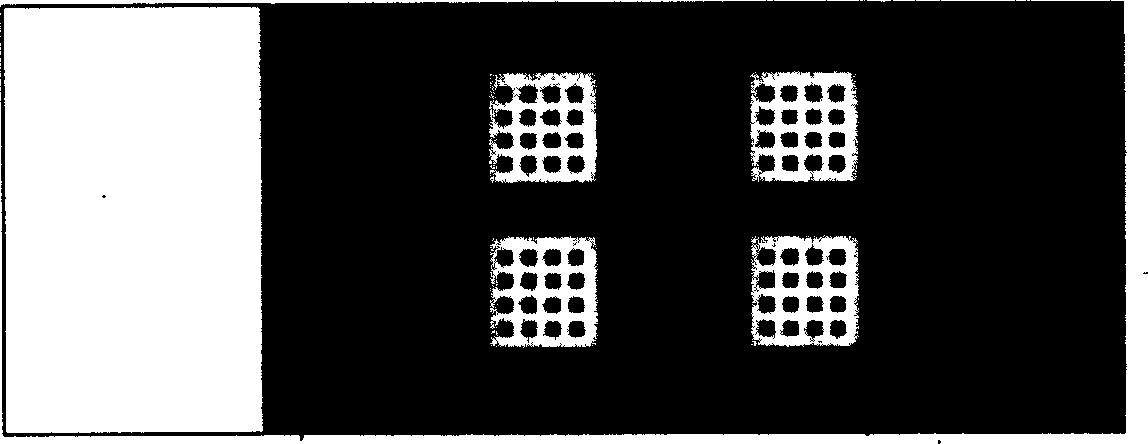
[0045] Example 1: Modification of slide and fixation of antigen, such as figure 1 The appearance of the protein chip for detecting serum cerebrospinal fluid pathogen antibodies, such as figure 2 Spotted array diagram.
[0046] Put the slides on a slide rack, put them into a glass jar filled with 350ml of cleaning solution (NaOH 100g, ethanol 600ml, water 400ml), place on a shaker at 60 rpm, and shake flatly for 2 hours; pour off the cleaning solution, fully Wash 4 times with water, 3 minutes each time; soak the slides in a glass jar filled with 350ml polylysine PBS solution (polylysine 35ml, PBS 35ml, water 280ml), and place on a shaker at 60 rpm , shake flatly for 1 hour; immerse the slide in water and wash it up and down 5 times; put it in a centrifuge, centrifuge at 800 rpm for 5 minutes, put it in a clean plastic box, place it vertically for 2 weeks or apply after baking in an oven use.
[0047] For 2 serum samples, choose a 2×2 microarray chip, (such as figure 1 ) wh...
Embodiment 2
[0049] Embodiment 2: Antigen-antibody reaction and detection
[0050] Add blocking solution (1% BSA, 0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / L NaCl, 0.1% Tween-20,) to seal on the chip where the antigen has been spotted, at 37°C hour, block the non-specific sites on the surface of the substrate; wash 3 times with PBST (0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / L NaCl, 0.1% Tween-20), each 10 Seconds after seeding, rinse with PBS (0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / LNaCl), and centrifuge at 800 rpm for 3 minutes to remove excess blocking solution; After diluting 10 times with PBS, take 3 μL and add it to the array. The first sample is added to the upper and lower arrays of the first column; the second sample is added to the upper and lower arrays of the second column, and placed in a hybridization box at 37°C 30 minutes to fully react the antigen and antibody; wash 3 times with PBST, 10 seconds each time, rinse with PBS, and centrifuge at 800 rpm for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com