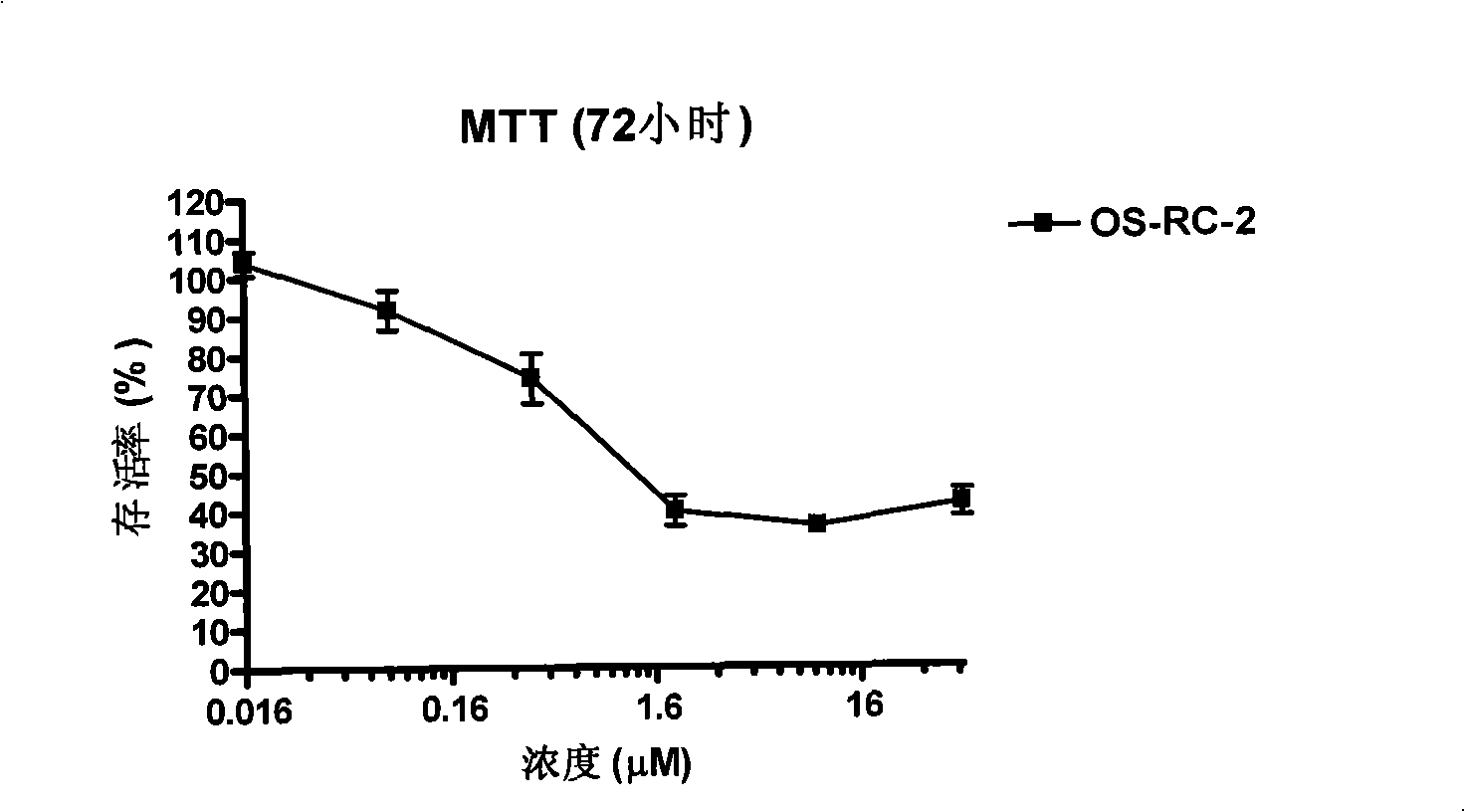
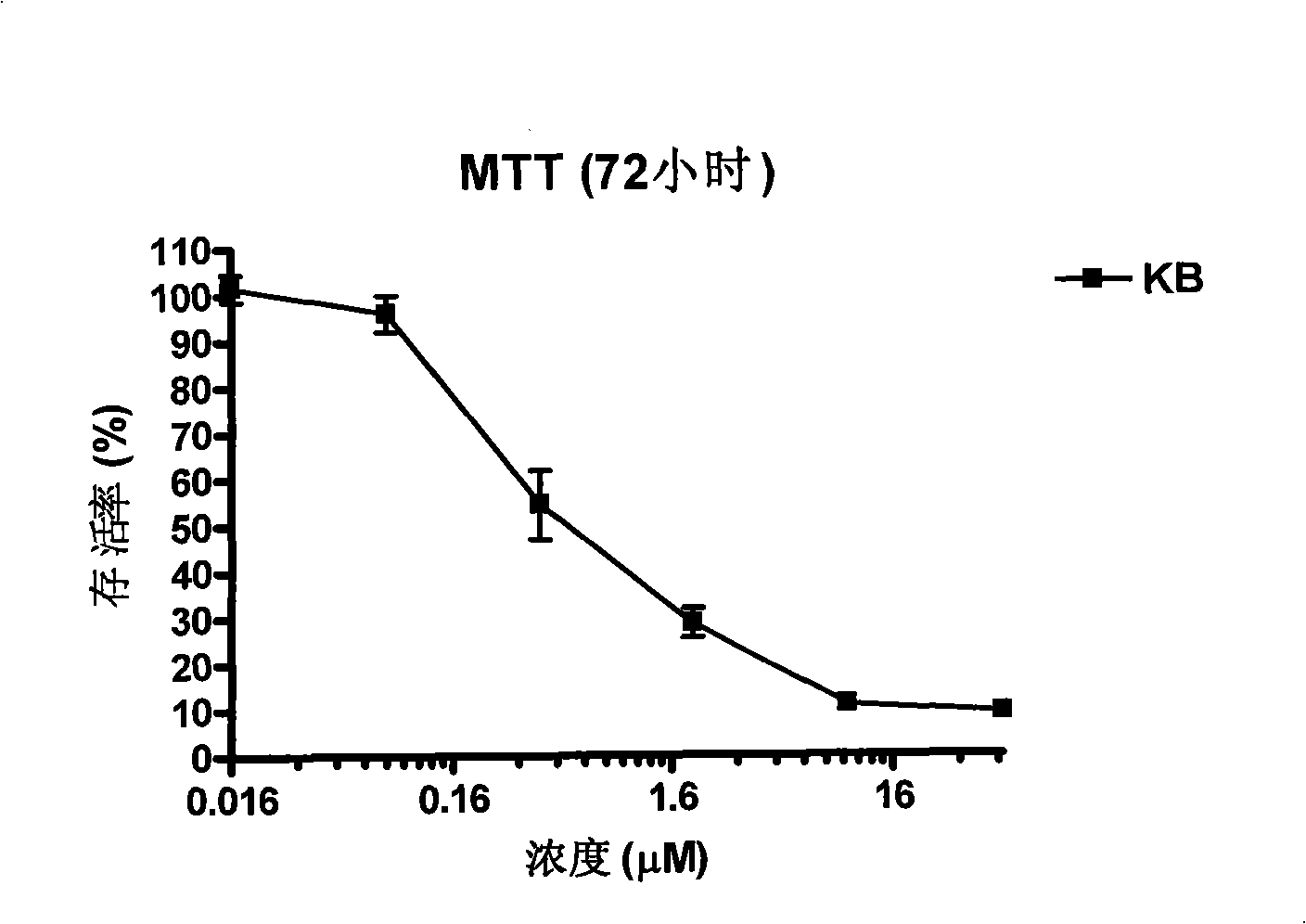
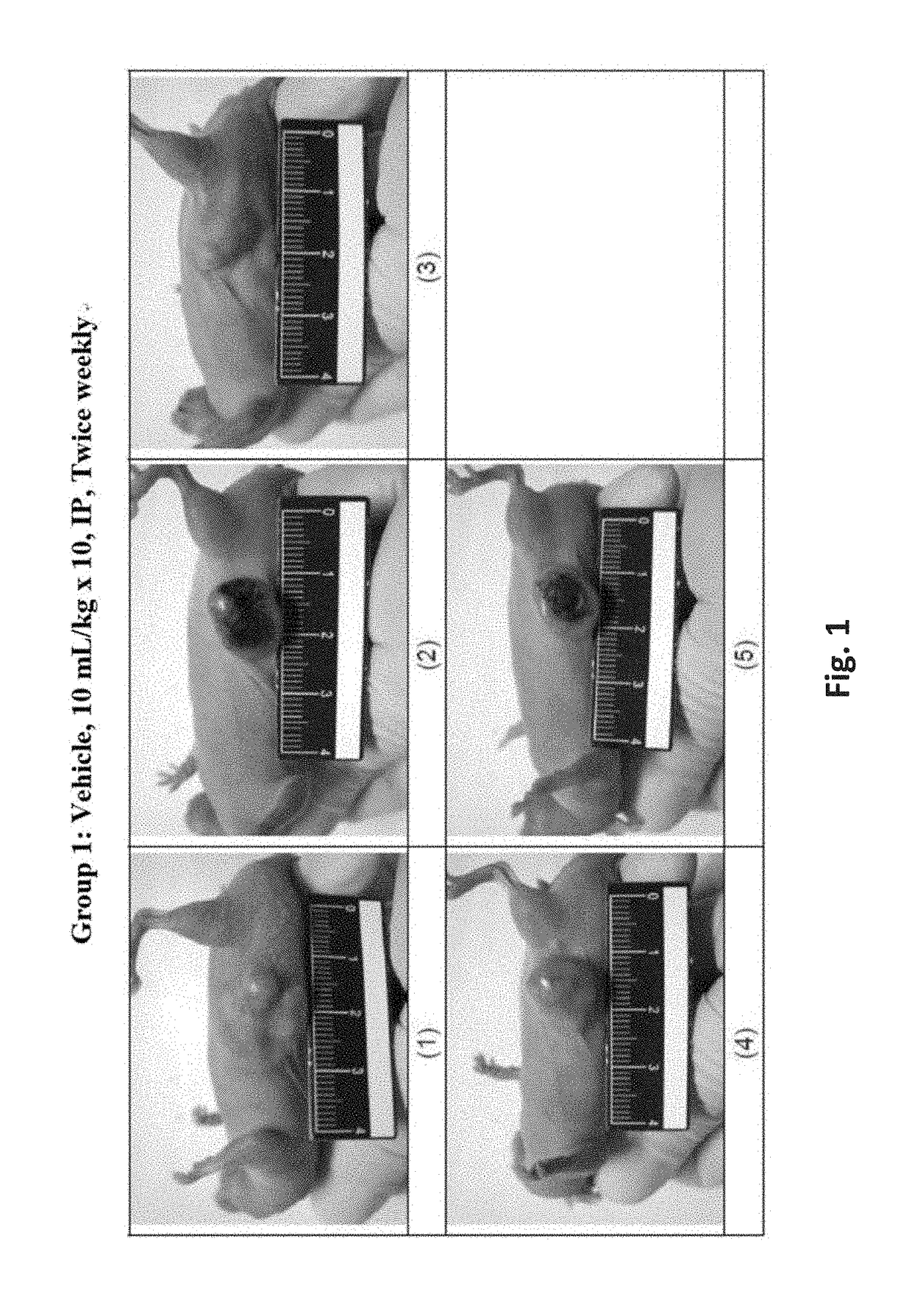
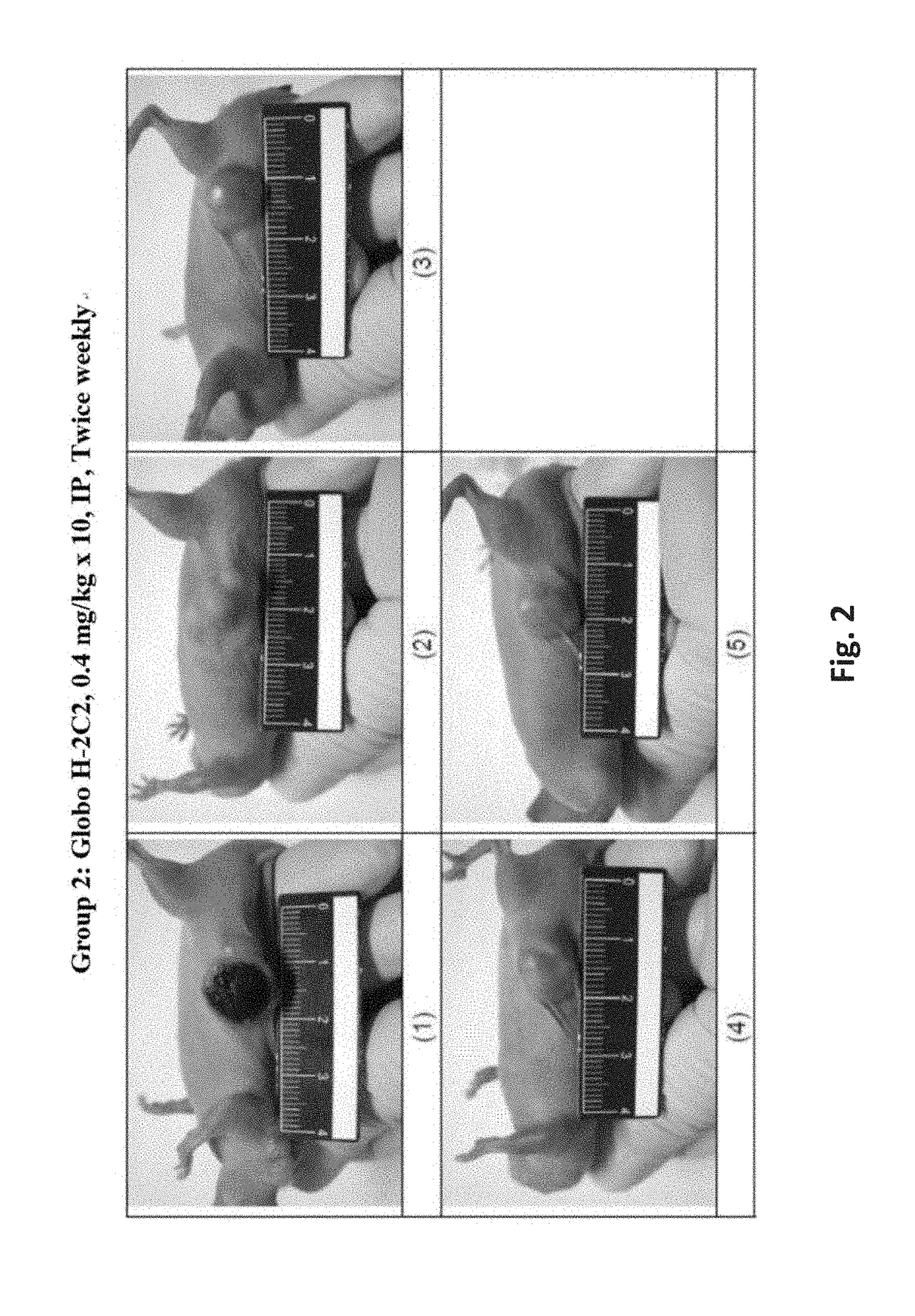
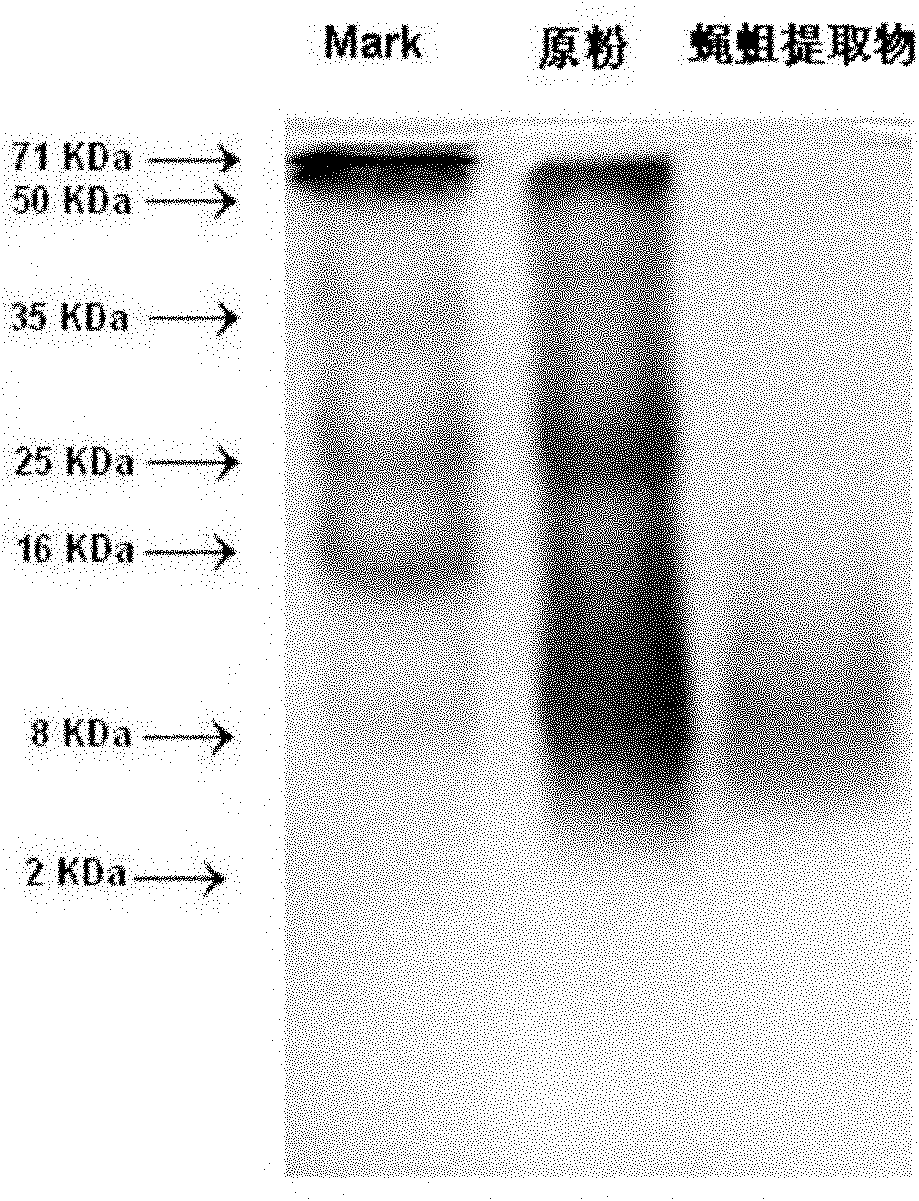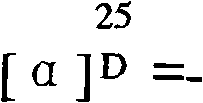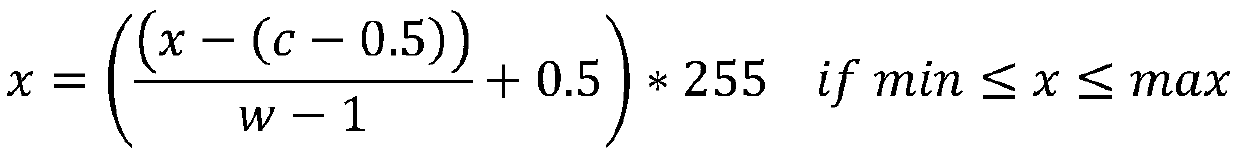Patents
Literature
123 results about "Nasopharyngeal Cancers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
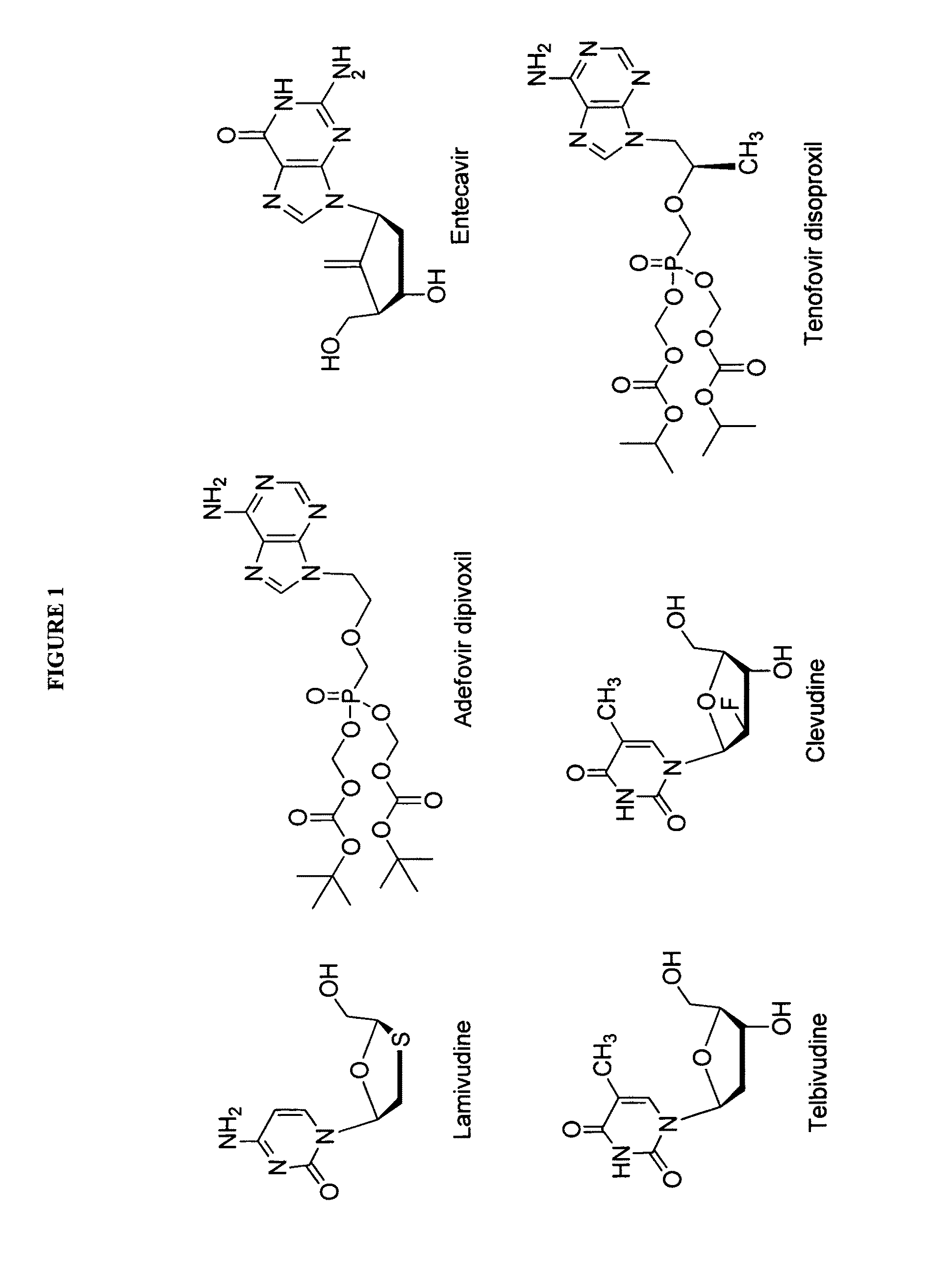
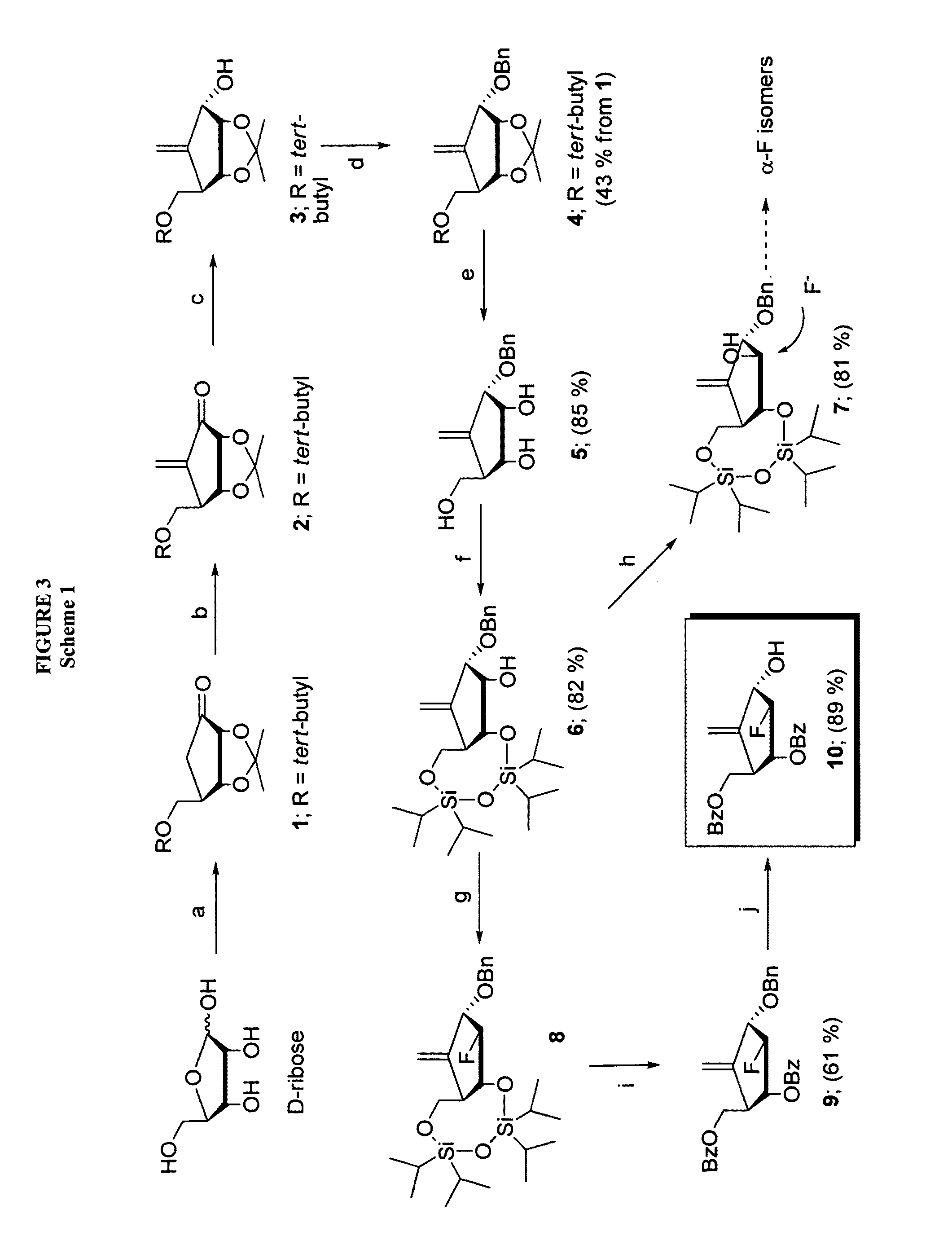
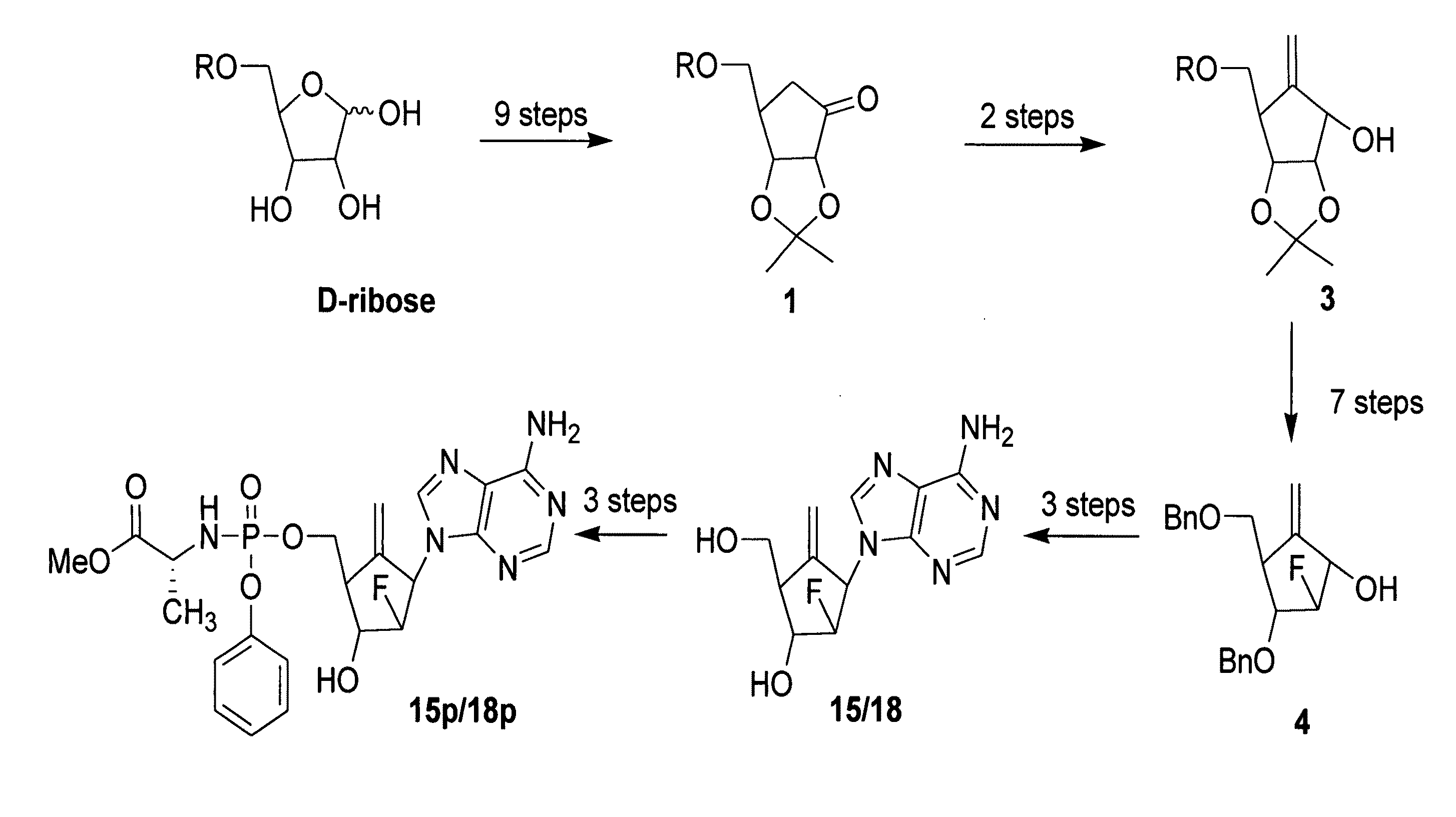
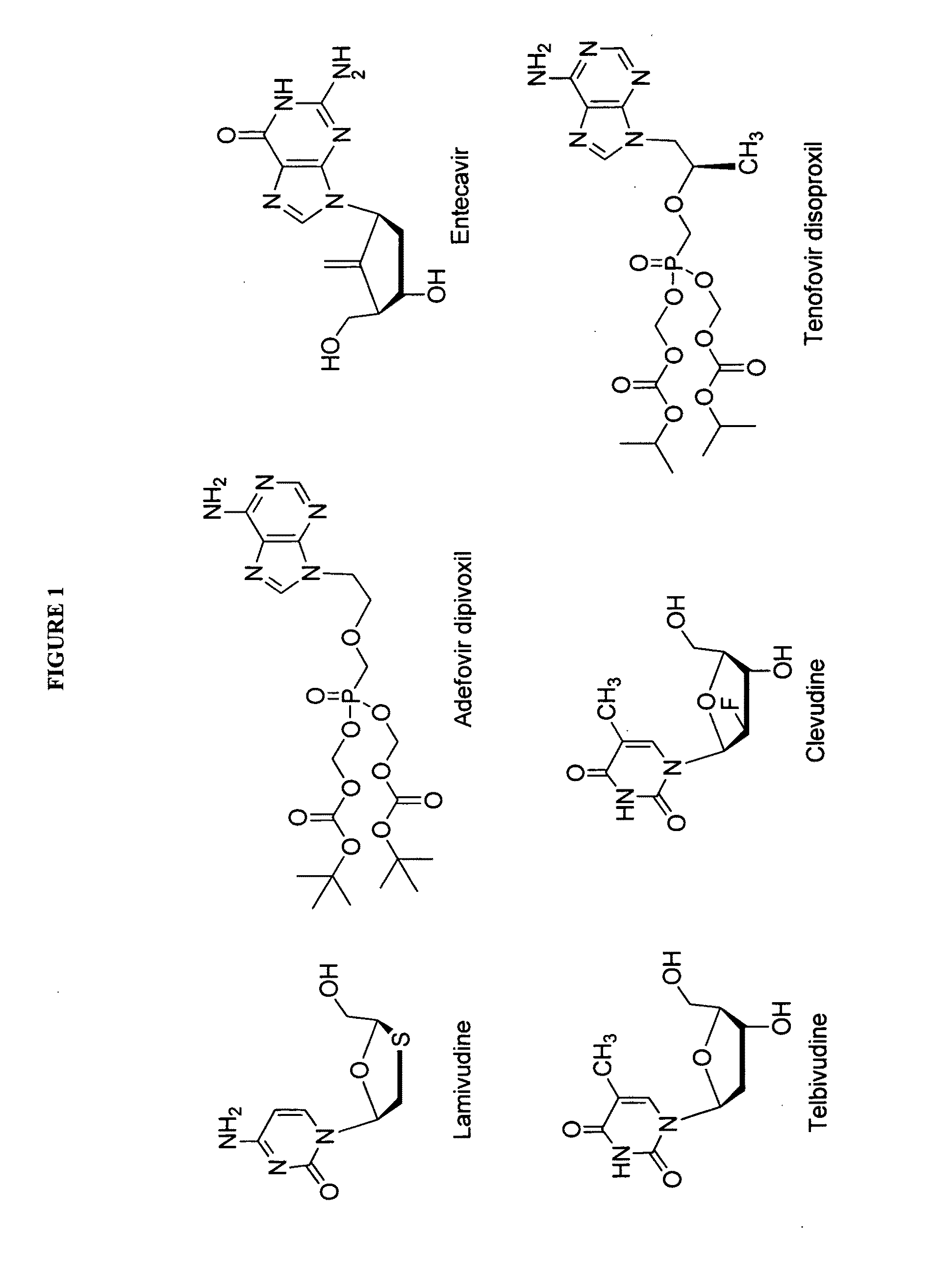
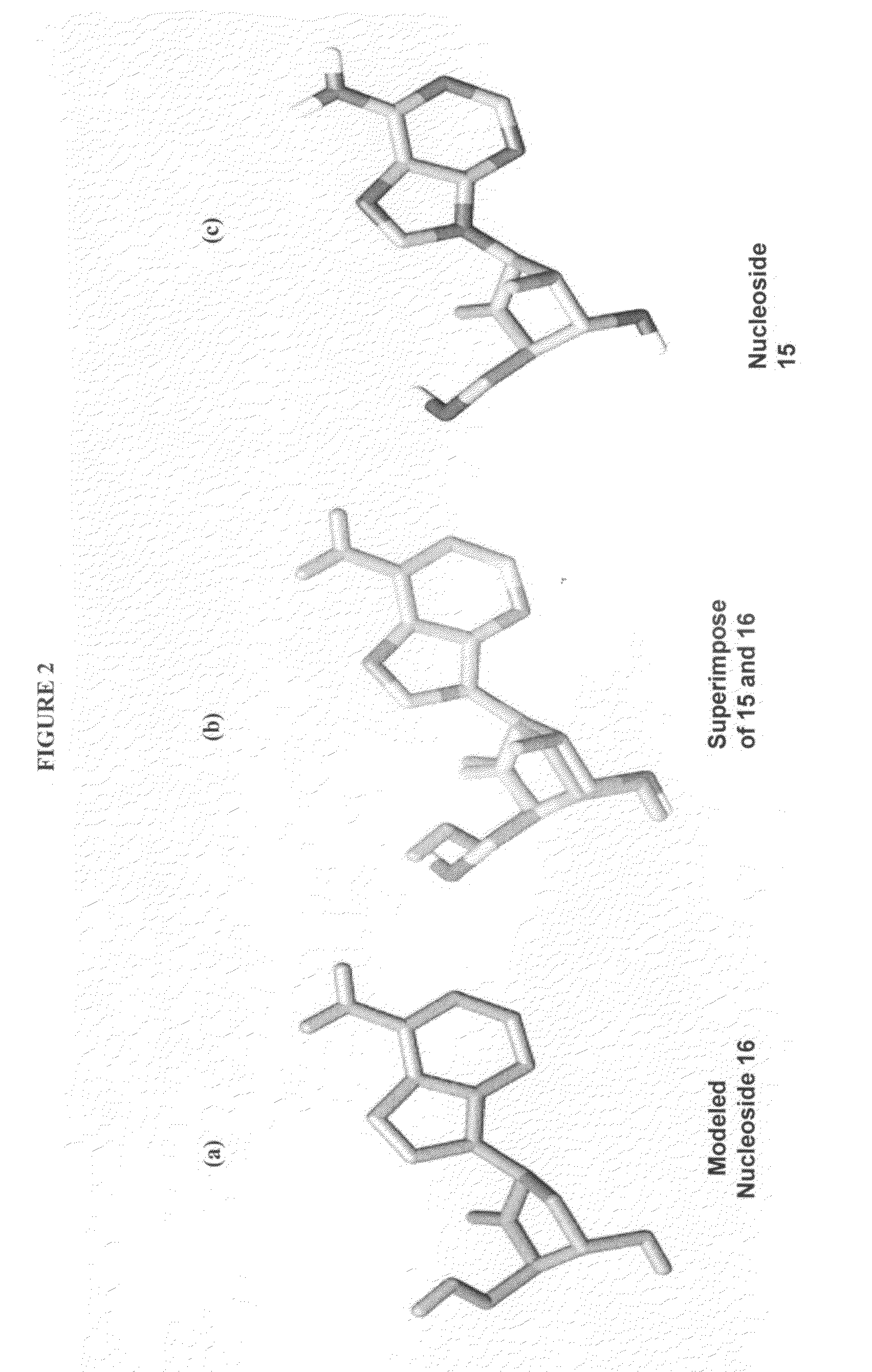
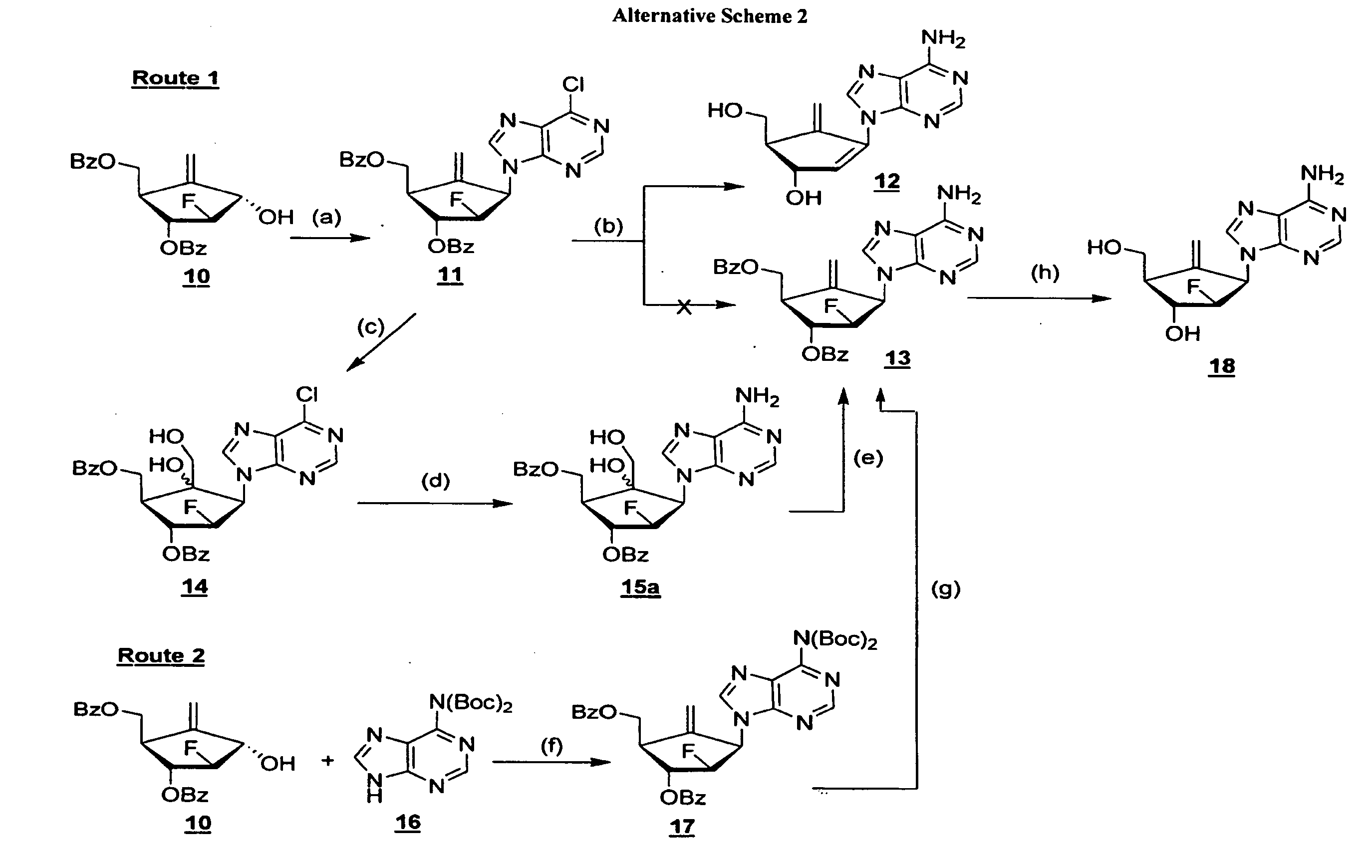
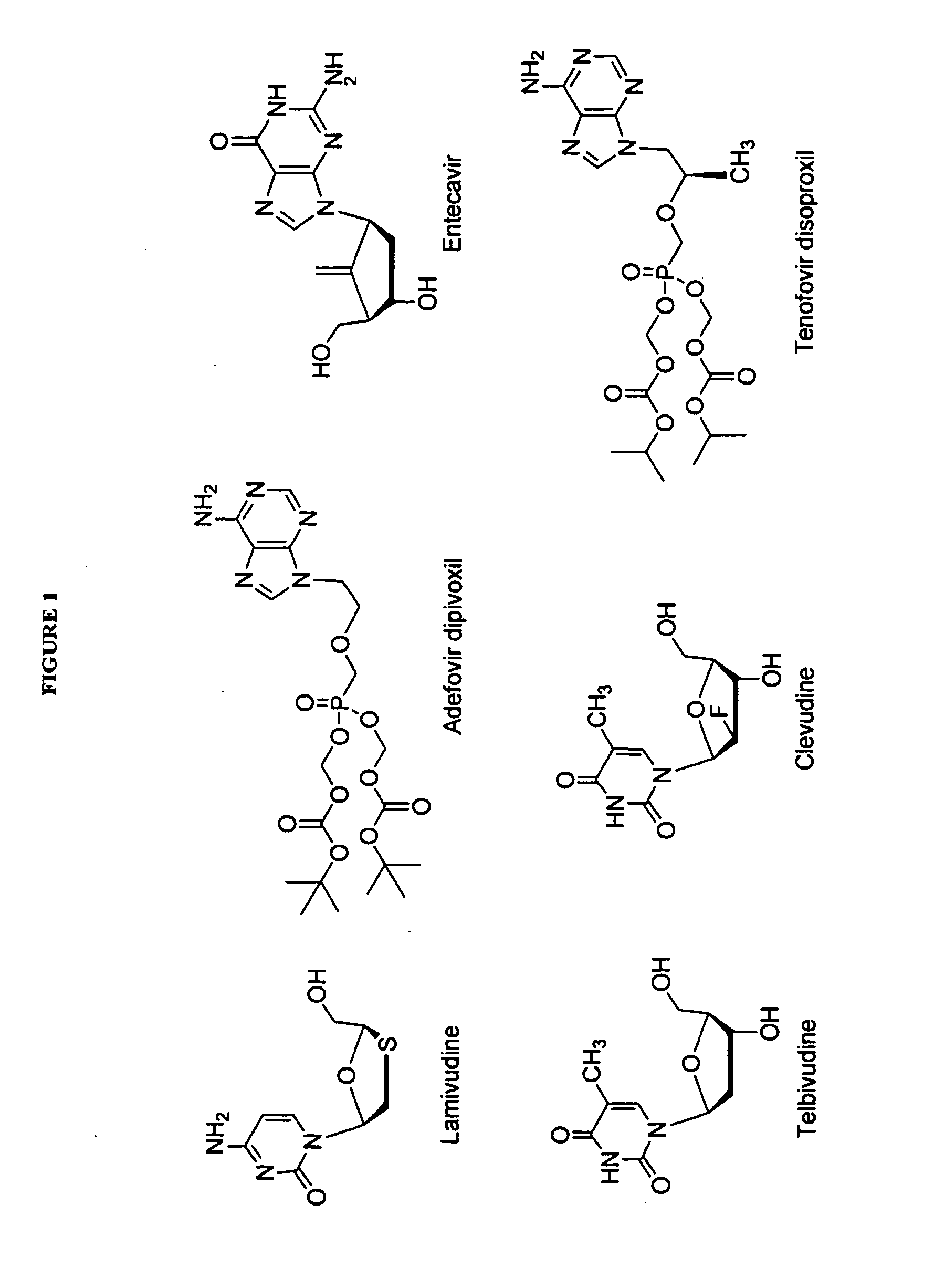
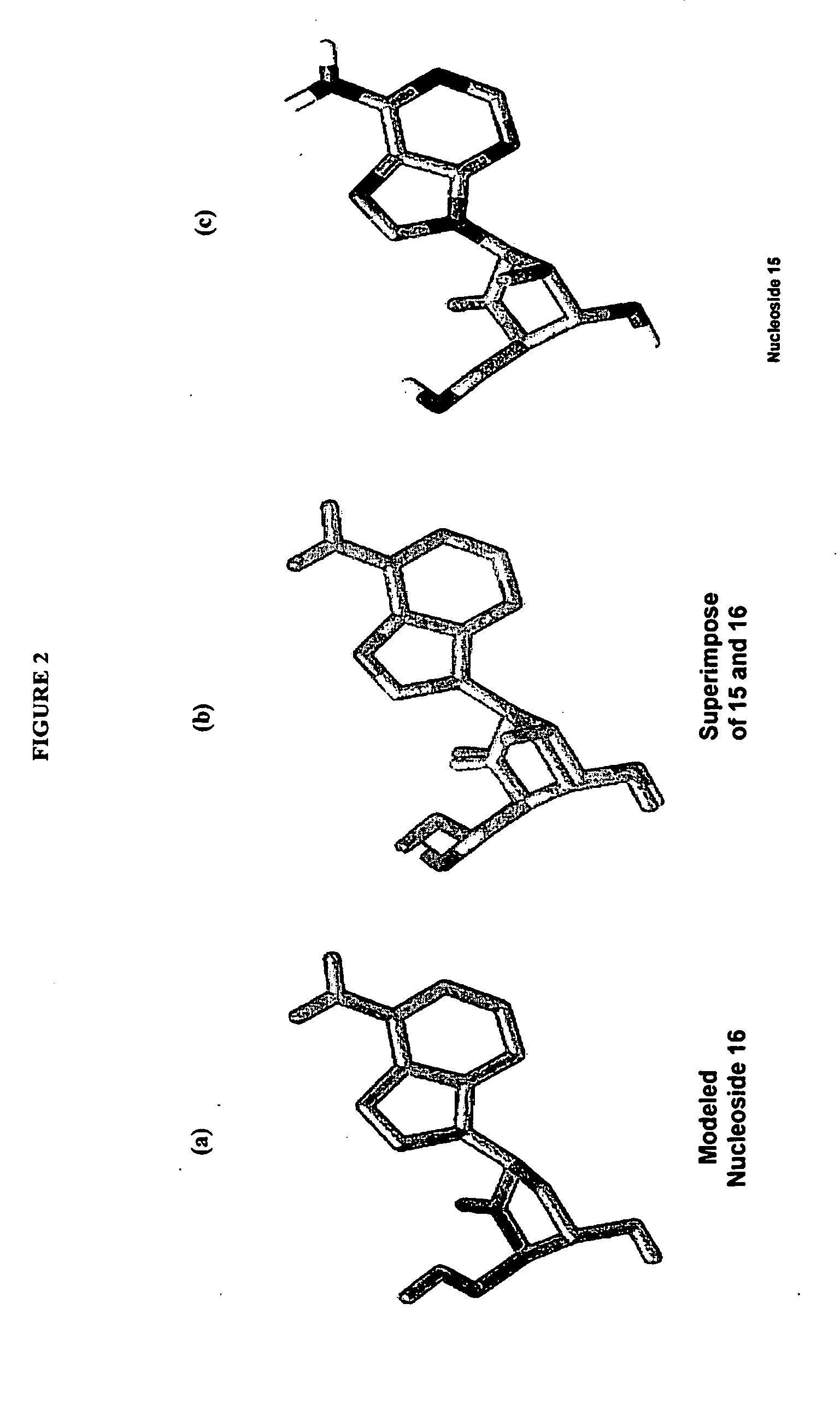
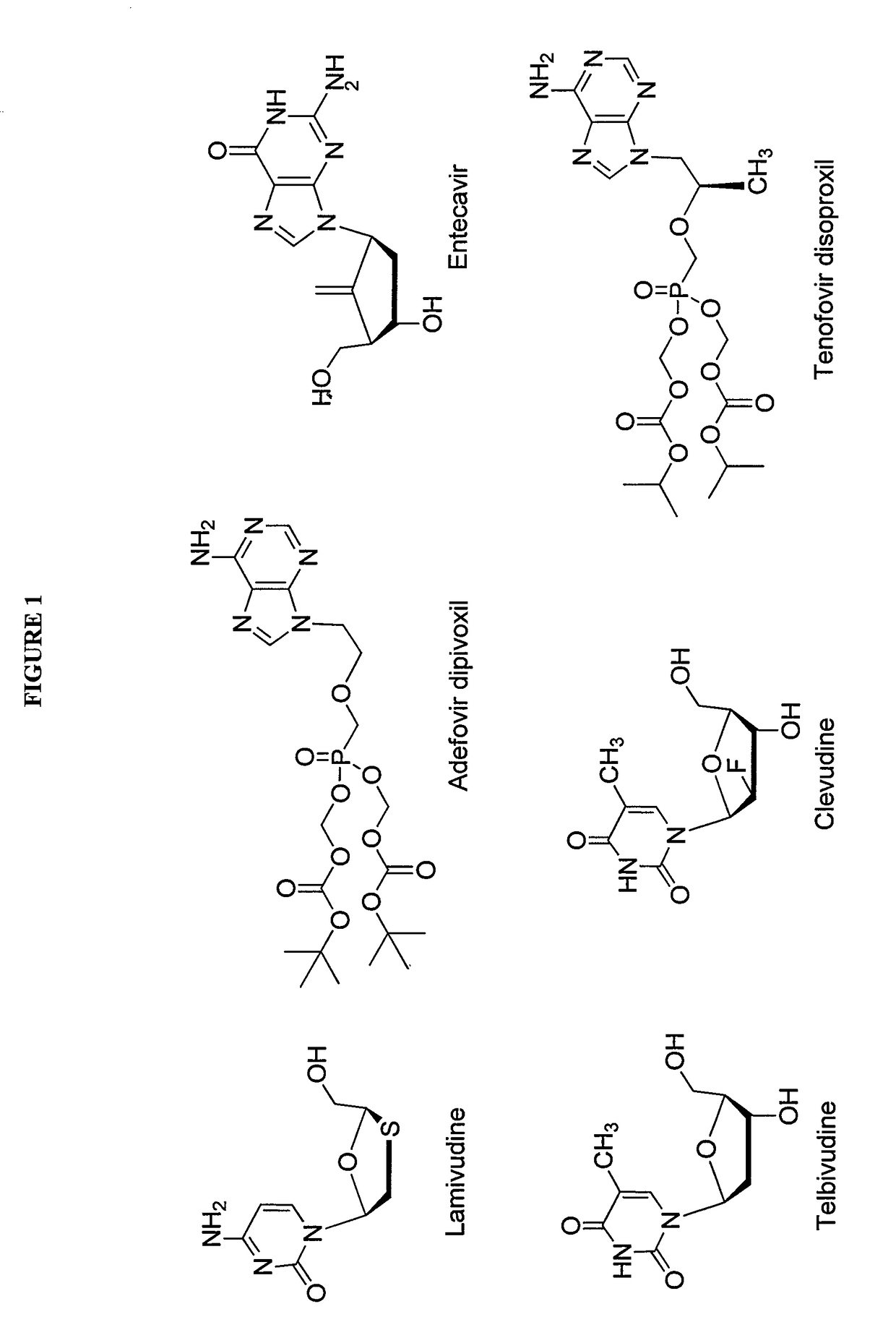
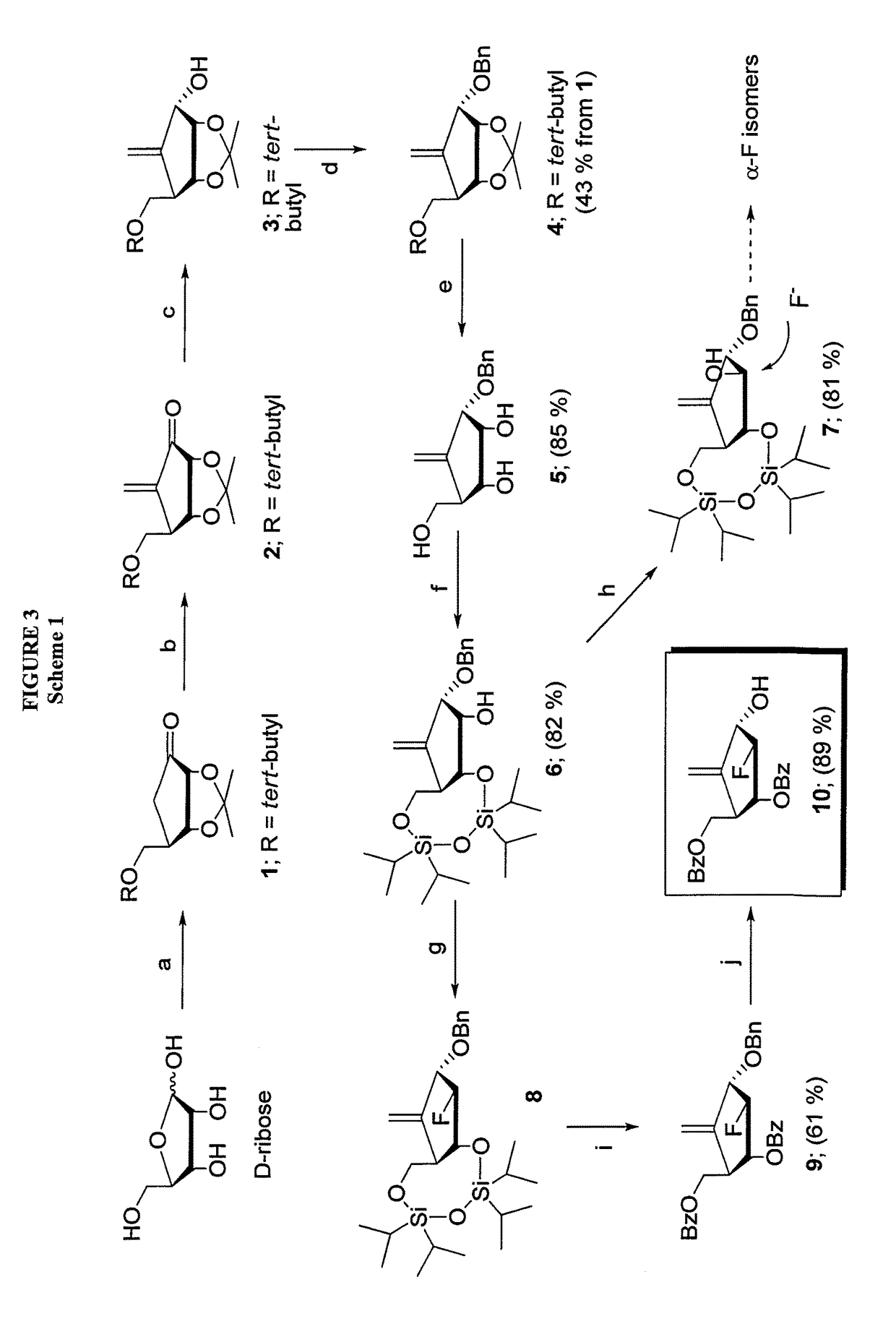
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
2'-Fluoro-6'-Methylene Carbocyclic Nucleosides and Methods of Treating Viral Infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
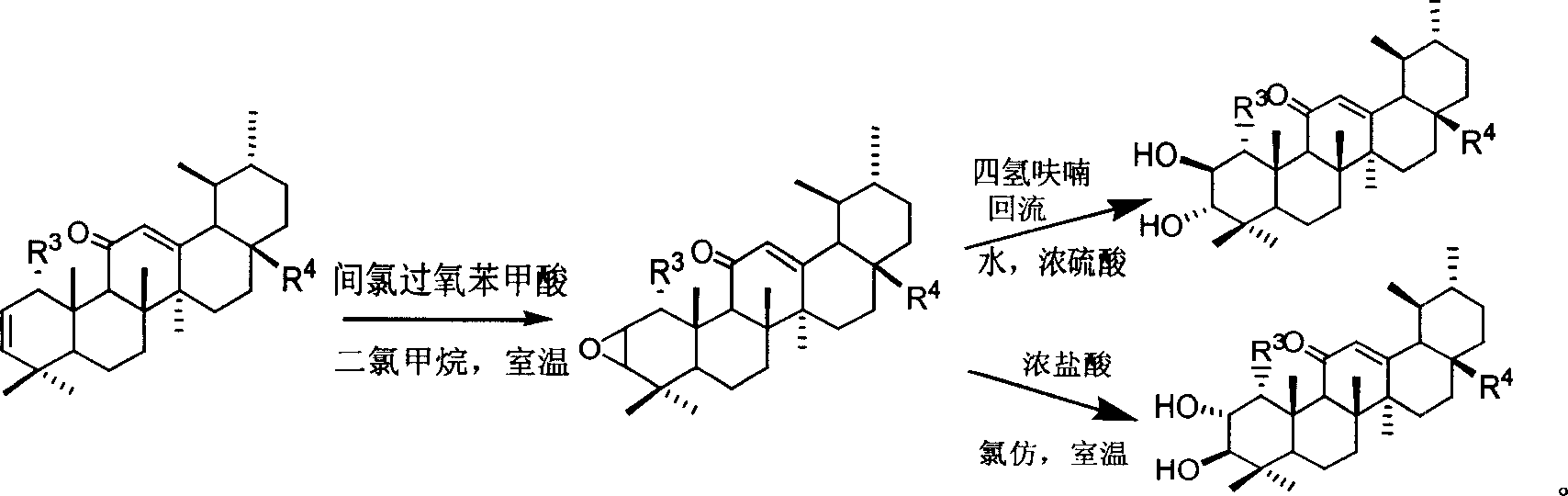
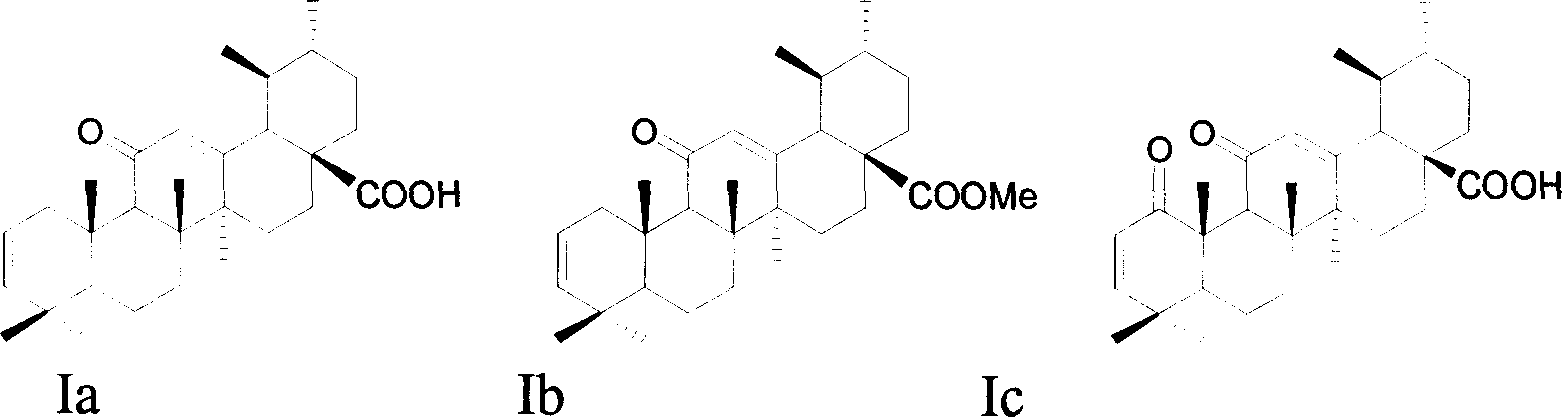
A macrocyclic oxidation substituted pentacyclic triterpanoids derivative and preparation method and use thereof
InactiveCN101117349AStrong inhibitory activityOrganic active ingredientsMetabolism disorderAlgluceraseAnti-Tumor Drugs
The present invention relates to a pentacyclic triterpanoid derivative of multiple-oxide substitution of the A ring and the medicine salt or solvate of the derivative, and the present invention also relates to the preparation method, the drug combination, and medical use of the derivative. The compound of the present invention has the functions of inhibiting the activity of six human tumor cell strains in vitro, such as human prostate cancer cell (PC-3), nasopharyngeal carcinoma cells (CNE), oral squamous carcinoma cell(KB), human lung cancer cell (A549), human hepatoma cell (BEL-7404), and human cervix cancer cell (Hela), and the function of the invention is at the same magnitude of the positive control of cisplatin, thereby the compound can be used as expected antitumor drug. The compound of the present invention also inhibits the alpha glucosidase strongly, and the inhibiting effect is greater than the positive control of acarbose, thereby the compound can be used as expected medicine for preventing and treating diabetes and the treatment of the virus diseases.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Antibodies, pharmaceutical compositions and methods
ActiveUS20170283488A1Polypeptide with localisation/targeting motifAntibody mimetics/scaffoldsURINARY BLADDER CARCINOMASquamous Carcinomas
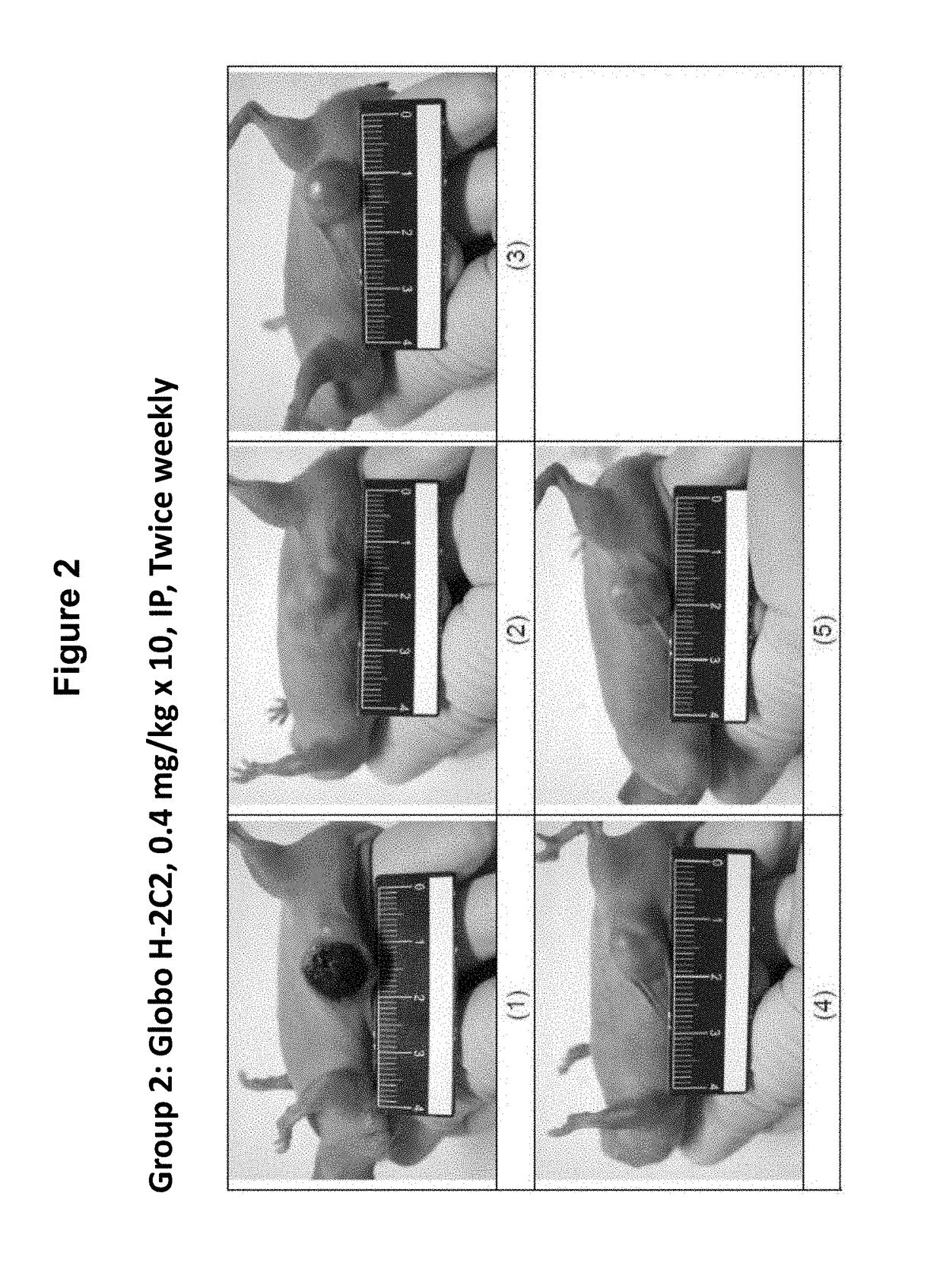
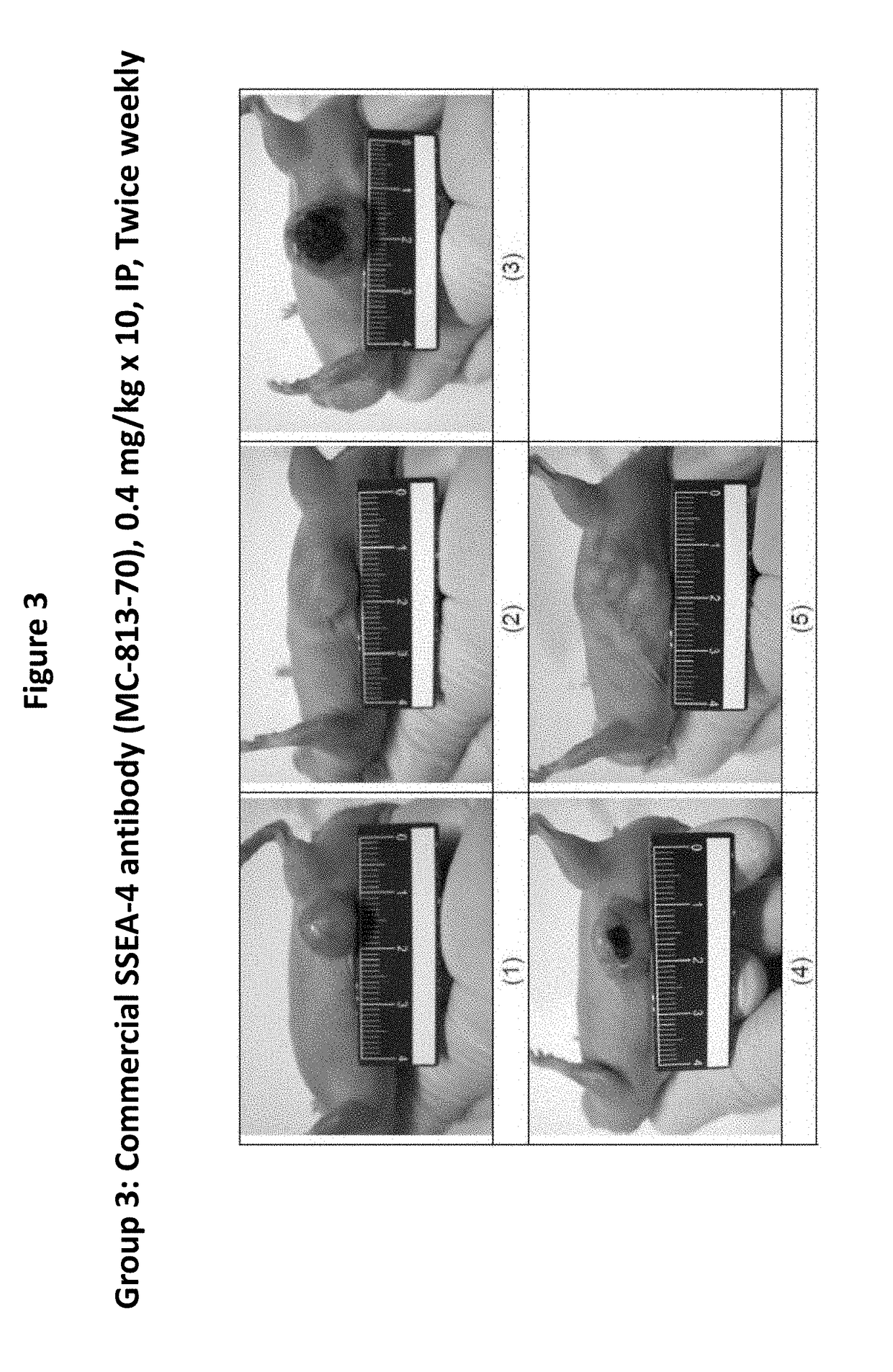
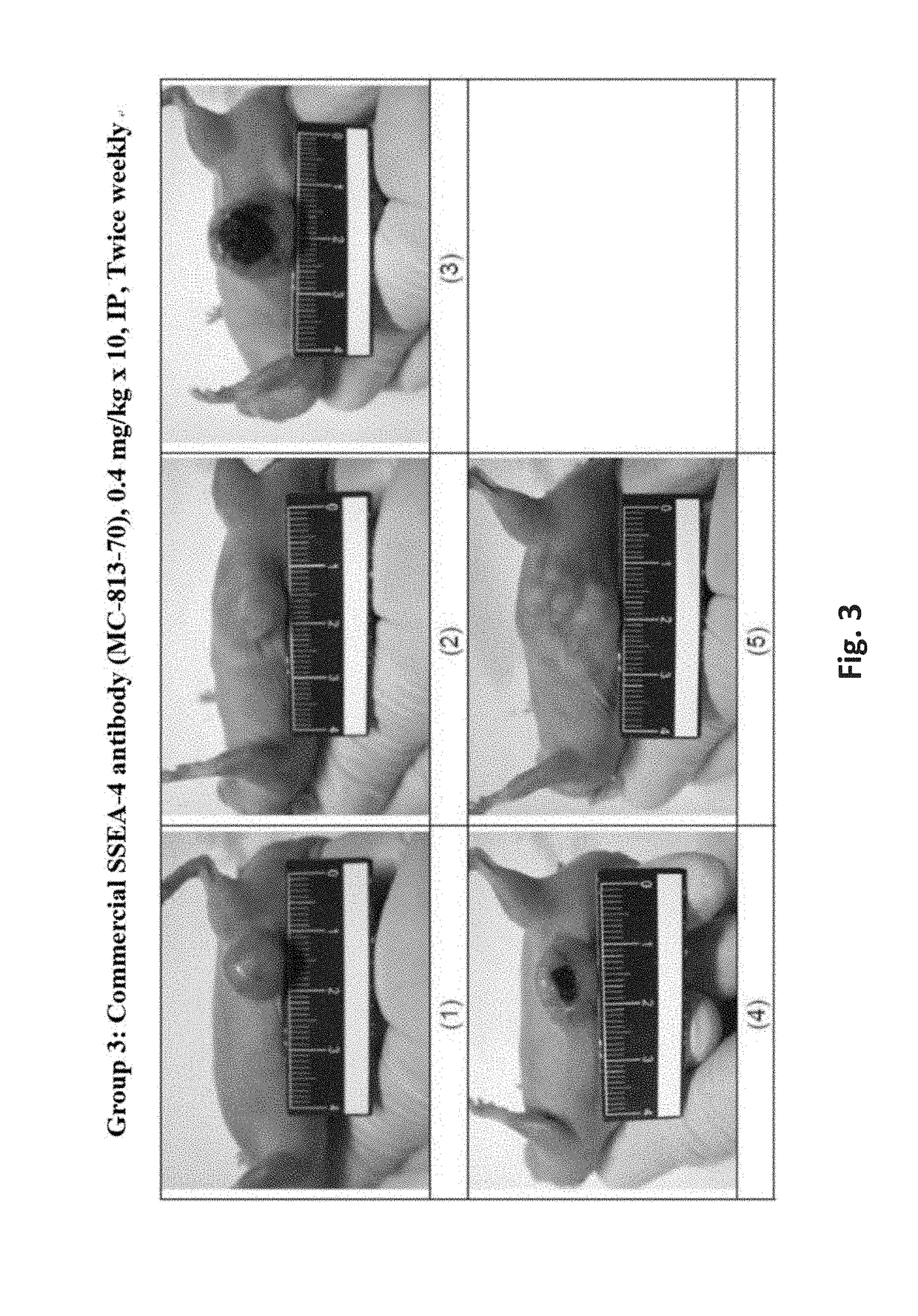
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA INC
2'-fluoro-6'-methylene carbocyclic nucleosides and methods of treating viral infections
ActiveUS20130005677A1Reduce infectious virus titerReduce cell viabilityBiocideSugar derivativesHerpes simplex diseaseCirrhosis
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Heptatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses.
Owner:UNIV OF GEORGIA RES FOUND INC
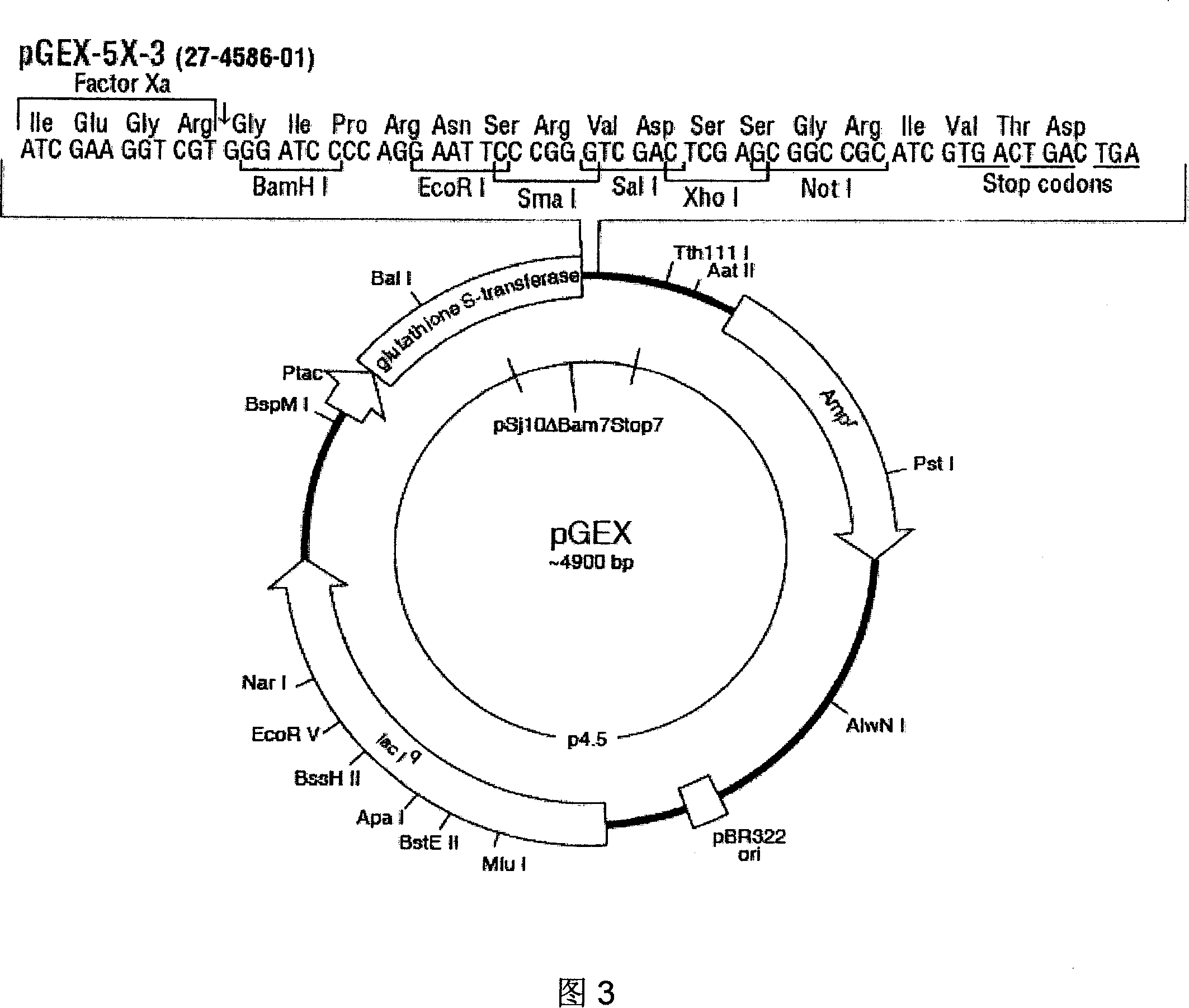
ELISA reagent kit for screening, diagnosis and treatment effect forecast of nasopharyngeal carcinoma
The present invention discloses two antigen protein fragments of protein product Rta of nasopharyngeal specific gene BRLF1, encoding gene, recombinant carrier, fusion protein (GST and GST-R185-R150), nasopharyngeal cancer diagnosis agent containing the compounds and the ELISA agent box, as well as the application of the compounds in detection of EB virus in vitro. The ELISA agent box of the present invention can be used for nasopharyngeal cancer screening, early diagnosis and forecasts of treatment results. The present invention has the advantages of simplicity, sensitivity and specificity, and is suitable for early diagnosis and forecasts of nasopharyngeal carcinoma, and the large-scale screening of high-risk groups. The present invention is easy to be widely promoted and used; the use is safe and clean; the present invention has broad market prospects.
Owner:同昕生物技术(北京)有限公司
Tissue chip used for tumour early stage diagnosis and preparation device
Three kinds of tissues including cancer tissue, precancerosis and corresponding normal tissue are sliced up, dyed, marked, and positioned. Receptor holes are prepared by leading designed lattice array mould paper to paste on surface of wax block of receptor. Wax block with tissue core bar is prepared by using perforating needle and puncture needle for tissue. Common cancer such as lung cancer, nasopharyngeal carcinoma, oesophagus cancer etc. and having integrated clinical data and pathology features are selected. Through in situ hybridization, testing mRNA of relevant gene and expression of protein on tissue chip, consistent result between the invented product and traditional test is validated. In the product, cellular morphology is clear and even, and there is no fallen off tissue point. The invention is applicable to filter cancers, early diagnosis and forecasting prognosis.
Owner:中南大学湘雅医学院肿瘤研究所
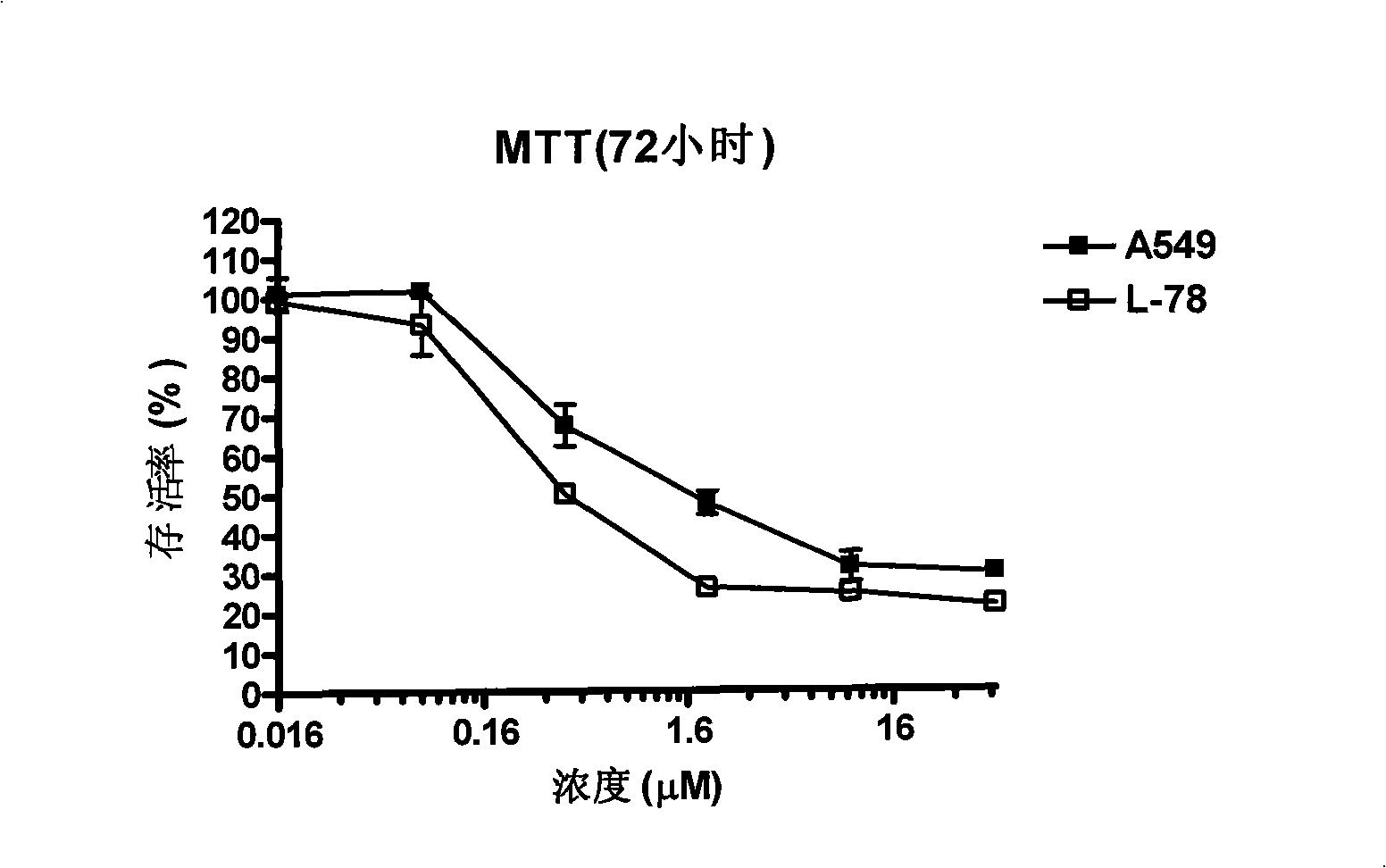
Fly maggot extractive as well as preparation method and application thereof
ActiveCN101991608AEnhance the body's humoral immunitySimple and efficient operationAnthropod material medical ingredientsAntineoplastic agentsMaggotWater soluble
The invention discloses a fly maggot extractive containing the principal components of water-soluble proteins, and the water-soluble proteins contained in the fly maggot extractive have the mass percentage of 50-70 percent and the molecular weight of 2-16 KDa. The fly maggot extractive not only has obvious inhibiting effect on human promyelocytic leukemia HL-60 cells, human erythroleukemia K562 cells, human liver cancers SMMC-7721, mouse leukemia P388 cells, human lung adenocarcinoma A549 cells, human nasopharyngeal darcinoma CNE cells, human prostatic carcinoma PC3 cells, human cervical carcinoma HeLa in vitro, but also has outstanding inhibiting effect on mouse S180 sarcomas and mouse Heps liver cancer solid tumors, and also has the effect on enhancing the humoral immunity of organisms. The invention also discloses a preparation method of the fly maggot extractive. The preparation method is easy and convenient for operation and control and low in cost and is suitable for industrialized production.
Owner:浙江佰科堂生物科技股份有限公司
Novel use of niclosamide and pharmaceutically acceptable salt thereof
InactiveCN101254183AGood curative effectImproved prognosisOrganic active ingredientsAntineoplastic agentsProstate cancerTherapeutic effect
The invention discloses an application of niclosamide or pharmaceutically-acceptable salts thereof in preparing anti-tumor drugs, which provides a candidate drug for tumor patients and may further improve the therapeutic effect and the prognosis to patients. The effectiveness of niclosamide or pharmaceutically-acceptable salts thereof on various tumor cells indicates that the composition may be used for treating various cancers such as cerebroma, genitourinary tumor, lymphatic system, stomach cancer, cancer of larynx, nasopharyngeal cancer, skin cancer, bone cancer, leukaemia, leukaemia, breast cancer, histiocytic lymphoma, non-small-cell lung cancer, small-cell lung cancer, lung adenocarcinoma, epidermoid cancer, pancreatic cancer, prostatic cancer, liver cancer and epithelial cell cancer.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Antibodies, pharmaceutical compositions and methods
ActiveUS20180339061A1Effective preventionEffective treatmentOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSquamous CarcinomasProstate cancer
Pharmaceutical composition comprising antibodies or antigen binding fragments thereof that bind to stage-specific embryonic antigen 4 (SSEA-4) are disclosed herein, as well as methods of use thereof. Methods of use include, without limitation, cancer therapies and diagnostics. The antibodies of the disclosure can bind to certain cancer cell surfaces. Exemplary targets of the antibodies disclosed herein can include carcinomas, such as breast cancer, lung cancer, esophageal cancer, rectal cancer, biliary cancer, liver cancer, buccal cancer, gastric cancer, colon cancer, nasopharyngeal cancer, kidney cancer, prostate cancer, ovarian cancer, cervical cancer, endometrial cancer, pancreatic cancer, testicular cancer, bladder cancer, head and neck cancer, oral cancer, neuroendocrine cancer, adrenal cancer, thyroid cancer, bone cancer, skin cancer, basal cell carcinoma, squamous cell carcinoma, melanoma, and / or brain tumor.
Owner:OBI PHARMA
Antineoplastic composition and use thereof
InactiveCN101332301AIncreased drug resistanceEnhanced inhibitory effectHeavy metal active ingredientsAntineoplastic agentsMalignant lymphomaProstate cancer
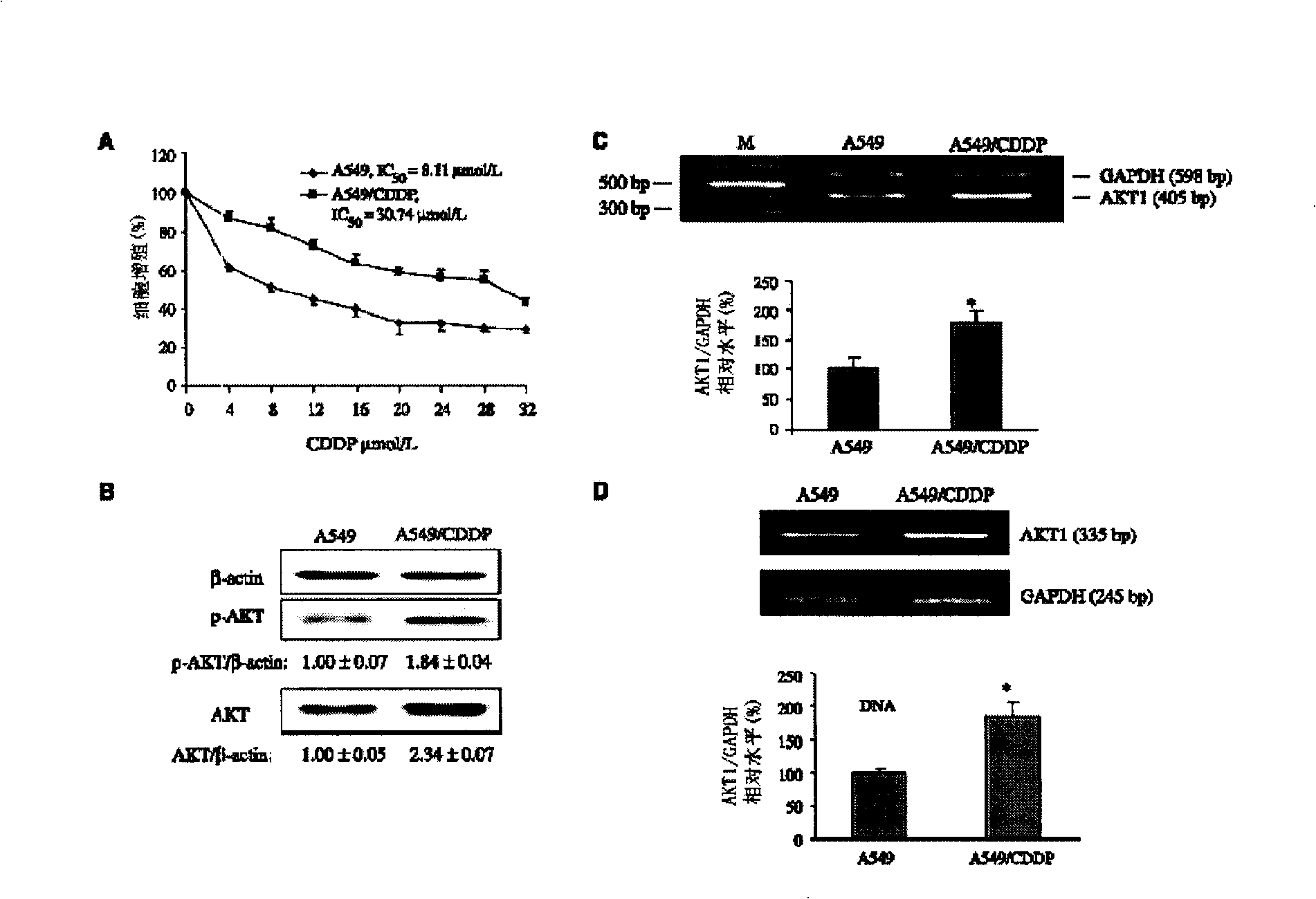
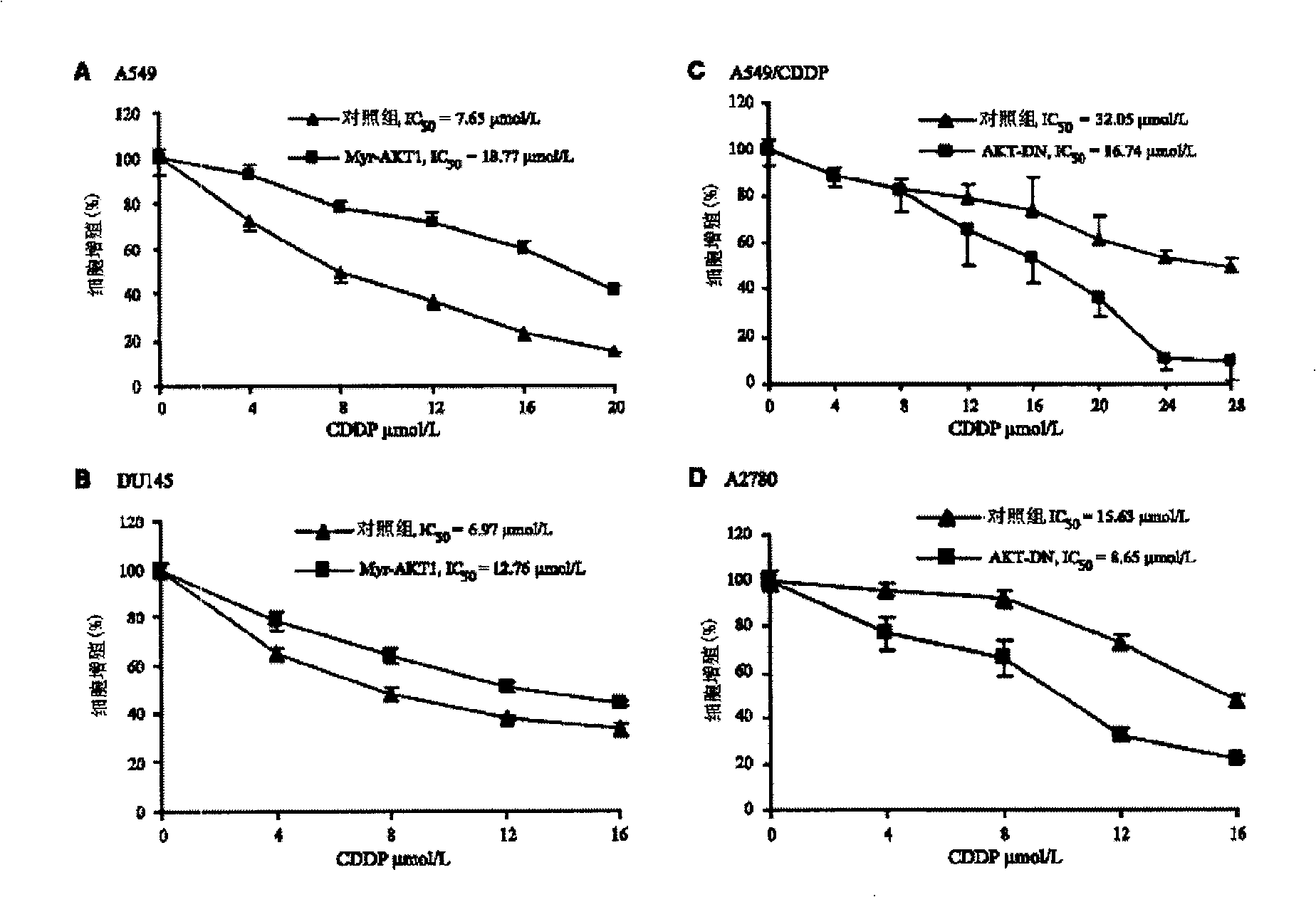
The invention discloses an anti-tumor combination and the application thereof; the anti-tumor combination which includes effective dose of platinum-based chemotherapy and AKT inhibitor and / or p70S6K1 inhibitor has the function of inhibiting the drug resistance of the tumor to the platinum-based chemotherapy, improves the effects of anti-tumor drugs, and can be used for preparing drugs that can resist various tumors, such as lung cancer, ovarian cancer, prostate cancer, breast cancer, stomach cancer, nasopharyngeal cancer, esophageal cancer, malignant lymphoma, head and neck squamous cell carcinoma, thyroid cancer, osteosarcoma, etc.
Owner:NANJING MEDICAL UNIV
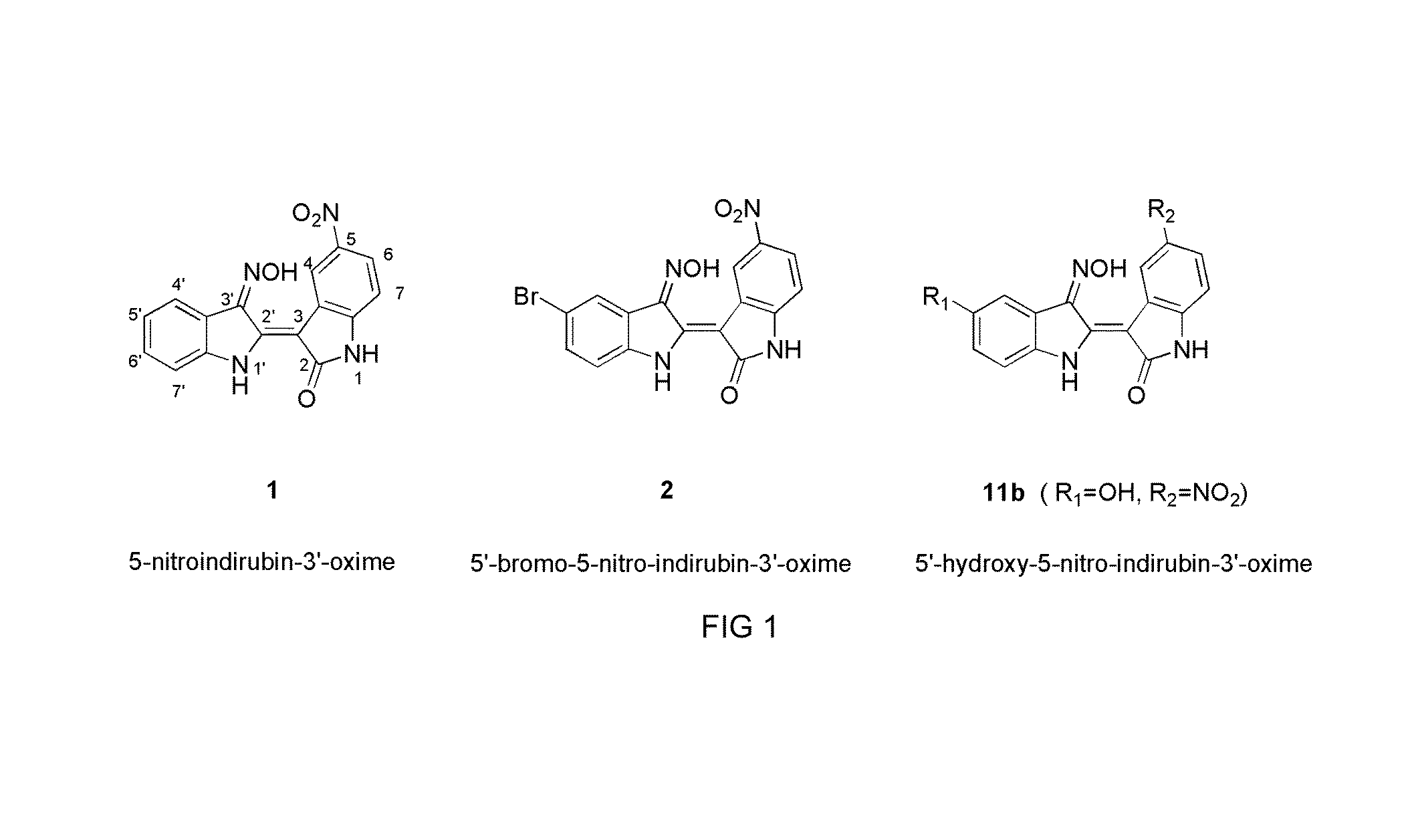
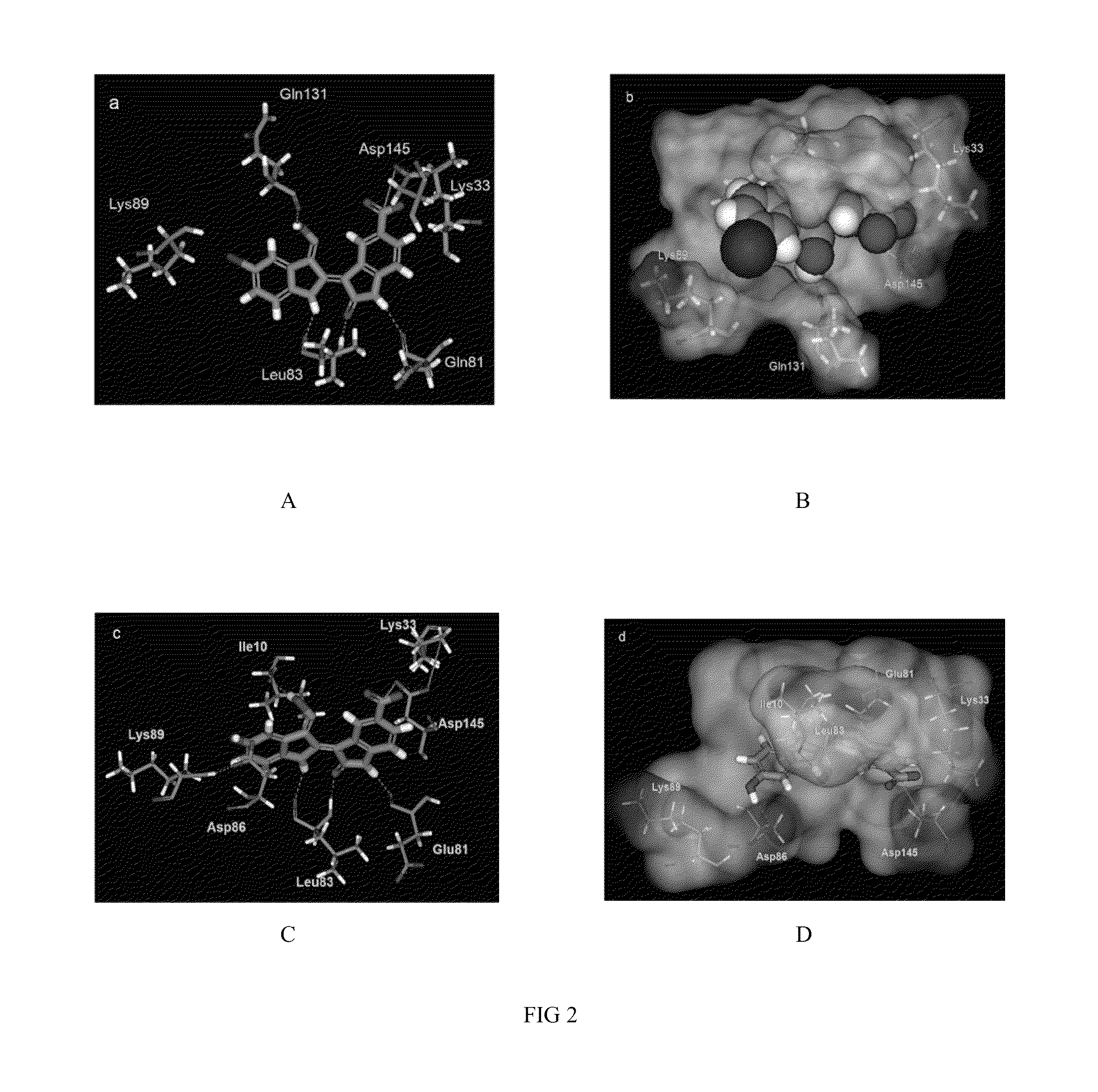
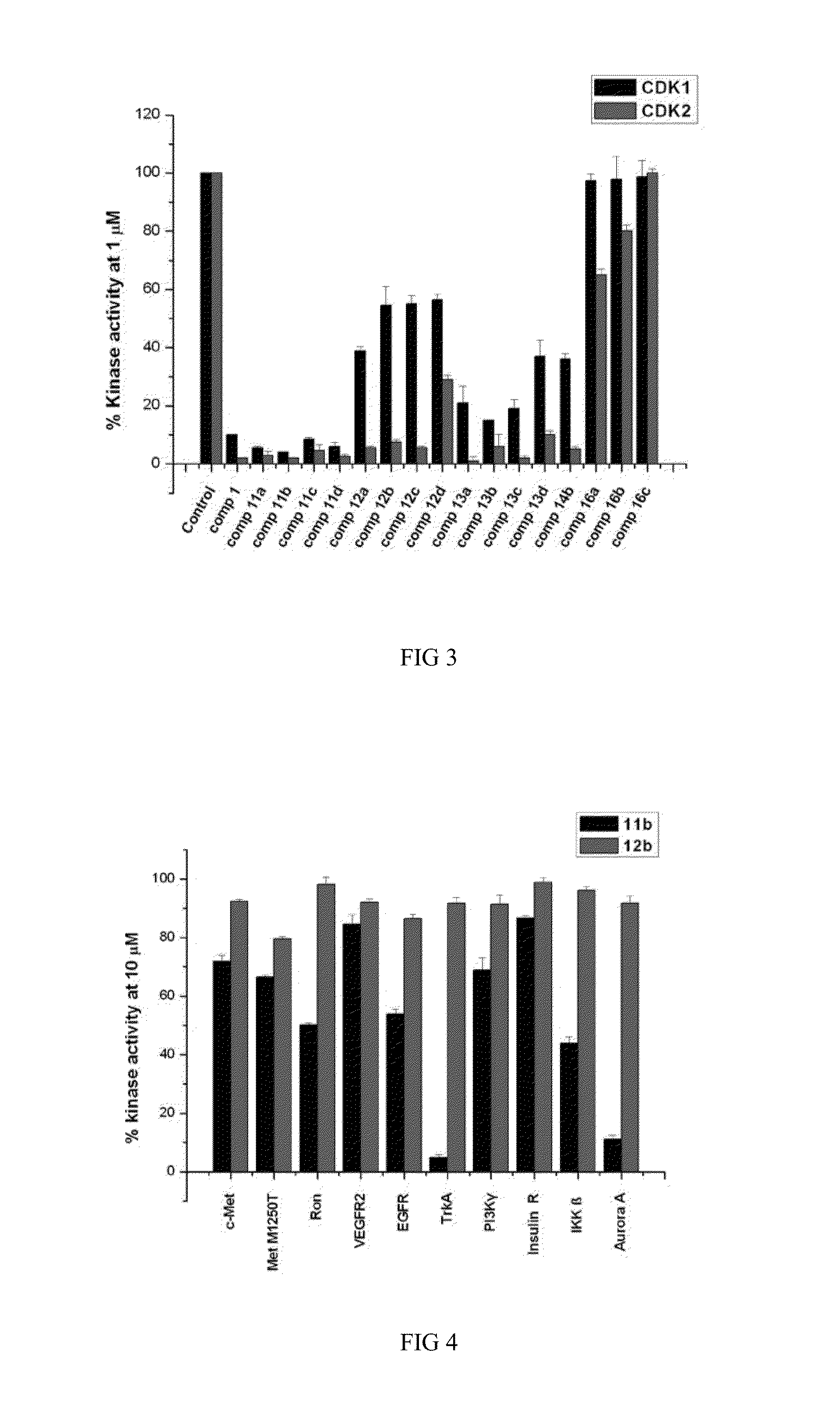
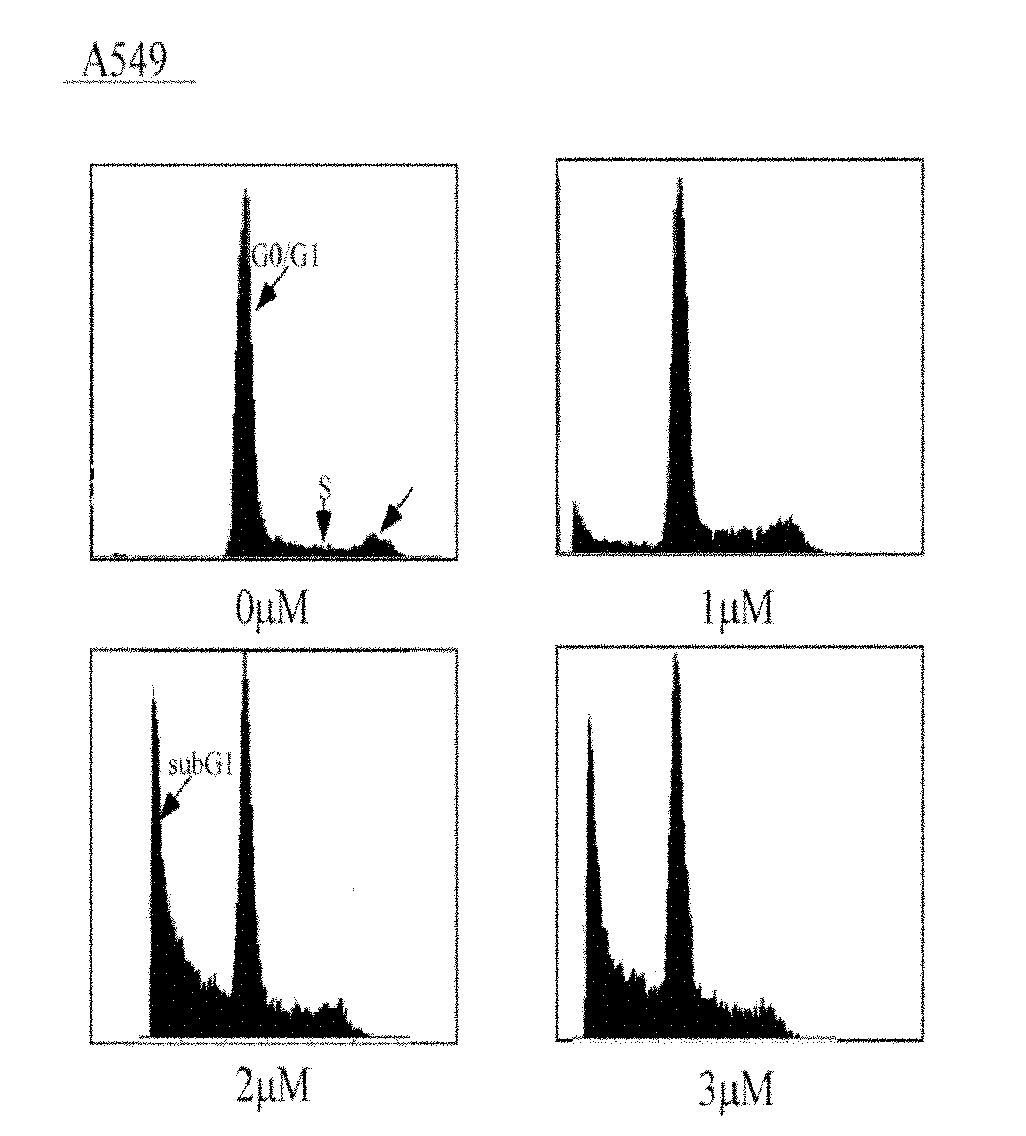
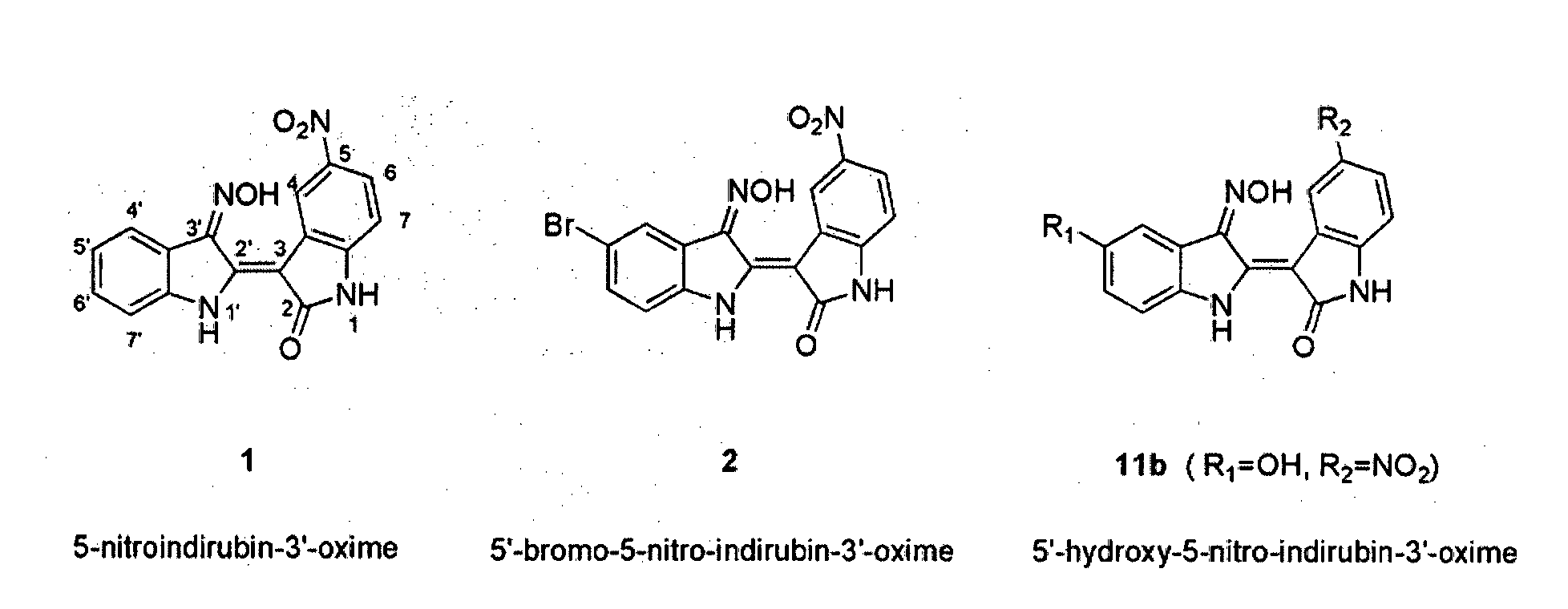
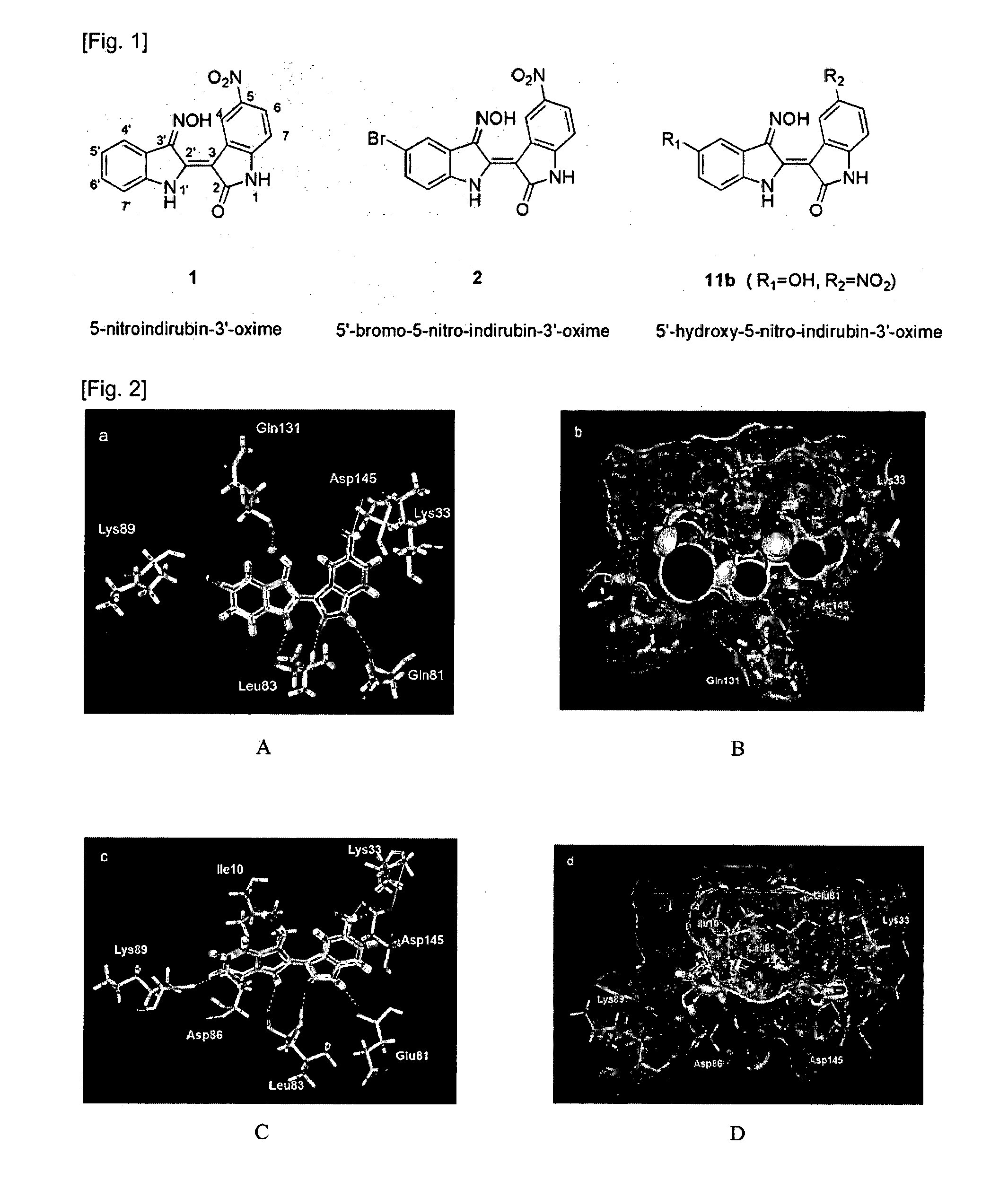
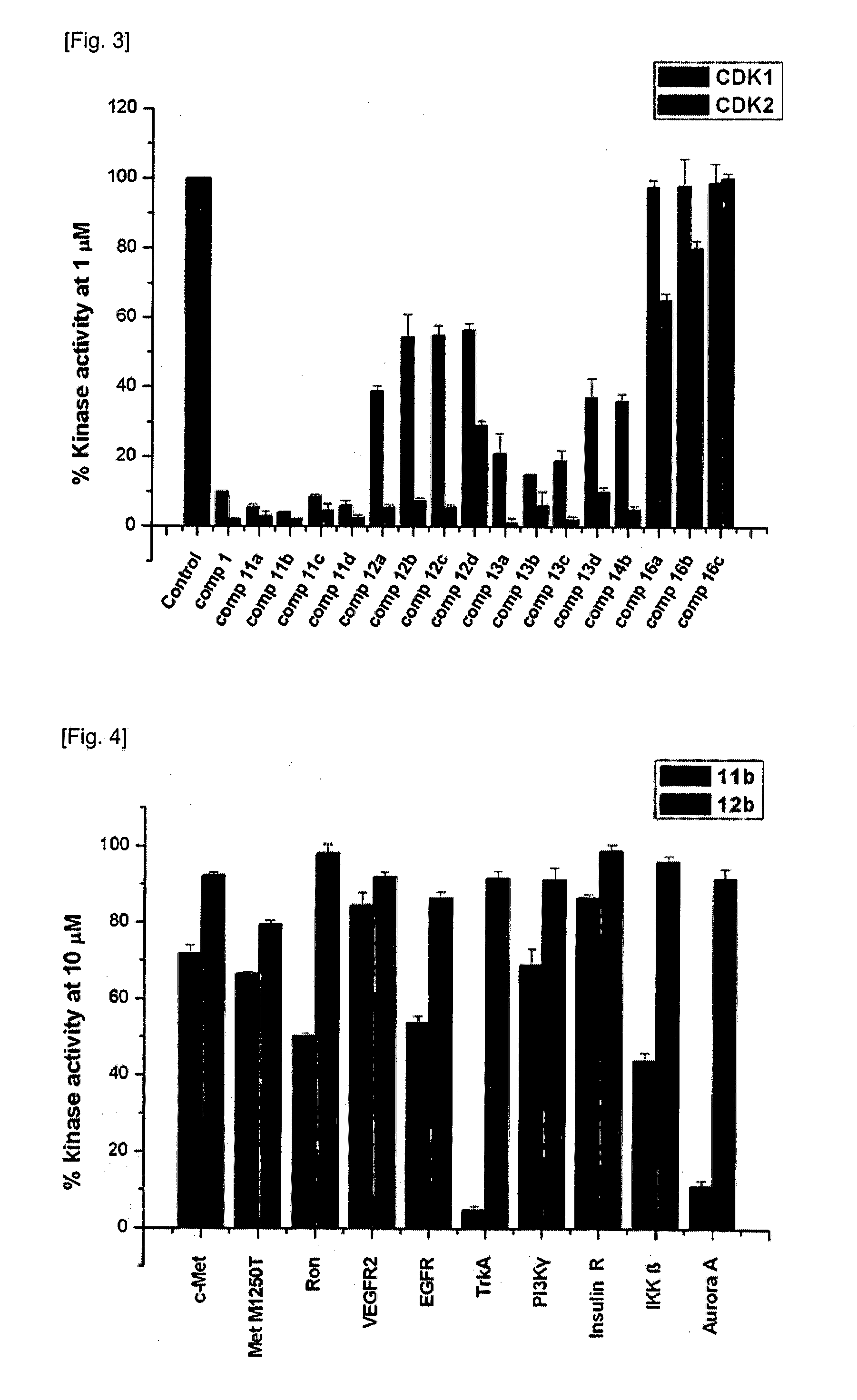
Indirubin-3'-oxime derivatives as potent cyclin dependent kinase inhibitors
The present invention relates to an indirubin-3′-oxime derivative as potent cyclin dependent kinase inhibitor with anti-cancer activity. More particularly, this invention relates to an indirubin-3′-oxime derivative as potent cyclin dependent kinase inhibitor having excellent anti-cancer activity against human lung cancer cell, human fibro sarcoma cell, human colon cancer cell, human leukemia cell, human stomach cancer cell, human nasopharyngeal cancer cell and / or human breast cancer cell.
Owner:ANYGEN
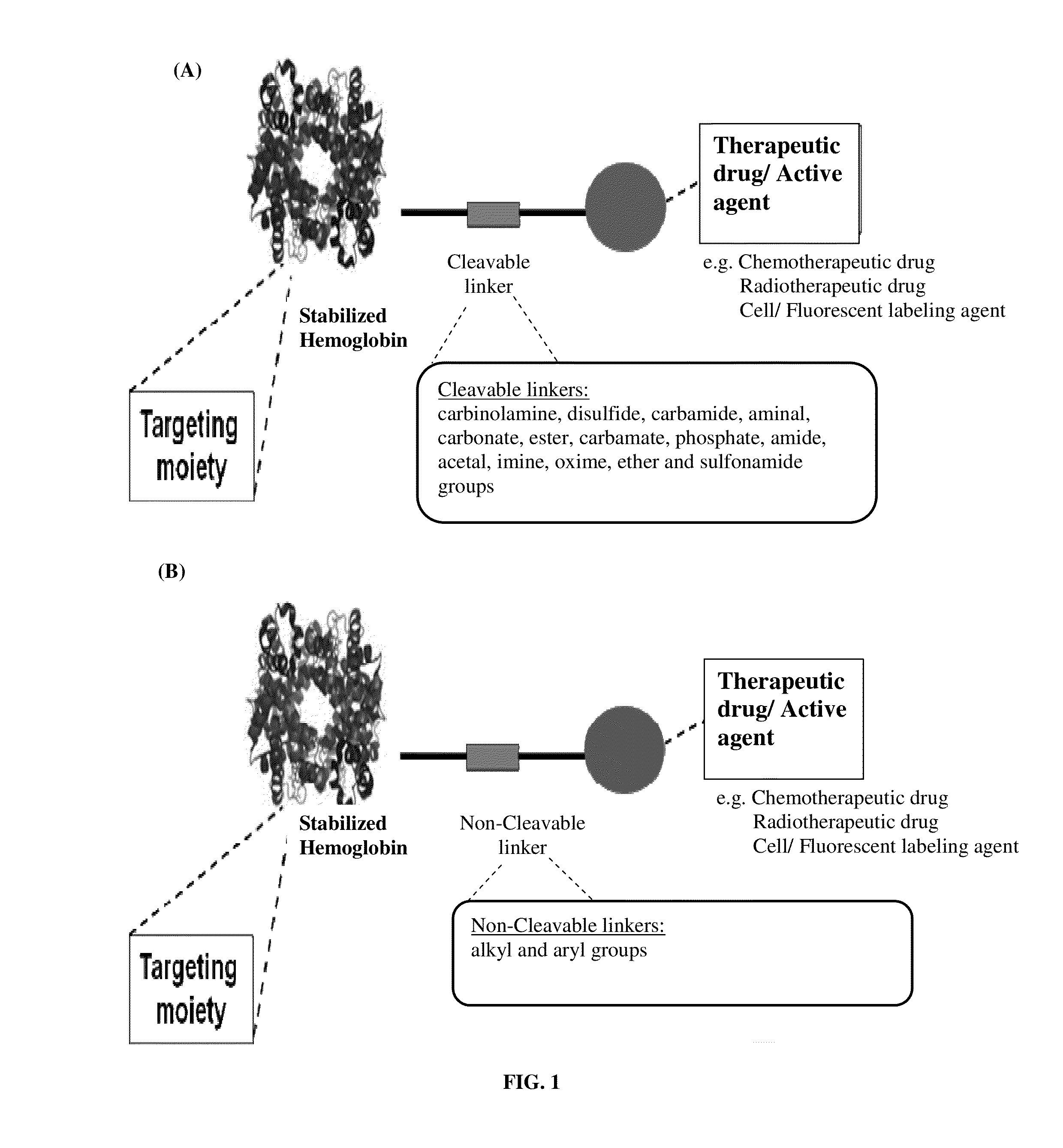
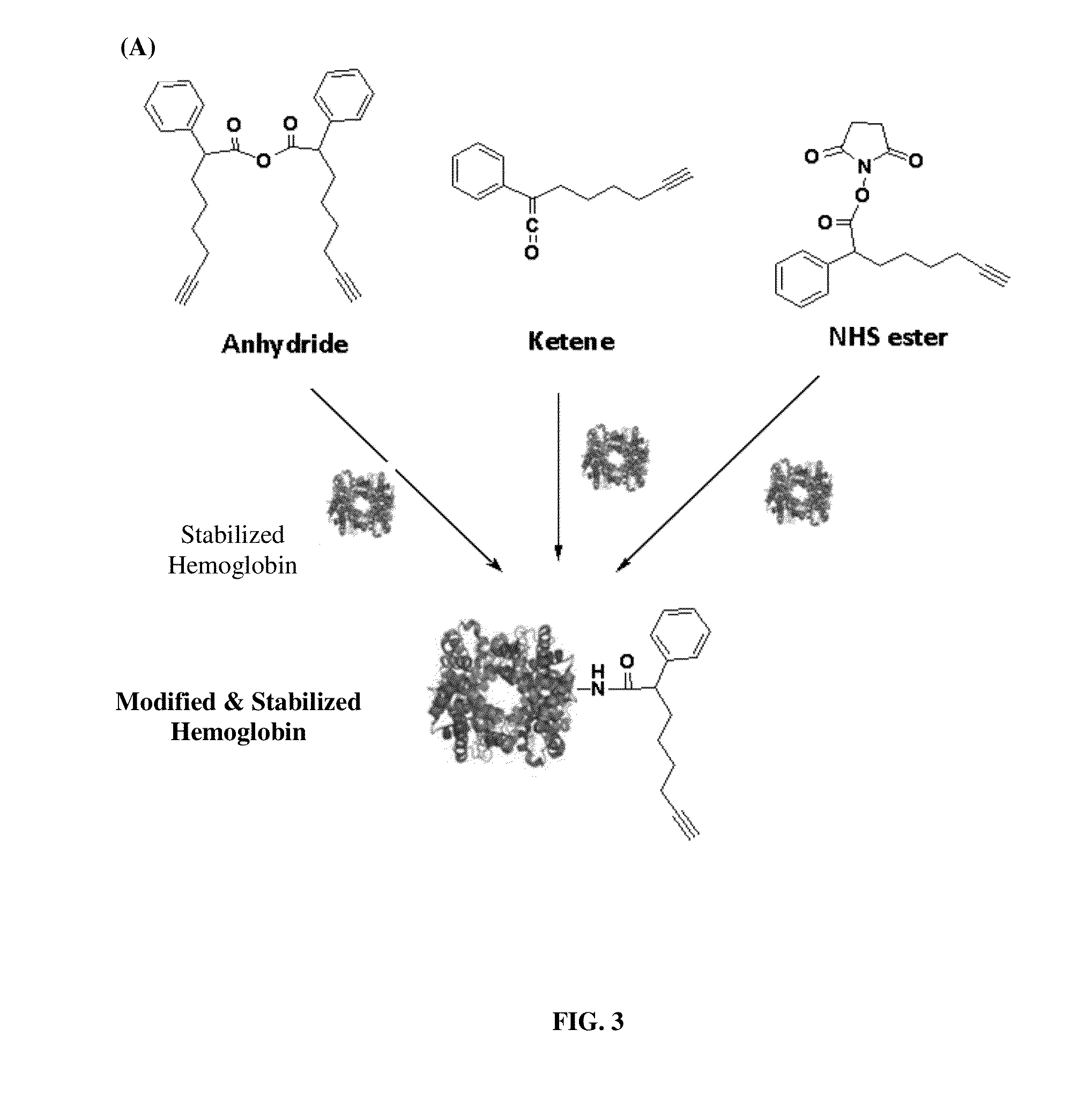
Pharmaceutical composition comprising modified hemoglobin-based therapeutic agent for cancer targeting treatment and diagnostic imaging
ActiveUS20140335018A1Synergistic effectEfficient killingUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsLymphatic SpreadCancer targeting
The present invention provides a pharmaceutical composition containing hemoglobin-based therapeutic agent for treating cancer. The hemoglobin moiety can target cancer cells and the therapeutic moiety (i.e. active agent / therapeutic drug) can kill the cancer cells efficiently. The hemoglobin-based therapeutic agent used in the present invention can be used in the treatment of various cancers such as pancreatic cancer, leukemia, head and neck cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal cancer, esophageal cancer, prostate cancer, stomach cancer and brain cancer. The composition can be used alone or in combination with other therapeutic agent(s) such as chemotherapeutic agent to give a synergistic effect on cancer treatment, inhibiting metastasis and / or reducing recurrence. The presently claimed hemoglobin-based 5FU-two-dye conjugate and / or hemoglobin-based 5FU-one-dye conjugate can also be used in live-cell imaging and diagnostic imaging.
Owner:VISION GLOBAL HLDG
Preparation method for replication and transcription activator (Rta) protein and application of Rta protein to nasopharynx cancer detection reagent
The invention discloses a preparation method for a replication and transcription activator (Rta) protein and the application of the Rta protein to a nasopharynx cancer detection reagent and relates to a medical diagnosis reagent. The preparation method disclosed by the invention comprises the following steps of: 1, constructing a recombinant expression vector by taking a BRLF1 full-length gene as an exogenous gene; 2, transfecting: transfecting the recombinant expression vector into an eukaryotic expression system to obtain a positive transfected cell; and 3, expressing and purifying: culturing the positive transfected cell so as to enable the positive transfected cell to express an interest protein, and separating and purifying the interest protein, wherein the eukaryotic expression system refers to a Chinese hamster ovary (CHO) cell. The Rta protein prepared by the method disclosed by the invention is used for detecting nasopharynx cancer; the sensitivity of the Rta protein is 96 percent (288 / 300), and the specificity of the Rta protein is 96.7 percent (290 / 300). The sensitivity and the specificity are superior to those of antigens respectively prepared by a prokaryotic expression system and a pichia expression system, and the sensitivity and the specificity on clinical early diagnosis on the nasopharynx cancer are greatly improved.
Owner:同昕生物技术(北京)有限公司
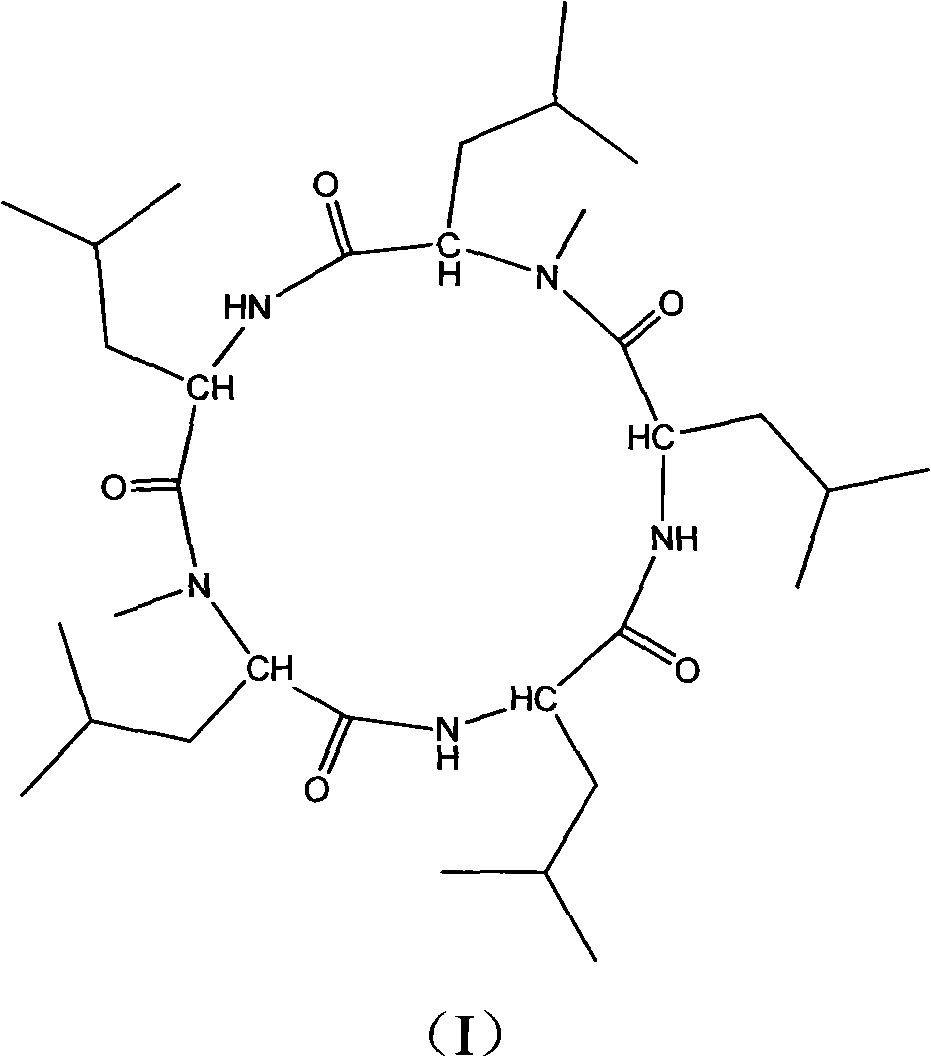
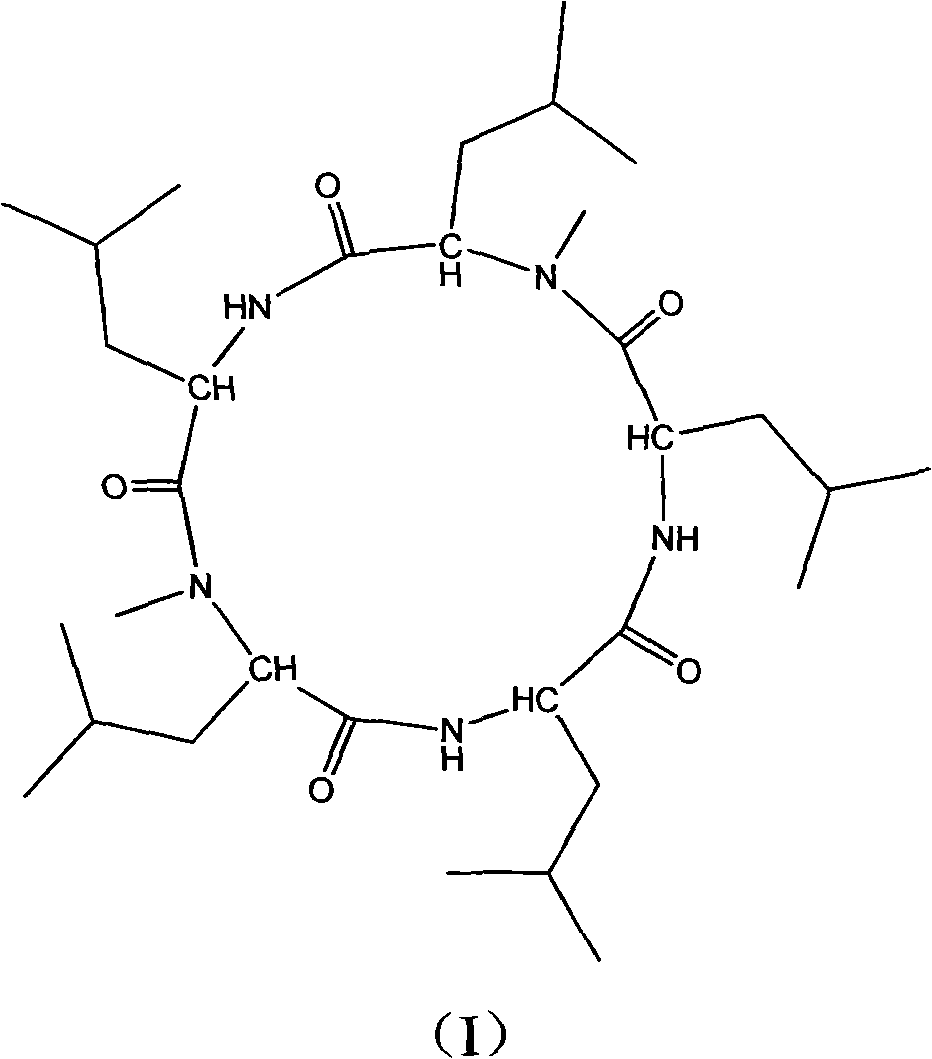
Cyclo-pentapeptide with antineoplastic activity
InactiveCN101270154AGood antitumor activitySimple preparation processPeptidesCyclic peptide ingredientsPharmaceutical ResourcesWilms' tumor
The present invention discloses a cyclic pentapeptide with anti-tumour activity, which is cyclic (leucyl-N-methylleucyl-leucyl-leucyl-N-methylleucyl) and the constitutional formula of which is shown in the formula (I). The cyclic pentapeptide of the present invention is separated from galaxaura filamentosa in the South China Sea, and in vitro experiments show that the cyclic pentapeptide has strong inhibiting effect on human hepatoma cell lines (HepG2), human hepatoma cell lines (BEL-7402), human mammary cancer cell lines (MCF-7), human colon cancer cell lines (LOVO), human lung cancer cell lines (PC84045) and human nasopharyngeal cancer (CNE) and can keep the mitotic cycle of BEL-7402 hepatoma cells in G2 / M phase. The present invention provides a pilot compound for the research and the development of new anti-tumour drugs and is valuable for the exploitation of the marine pharmaceutical resources of China.
Owner:JINAN UNIVERSITY
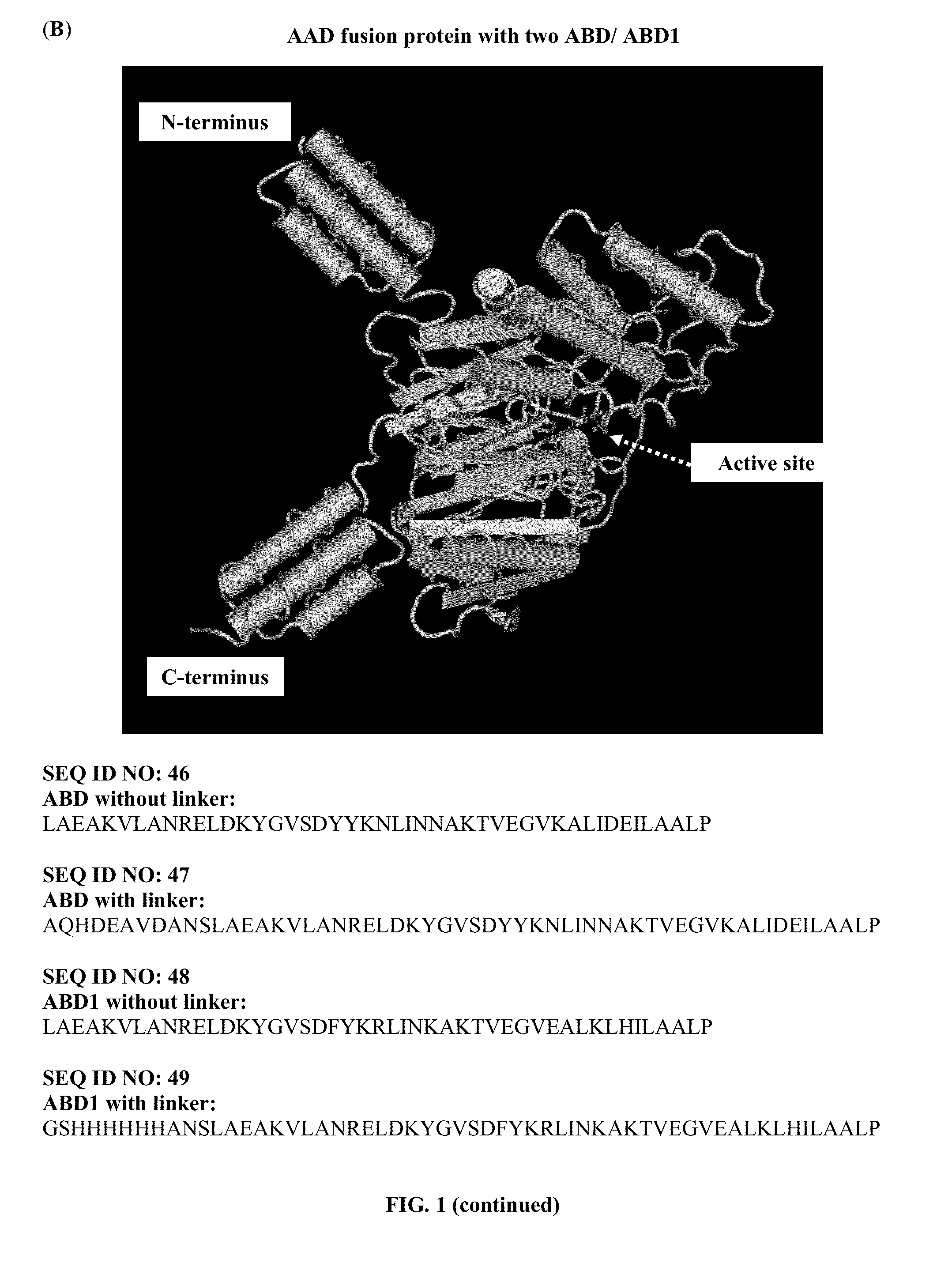
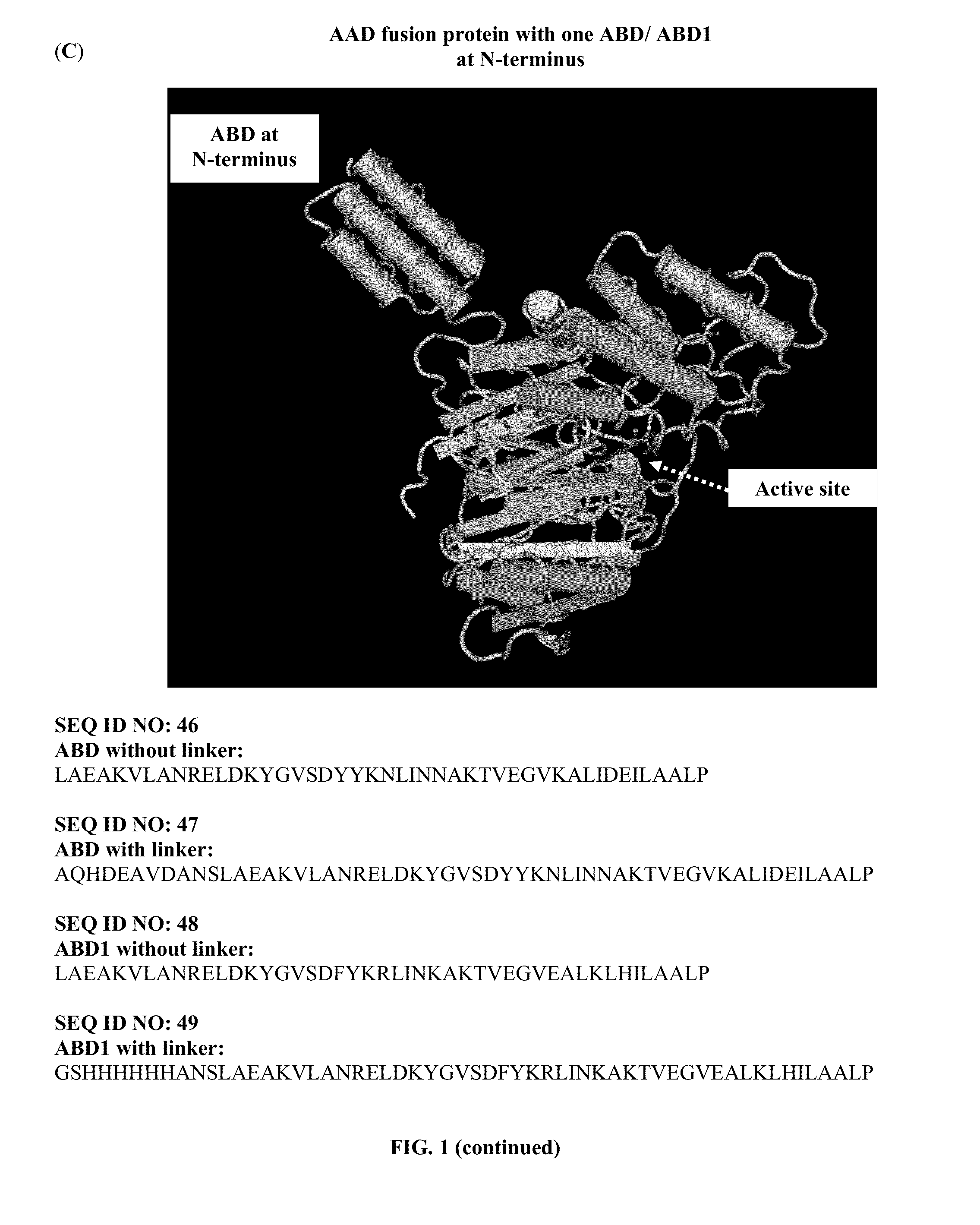
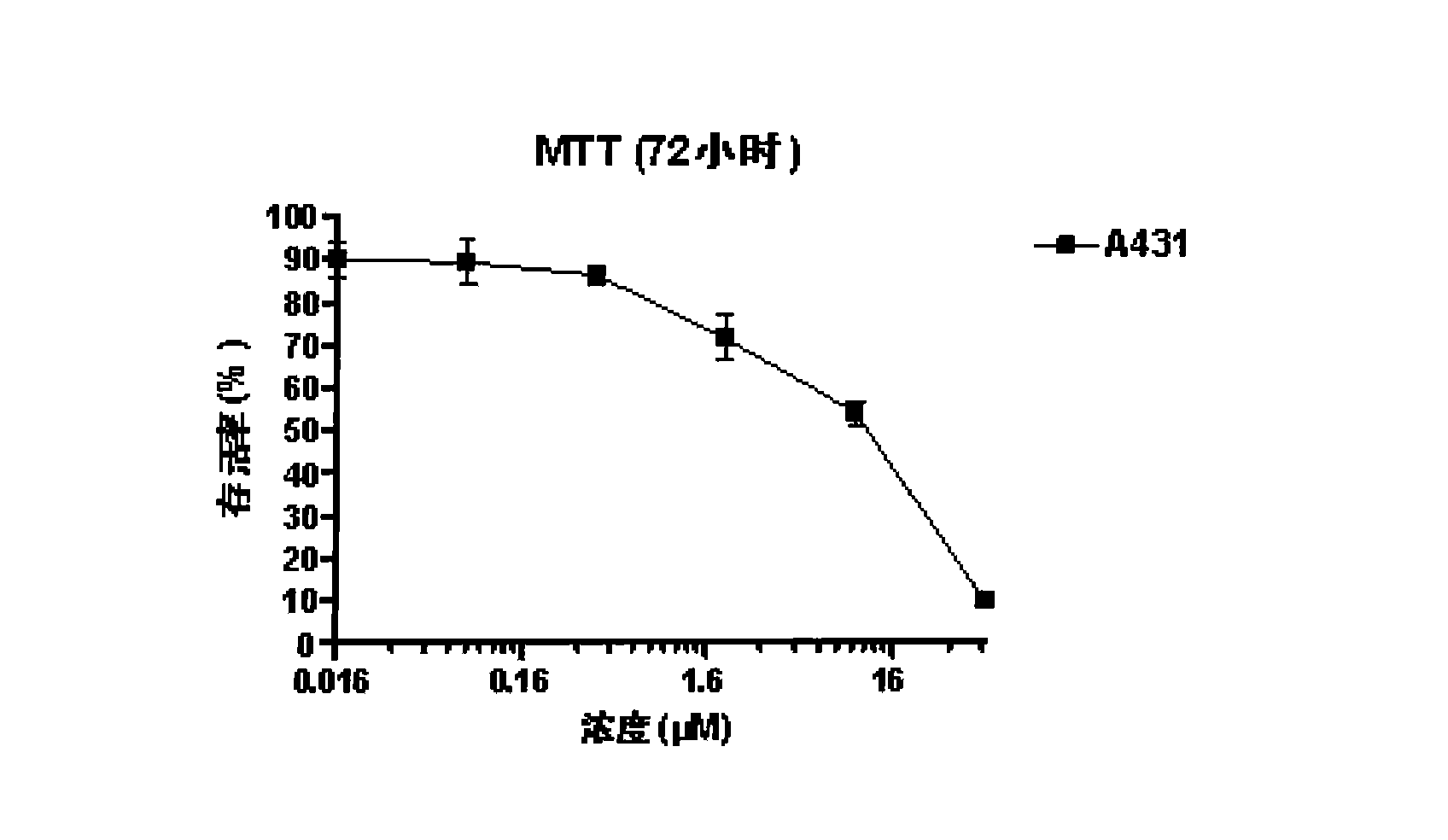
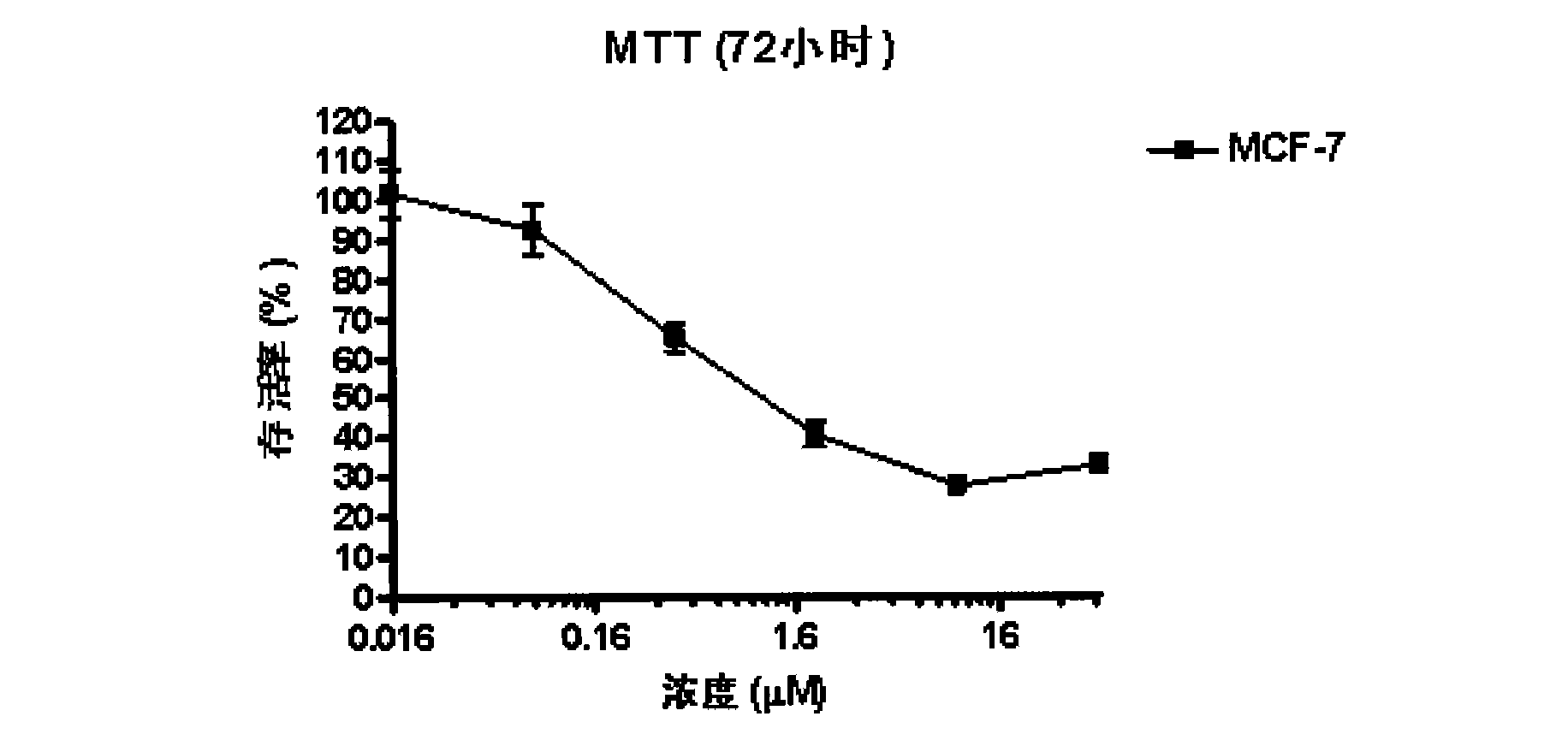
Pharmaceutical composition comprising albumin-binding arginine deiminase for cancer targeting treatment
ActiveUS20140255377A1Efficiently depletedHigh activityHydrolasesPeptide/protein ingredientsDiseaseHalf-life
The present invention provides a pharmaceutical composition containing albumin-binding arginine deiminase fusion protein (AAD) for treating cancer or other arginine-dependent diseases. The AAD fusion protein can be purified from both soluble and insoluble fractions of crude proteins, it binds to human serum albumin (HSA) and has its high activity with longer half life for efficient depletion of arginine in cancer cells. The specific activities of wild-type ADI and AAD in the present invention are 8.4 and 9.2 U / mg (at physiological pH 7.4), respectively. The AAD used in the present invention can be used in the treatment of various cancers (e.g. pancreatic cancer, leukemia, head and neck cancer, colorectal cancer, lung cancer, breast cancer, liver cancer, nasopharyngeal cancer, esophageal cancer, prostate cancer, stomach cancer & brain cancer) and curing arginine-dependent diseases. The composition can be used alone or in combination with at least one chemotherapeutic agent to give a synergistic effect on cancer treatment and / or inhibiting metastasis.
Owner:VISION GLOBAL HLDG
Application of compound as JAK-STAT3 signal passage inhibitor
InactiveCN101537001AGood curative effectImproved prognosisOrganic active ingredientsAntineoplastic agentsProstate cancerTherapeutic effect
The invention discloses an application of a compound as a JAK-STAT3 signal passage inhibitor, and particularly relates to an application of a compound with a formula I or pharmaceutically acceptable salt of the compound in the process of preparing an anti-tumor medicament. The application provides a novel treating candidate medicament for a tumor patient, thereby probably further improving the treatment effect on the patient and improving the prognosis of the patient. The compound with the formula I or the pharmaceutically acceptable salt of the compound has the effect on various tumor cells, which indicates that the compound can be used for treating various cancers including cerebral tumor, genitourinary system tumor, lymphatic system tumor, stomach cancer, laryngeal cancer, nasopharyngeal cancer, skin cancer, bone cancer, blood cancer, leukemia, breast cancer and histiocytic lymphoma, non-small cell lung cancer, small-cell lung cancer, lung adenocarcinoma, lung squamous cell cancer, pancreatic cancer, prostatic cancer, liver cancer, epithelial cell cancer and the like. The definition of genes in the formula refers to description for details.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Application of macrolide compound in reversing tumor multidrug resistance and enhancing anti-tumor curative effect
The invention relates to an application of a macrolide compound in reversing tumor multidrug resistance and enhancing an anti-tumor curative effect. The compound has the functions of inhibiting ABC transport protein activity, improving the concentration of a substrate chemotherapeutic drug of the ABC transport protein in tumor cells, reversing tumor multidrug resistance, and enhancing curative effects of the ABC transport protein substrate chemotherapeutic drugs such as antibiotic tumor chemotherapeutic drugs, plant-derived tumor chemotherapeutic drugs and small-molecular tyrosine kinase inhibitors, and has an effect of enhancing curative effects when being combined with antitumor drugs. Effective dose of the compound is combined with effective dose of an anti-tumor drug for application, apharmaceutically acceptable compound preparation is prepared by mixing with an effective dose of the antitumor drug, and is used for treating tumors including leukemia, lymphoma, gastric cancer, colon cancer, liver cancer, pancreatic cancer, breast cancer, nasopharyngeal carcinoma, cervical carcinoma, melanoma, multiple myeloma, and sarcoma.
Owner:FUJIAN MEDICAL UNIV
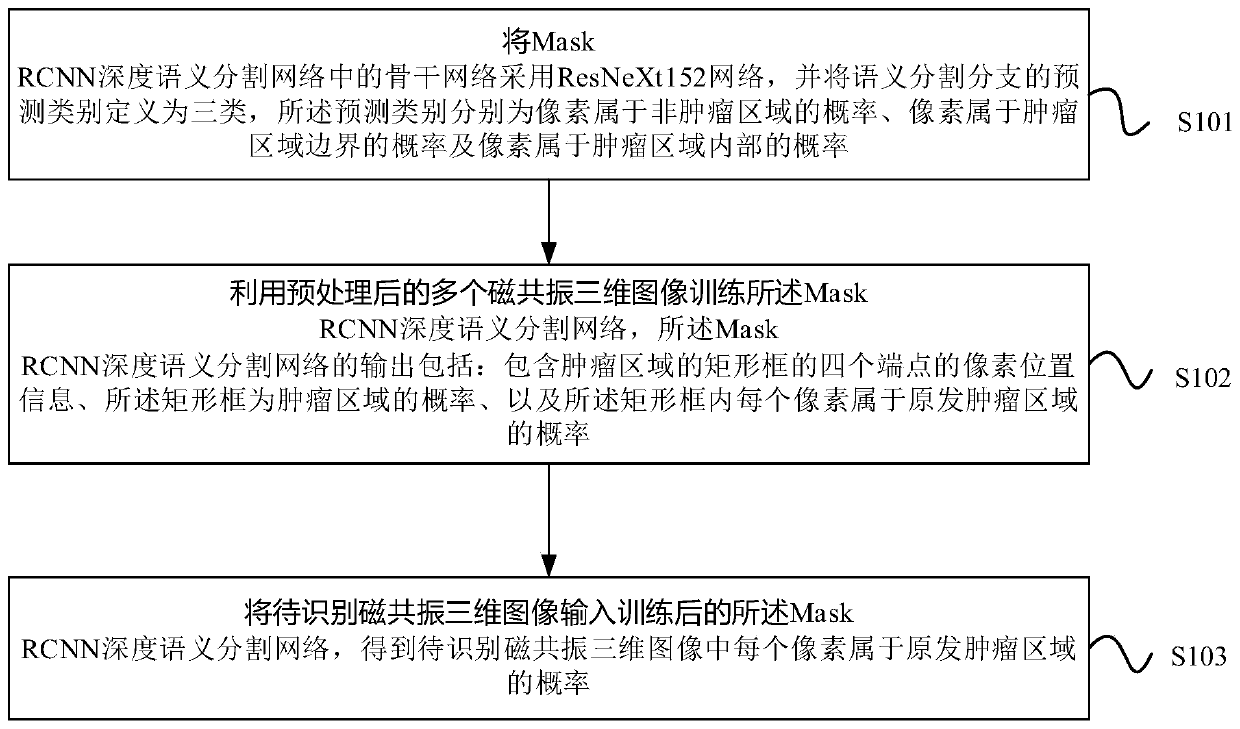
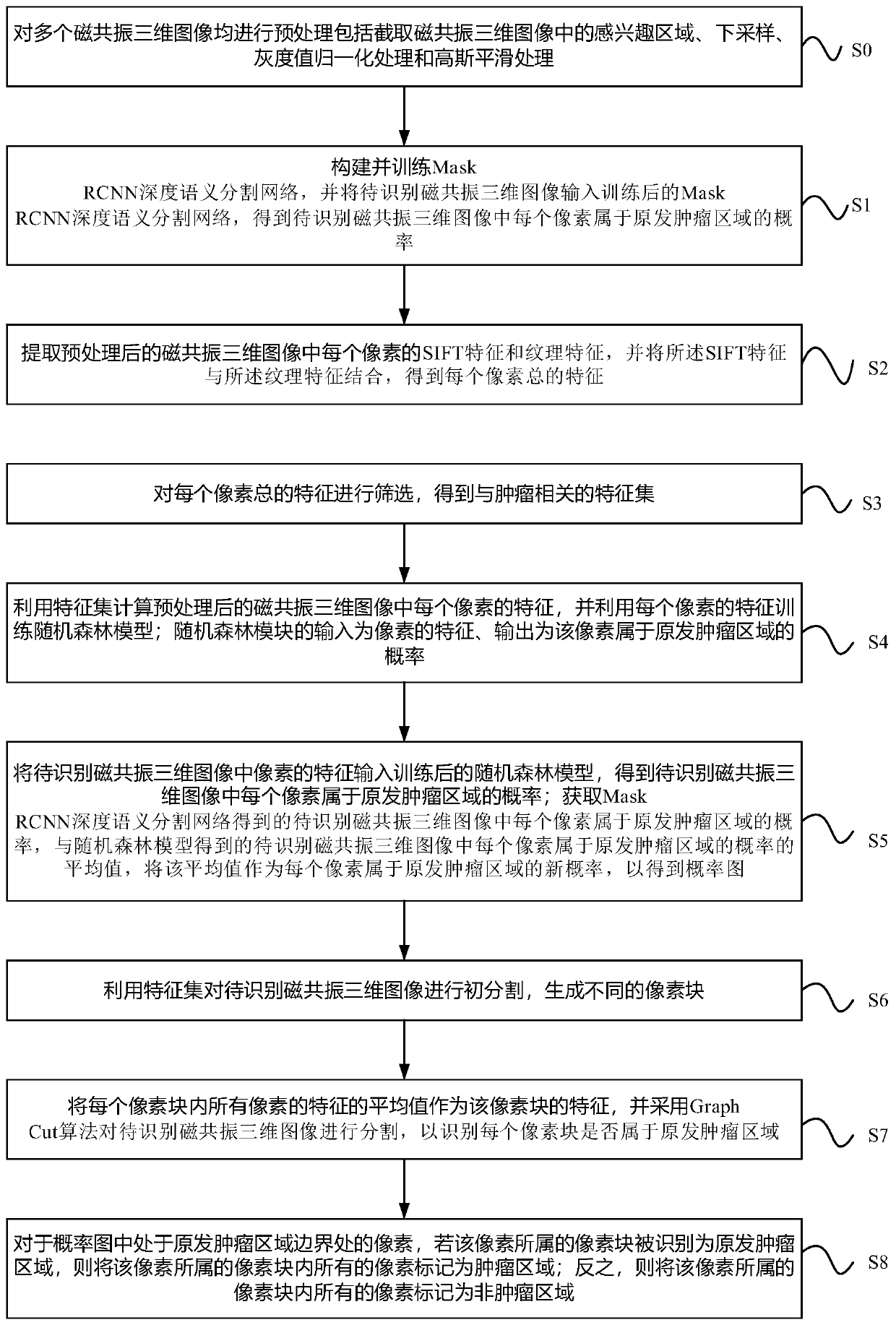
Method and device for automatically identifying nasopharyngeal carcinoma primary tumors
ActiveCN110969619AImprove generalization abilityImprove accuracyImage enhancementImage analysisParanasal Sinus CarcinomaImage manipulation
The invention relates to the field of image processing, and provides a method and device for automatically identifying nasopharyngeal carcinoma primary tumors. The method comprises the steps: employing a ResNeXt152 network as a backbone network in a Mask RCNN deep semantic segmentation network, and defining the prediction types of semantic segmentation branches into three types; training the deepsemantic segmentation network by utilizing a plurality of preprocessed magnetic resonance three-dimensional images, and outputting pixel position information of four end points of a rectangular framecontaining a tumor region, the probability that the rectangular frame is the tumor region and the probability that each pixel in the rectangular frame belongs to a primary tumor region; and inputtinga to-be-recognized magnetic resonance three-dimensional image into the deep semantic segmentation network to obtain the probability that each pixel belongs to a primary tumor region. According to themethod, through a deep learning method, a Mask RCNN network architecture is utilized and improved, so that the prediction accuracy and the generalization ability of the model can be effectively improved.
Owner:PERCEPTION VISION MEDICAL TECH CO LTD
7-oxy, thio or imino substituted coumarin, and derivatives and applications thereof
InactiveCN103420990AGood reference valueStrong inhibitory activityOrganic chemistryAntineoplastic agentsProstate cancer cellThio-
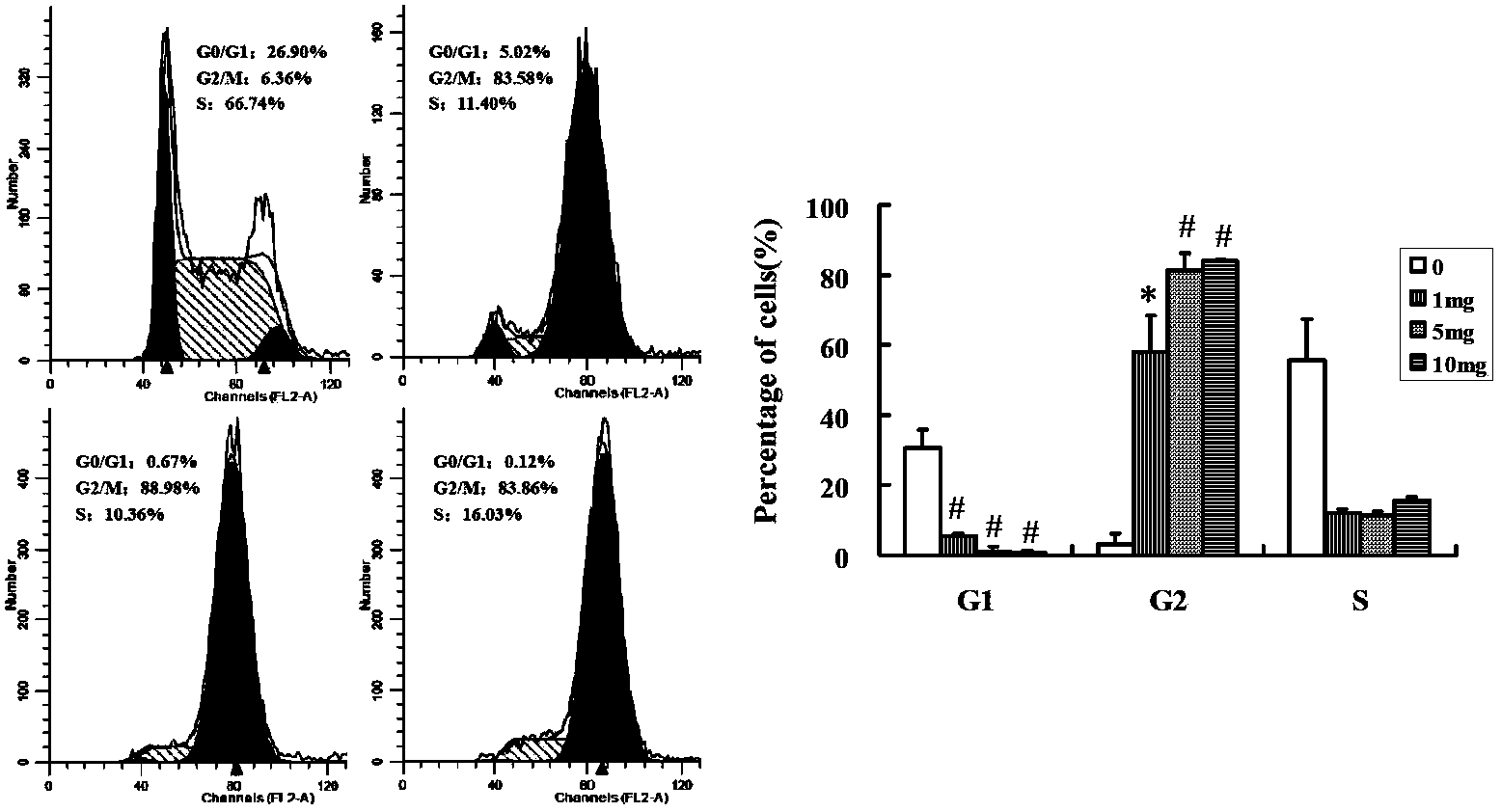
The invention belongs to the field of chemical pharmaceuticals, and more specifically relates to 7-oxy, thio or imino substituted coumarin with a structure represented by formula 8 or 12, and derivatives and antineoplastic activity thereof. The results of antineoplastic activity and pharmacological target experiments show that 7-oxy, thio or imino substituted coumarin derivatives possess inhibitory activity on the growth of nasopharyngeal carcinoma cells and drug-resistant tumor strains of nasopharyngeal carcinoma cells, lung cancer cells and prostatic cancer cells; and the results of pharmacological research show that 7-oxy, thio or imino substituted coumarin is capable of promoting cell apoptosis and influencing cell division cycle G2 / M. 7-oxygen, sulfur or nitrogen substituted coumarin is novel in structure, and is capable of providing basis for intensive study on target sports and related pharmacological mechanisms such as reversing drug-resistance mechanism. X is selected from oxygen, sulfur or nitrogen atoms; R2 is selected from alkyl, alkoxy, halogenated alkyl groups or ester group; and R3 is selected from alkyl, and substituted aromatic heterocyclic compounds including 6-chloropyridine and substituted aralkyl.
Owner:FUDAN UNIV
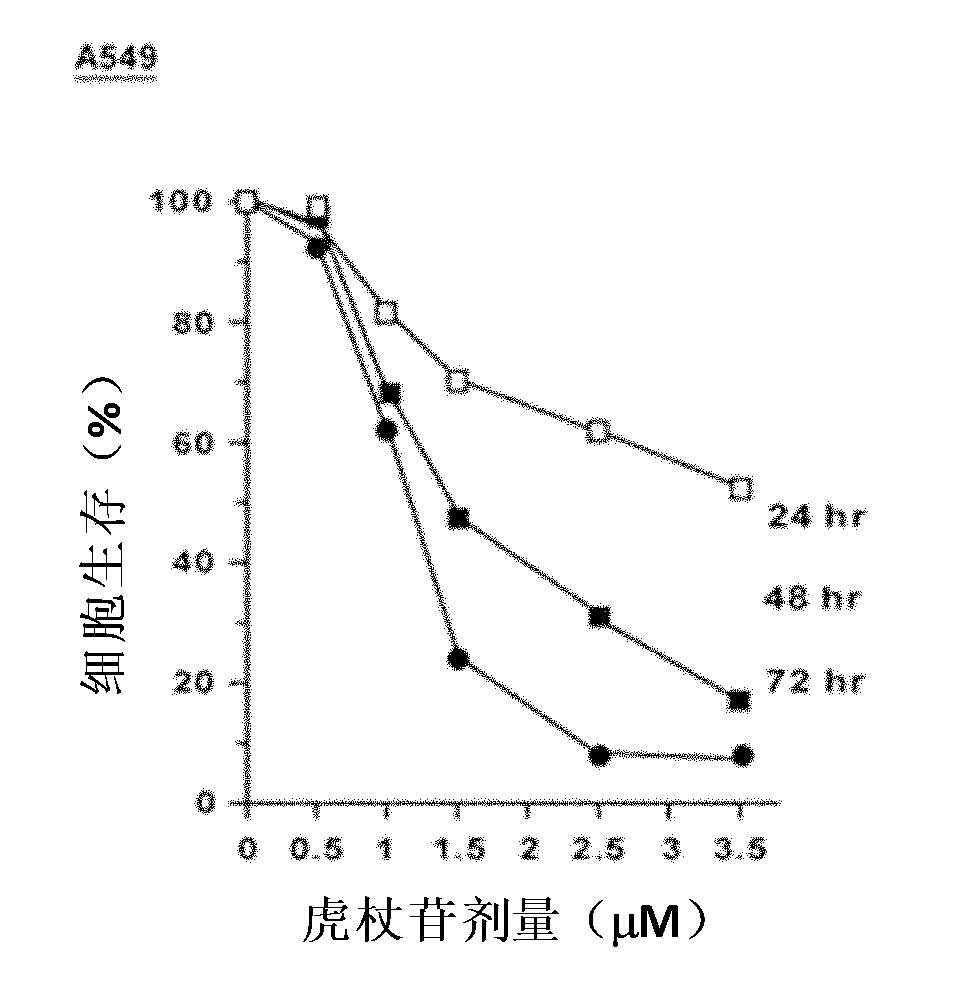
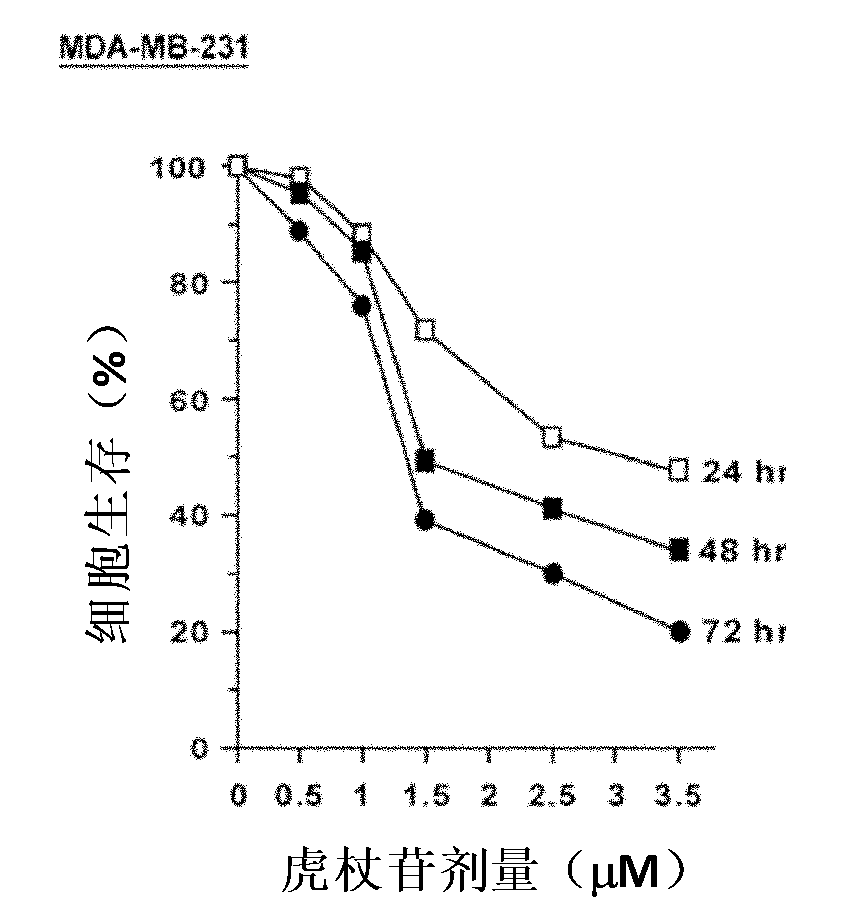
Application of polydatin to preparing antineoplastic drug
InactiveCN102058609APrevent proliferationInhibit migrationOrganic active ingredientsAntineoplastic agentsSide effectLife quality
The invention belongs to the field of antineoplastic drug preparation and relates to novel application of polydatin to preparing the antineoplastic drugs. The invention also provides an antineoplastic drug, wherein the main active component of the antineoplastic drug is polydatin. In the invention, the proliferations of cells such as breast cancer, lung cancer, liver cancer, ovarian cancer, cervical cancer, nasopharyngeal cancer, leukemia and the like treated by the antineoplastic drug polydatin are all restrained obviously; a cell cycle analysis shows that the S phase in the cell cycle is retardant and cell apoptosis is induced; and the polydatin with low concentration has the effect of restraining the migration and the conglutination of a tumor cell. In the invention, while the antineoplastic drug polydatin increases the treatment effect of a malignant tumor and reduces the incidence rate of relapse and transfer of a tumor patient, the toxic and side effects are obviously reduced compared with the traditional clinical commonly used chemotherapeutic drugs, therefore, the lifetime of a malignant tumor patient is prolonged, and the life quality of the malignant tumor patient is improved.
Owner:SUZHOU UNIV
Implant agent treating for solid tumor
InactiveCN101204365ABoron compound active ingredientsPharmaceutical delivery mechanismNervous systemProstate cancer
The invention relates to a sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of bortezomib and sustained-release excipients. The solid tumor includes brain tumor, liver cancer, lung cancer, oesophagus cancer, gastric cancer, breast cancer, pancreatic cancer, thyroid cancer, nasopharyngeal cancer, ovarian cancer, endometrial cancer, cervical cancer, renal cancer, prostate cancer, bladder cancer, colon cancer, rectal cancer, skin cancer, head and neck cancer and primary or secondary cancer, caruncle or carcinosarcoma rooted at a peripheral nervous system, mucosa, glands, blood vessels, bone tissues and lymph nodes. The sustained-release excipients are mainly a biological polymer which is dissoluble and can be degraded and absorbed, in the degradation and absorption process of which carmustine is sustainedly released to part of the tumor, thus the entire toxicity of the carmustine is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the carmustine but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Indirubin-3'-oxime derivatives as potent cyclin dependent kinase inhibitors
The present invention relates to an indirubin-3′-oxime derivative as potent cyclin dependent kinase inhibitor with anti-cancer activity. More particularly, this invention relates to an indirubin-3′-oxime derivative as potent cyclin dependent kinase inhibitor having excellent anti-cancer activity against human lung cancer cell, human fibro sarcoma cell, human colon cancer cell, human leukemia cell, human stomach cancer cell, human nasopharyngeal cancer cell and / or human breast cancer cell.
Owner:ANYGEN
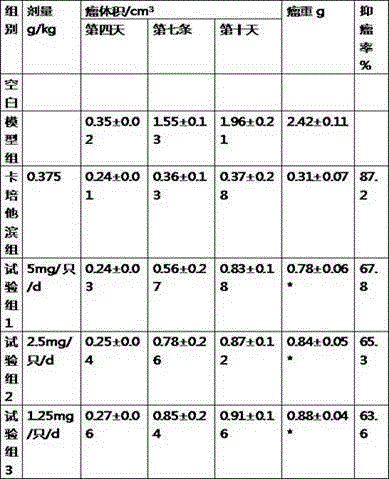
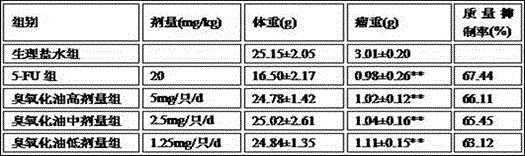
Application of ozonization oil to cancer prevention
The invention relates to ozonization oil for cancer prevention and treatment and a combination drug with an anti-cancer drug. Particularly, the ozonization oil is used for preventing and treating liver cancer, lung cancer, stomach cancer, colorectal cancer, rectal cancer, esophagus cancer, breast cancer, cervical cancer, prostatic cancer, skin cancer and nasopharyngeal cancer by means of oral administration.
Owner:侯建生
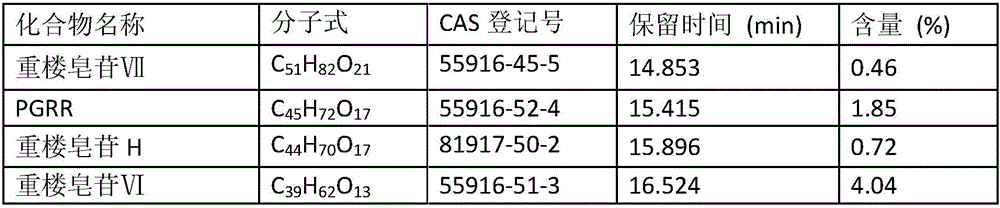
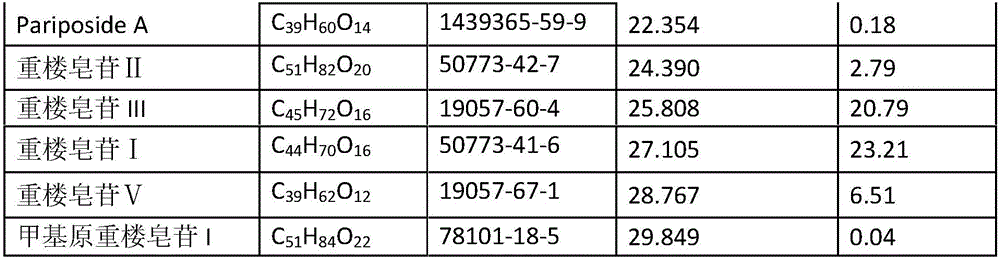
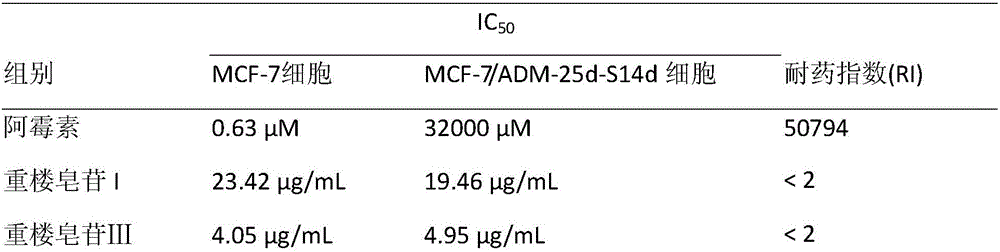
Paris forrestii (Takht.) H. Li monomer saponin combination, pharmaceutical composition thereof and application thereof in pharmaceutical industry
InactiveCN106074588AGood effectEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsNasopharyngeal carcinomaBULK ACTIVE INGREDIENT
The invention provides Paris forrestii (Takht.) H. Li total saponin PFE-PT3 and monomer saponin combination PFE-PT65, and provides a pharmaceutical composition using the combination as an active ingredient, and application in the preparation of a drug for preventing or treating drug-resistant breast cancer and drug-resistant nasopharyngeal carcinoma. The Paris forrestii (Takht.) H. Li total saponin PFE-PT3 and monomer saponin combination PFE-PT65 has an obvious inhibiting effect on adriamycin-resistant human breast cancer MCF-7 / ADM cells, with a resistance index of less than 2, and has an obvious inhibiting effect on paclitaxel-resistant human nasopharyngeal carcinoma KBvin cell growth, with a resistance index of less than 2.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
2′-fluoro-6′-methylene carbocyclic nucleosides and methods of treating viral infections
The present invention relates to 2′-Fluoro-6′-methylene carbocyclic nucleosides, pharmaceutical compositions containing these nucleosides and their use in the treatment or prophylaxis of a number of viral infections and secondary disease states and conditions thereof, especially including Hepatitis B virus (HBV) and secondary disease states and conditions thereof (cirrhosis and liver cancer), Hepatitis C virus (HCV), Herpes Simplex virus I and II (HSV-1 and HSV-2), cytomegalovirus (CMV), Varicella-Zoster Virus (VZV) and Epstein Barr virus (EBV) and secondary cancers which occur thereof (lymphoma, nasopharyngeal cancer, including drug resistant (especially including lamivudine and / or adefovir resistant) and other mutant forms of these viruses, especially HBV.
Owner:UNIV OF GEORGIA RES FOUND INC
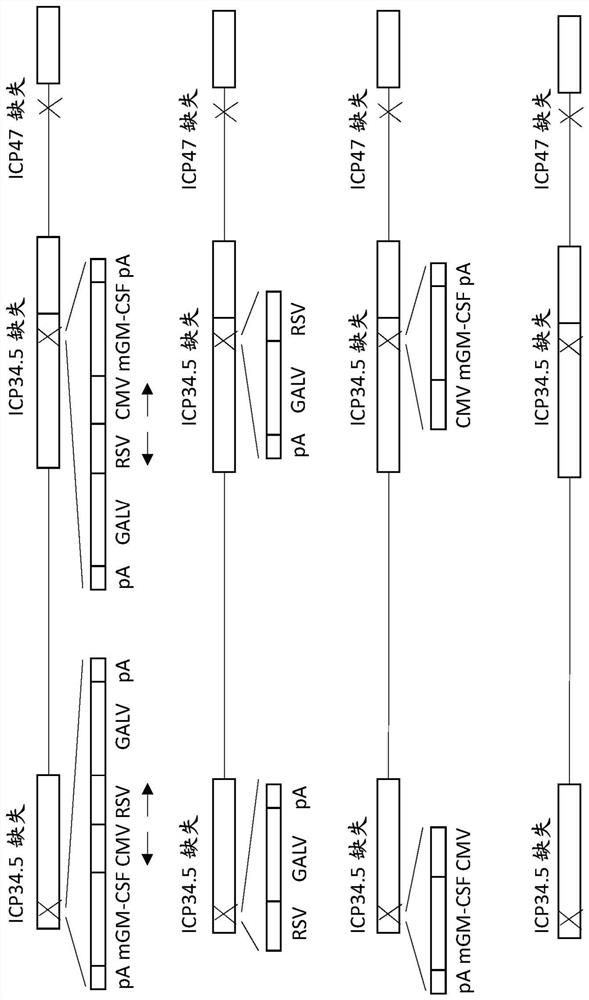
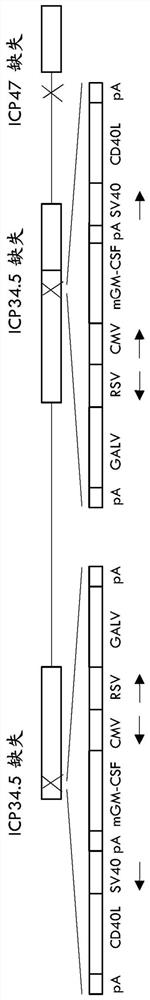
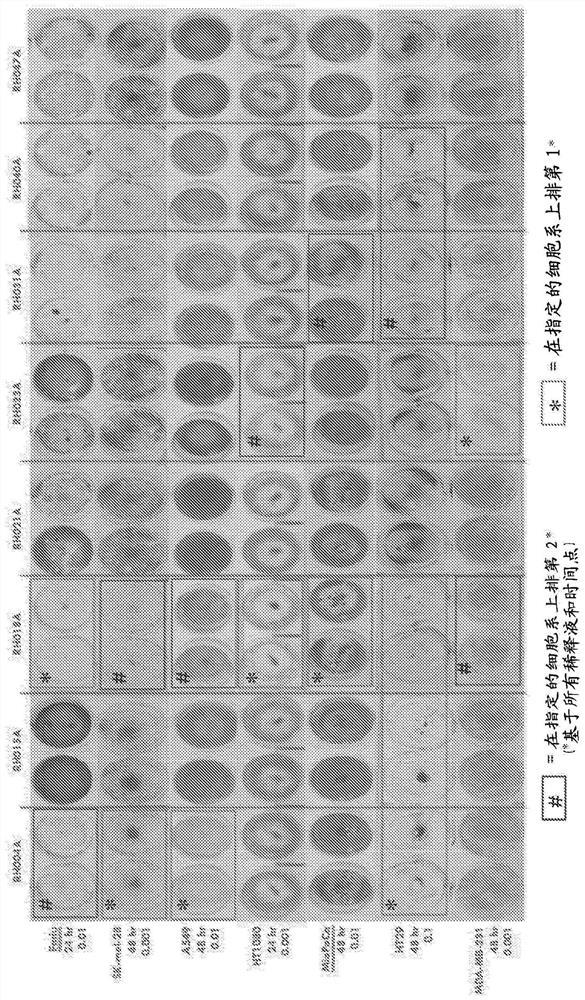
Treatment using oncolytic virus
An oncolytic virus is for use in a method of treating or preventing cutaneous squamous cell carcinoma (CSCC), renal cell carcinoma (RCC), non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), small cell lung cancer (SCLC), advanced recurrent head and neck cancer, squamous cell carcinoma of the head and neck (SCCHN), nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), anal cancer, colorectal cancer (CRC), basal cell carcinoma (BCC), Merkel cell carcinoma, appendiceal carcinoma, sarcoma of the skin, recurrent melanoma after surgery, advanced or metastatic urothelial carcinoma, liver metastases, microsatellite instability high cancer (MSI-H), mixed advanced solid tumors, virally caused cancer, locoregionally advanced cancer, pediatric cancer, cancer in patientswith no or minimal pre-existing anti-cancer immunity, cancer as first line therapy, cancer in previously treated patients, cancer in patients who have not received checkpoint blockade therapy, and / orcancer in patients who have received checkpoint blockade therapy, wherein the oncolytic virus is, or is derived from, a clinical isolate which has been selected by comparing the abilities of a panelof three or more clinical isolates of the same viral species to kill tumor cells of two or more tumor cell lines in vitro and selecting a clinical isolate which is capable of killing cells of two or more tumor cell lines more rapidly and / or at a lower dose in vitro than one or more of the other clinical isolates in the panel; comprises (i) a fusogenic protein-encoding gene; and (ii) an immune stimulatory molecule or an immune stimulatory molecule-encoding gene; comprises (i) a GM-CSF-encoding gene; and (ii) an immune co-stimulatory pathway activating molecule or an immune co-stimulatory pathway activating molecule-encoding gene; and / or comprises a gene encoding a CTLA-4 inhibitor.
Owner:REPLIMUNE
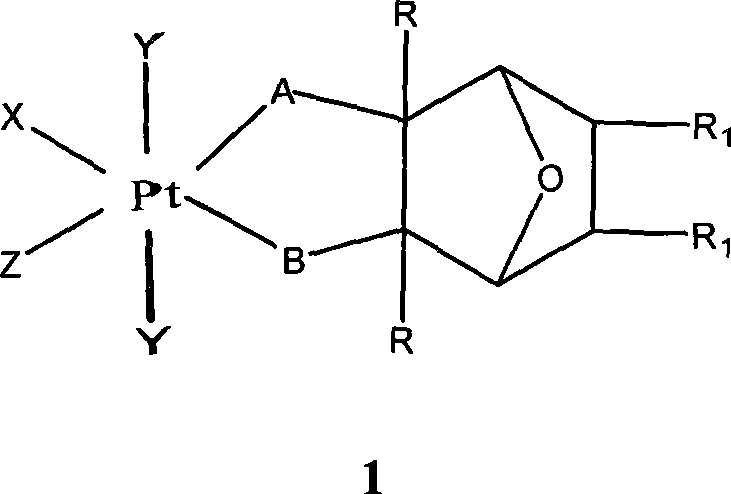
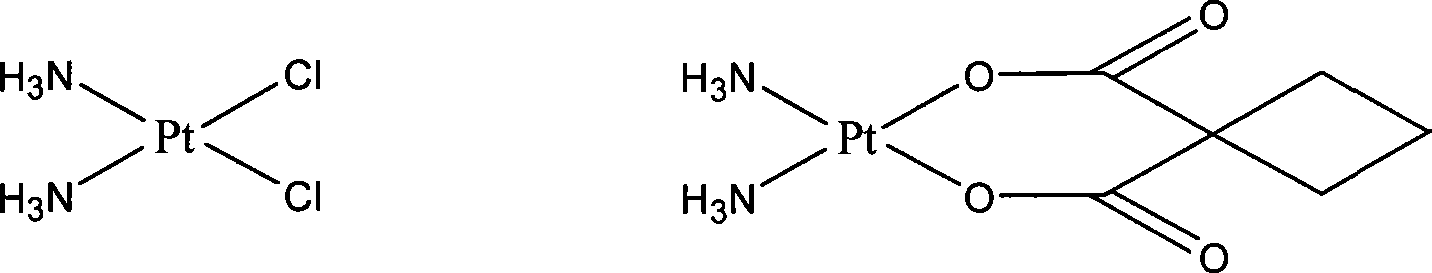
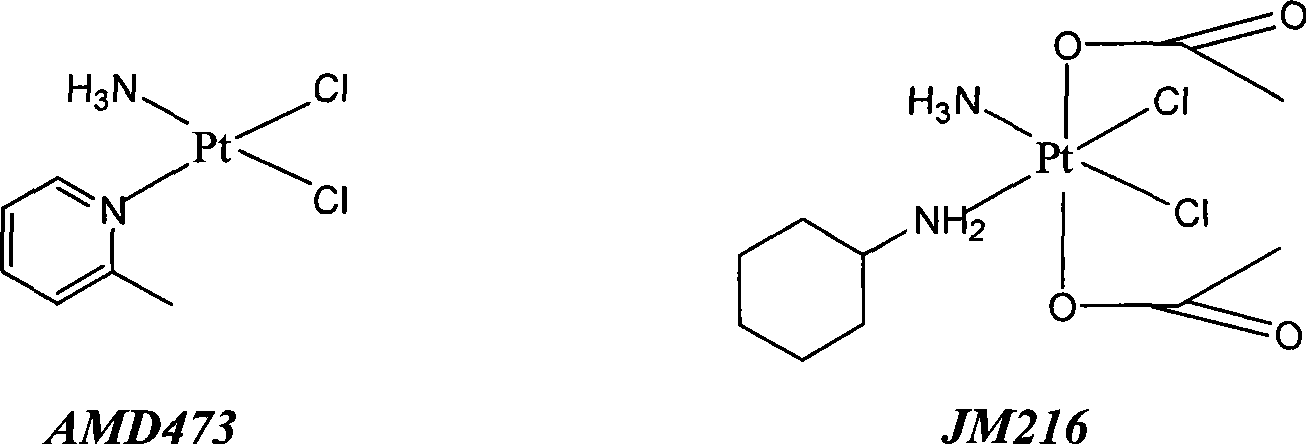
Preparation of novel organic platinum complex and uses thereof
InactiveCN101429218AGroup 8/9/10/18 element organic compoundsAntineoplastic agentsSide effectOrganoplatinum
The invention relates to preparation and application of a novel organo-antimony complex (1). The complex (1) has the characteristics of high anticancer activity, large water solubility, small toxic side effect and the like. A drug effect composition containing the complex (1) is used for treating various solid cancers, such as lung cancer, gastric carcinoma, liver cancer, breast carcinoma, cancer of colon, ovarian cancer, cervical carcinoma, leukemia, lymphoma, nasopharyngeal darcinoma and the like.
Owner:BAIBO FANGZHOU BEIJING TECH
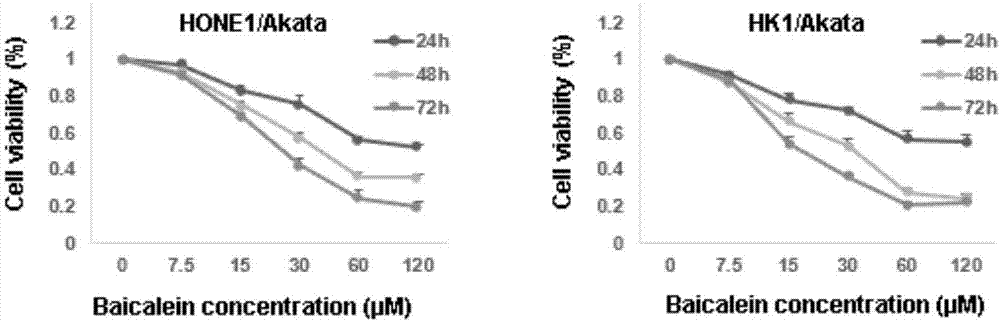
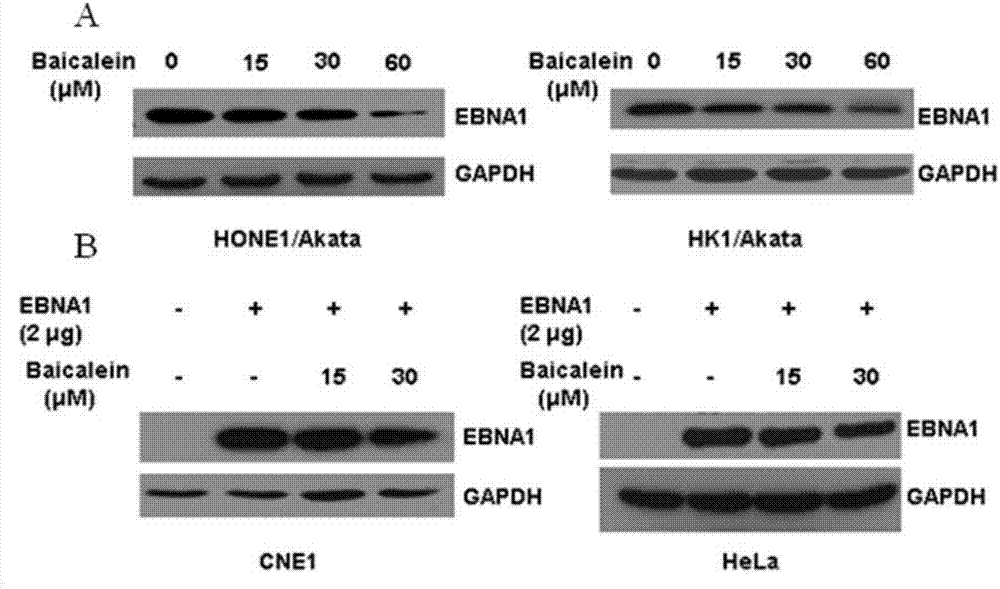
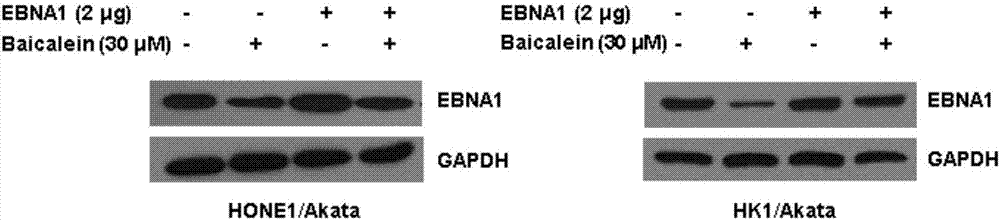
Application of baicalein to preparation of drug for preventing and/or treating nasopharyngeal carcinoma
InactiveCN107334763AConvenient treatmentHelps treat precancerous lesionsOrganic active ingredientsAntiviralsNasopharyngeal CancersBaicalein
The invention discloses an application of baicalein to the preparation of a drug for preventing and / or treating nasopharyngeal carcinoma. The application firstly proves that baicalein can remarkably inhibit a viral protein EBNA1 on which an Epstein-Barr virus exists, and a cell can become a normal cell due to tumor virus loss caused by overlow existing quantity of the protein in the cell. Baicalein can be favorably used for treating precancerous lesion, the conversion of severe rhinitis to nasopharyngeal carcinoma is effectively stopped, and the application has important significance for treating the precancerous lesion of nasopharyngeal carcinoma and reducing the occurrence rate and fatality rate of nasopharyngeal carcinoma.
Owner:WUHAN UNIV
Medical formula food for nasopharynx cancer
InactiveCN105029392ASolve inactivationSolve the coexistence problemSugar food ingredientsVitamin food ingredientsFormularyNutrition
The invention belongs to the technical field of affinal-drug-and-diet formulas and new-resource foods, and particularly provides a medical formula food, a special medical formula food or a non-special medical formula food for nasopharynx cancer patients. The formula is designed according to the requirement in "National Food Safety Standard General Rules on Formula Food for Special Medical Use". With consideration of the body characters of the nasopharynx cancer patients, various "affinal-drug-and-diet" traditional Chinese medicines are combined reasonably according to traditional Chinese medicine essential principles, and a plurality of affinal-drug-and-diet traditional Chinese medicine extract essences which are extracted through a pre-hydrolysis SBE technology, various micro-capsulated probiotics, oligopeptides extracted through bio-enzymolysis, prebiotics, amino acids, carbohydrates, grease having health-caring functions, various vitamins and minerals are all mixed together and are subjected to other processes to obtain the medical formula food. The medical formula food can be used for satisfying nutrition requirement of the nasopharynx cancer patients as a single nutritional source, and has the functions of nourishing yin and clearing heat, boosting qi and soothing throat, tonifying and boosting qi and blood, harmonizing ying and removing toxin, softening hardness and dissipating binds, and enhancing body immunity.
Owner:JINSHANMEI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com