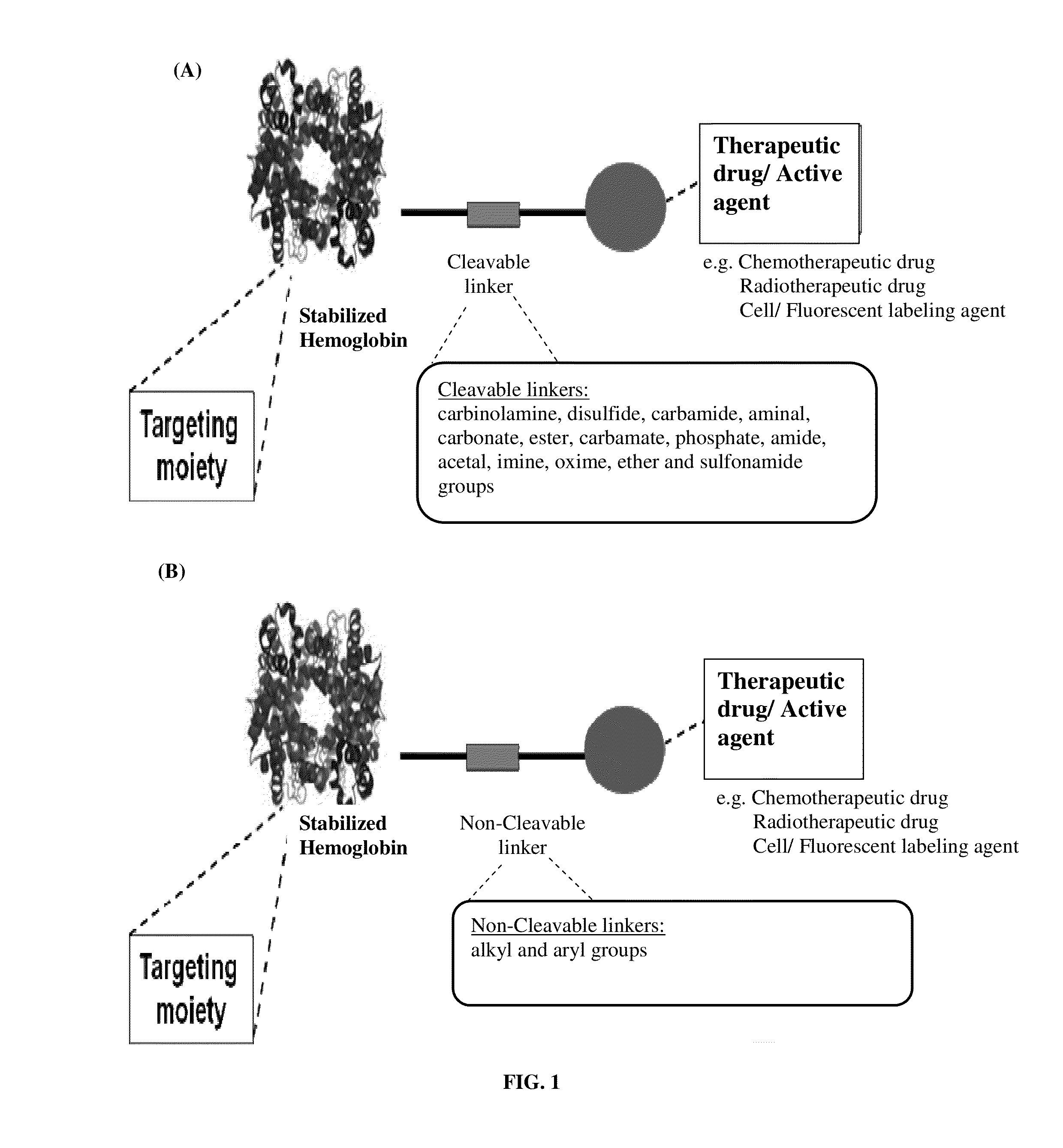
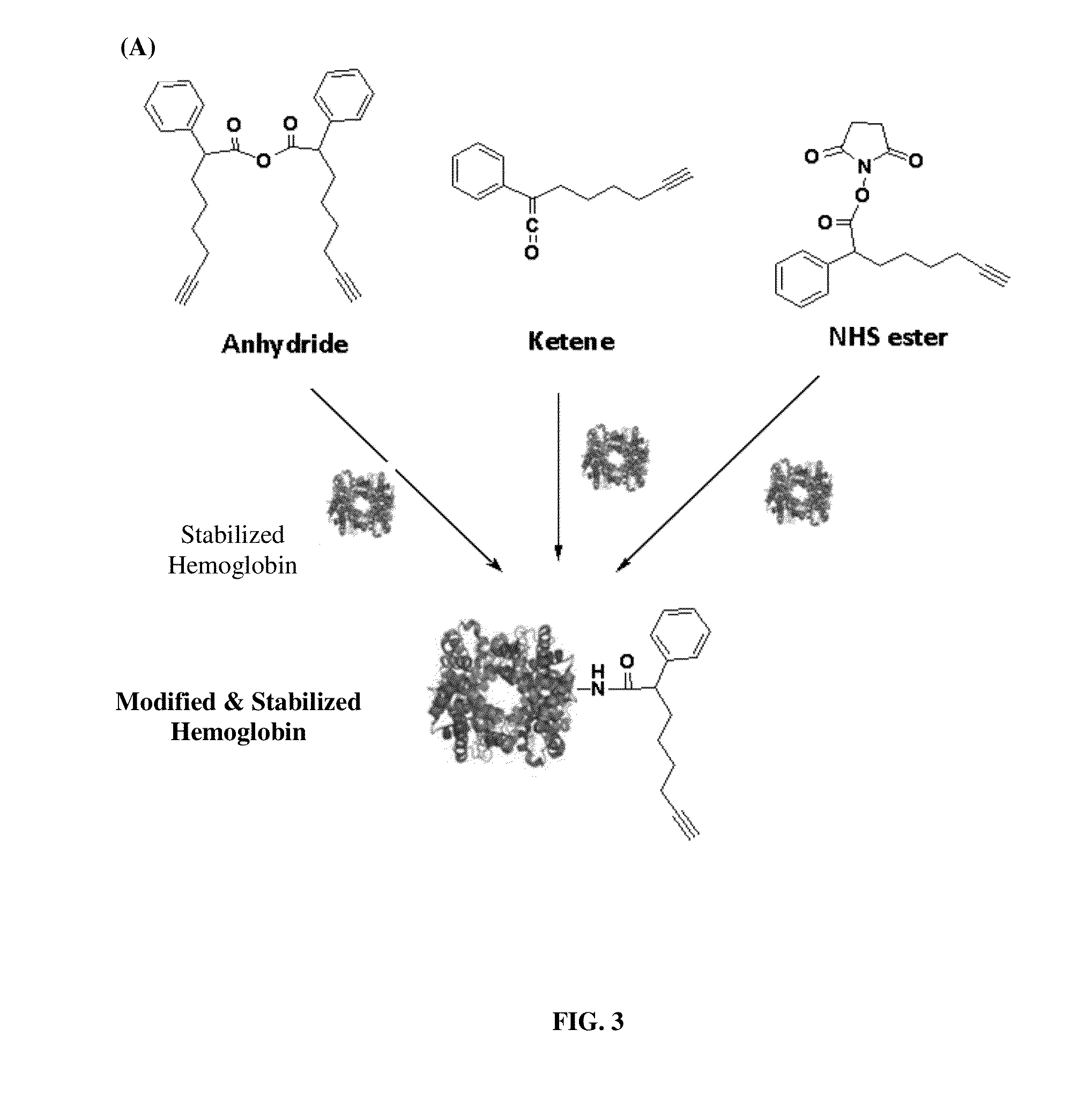
Pharmaceutical composition comprising modified hemoglobin-based therapeutic agent for cancer targeting treatment and diagnostic imaging
a technology of therapeutic agent and modified hemoglobin, which is applied in the direction of drug composition, peptide, medical preparation, etc., can solve the problems of high cost of most therapeutic drugs and many side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
Synthesis of Ketene
[0075]To a vigorously stirred solution of 5-hexyn-1-ol (4 mmol) and triethylamine (4.5 mmol) in dichloromethane (DCM) (100 mL), methylsulfonyl chloride (MsCl) (4.1 mmol in DCM) is added dropwise at 0° C. The mixture is then warmed to room temperature for stirring overnight. Sodium bicarbonate (aqueous) is poured into the reaction mixture and the organic phase is separated. The aqueous layer is extracted with DCM and the combined organic extracts are washed with water and brine, dried over magnesium sulfate (MgSO4) and the solvent evaporated. The crude mesylate is purified by flash column chromatography (20% ethyl acetate in n-hexane) to yield colorless oil.
[0076]To a solution of mesylate (2 mmol) in acetone (60 mL), potassium iodide (2.5 mmol) is added in the reaction mixture and heated to reflux for 20 h. After cooling to room temperature, the precipitate is filtered off. The filter cake is washed with acetone (20 mL) and the solvent is evaporated. The residual o...
example 1b
Modification of Peptide and Hemoglobin Using Ketene
[0080]In a 1.0 mL eppendorf tube, peptide YTSSSKNVVR solution in water (1 mM, 10 μL), ketene (10 equivalents, 1 μL of a 100 mM stock solution of ketene in dried tetrahydrofuran), and phosphate buffer (pH 6.3 and 7.4, 80 μL) are mixed. The reaction mixture is kept at room temperature for 2 h. The conversion of the peptide is determined from total ion count of LC-MS analysis of the reaction mixtures. Using MS / MS (tandem mass spectrometry) analysis, the N-terminal selectivity is determined.
[0081]In a 1.0 mL eppendorf tube, the stabilized hemoglobin solution in buffer (1.56 mM, 40 μL), ketene (10, 20, 30, 40, and 50 equivalents, 100 mM stock solution of ketene in dried tetrahydrofuran), and phosphate buffer (pH 6.3, 7.4 and 9, 160 μL) are mixed. The reaction mixture is kept at room temperature overnight (one set at pH 9 at 37° C.). The conversions of the protein are determined from total ion count of LC-MS analysis of the reaction mixtu...
example 2a
Synthesis of Anhydride
[0082]A solution of alkyne-functionalized carboxylic acid (100 mg, 0.46 mmol), (3-dimethyl aminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (180.5 mg, 0.94 mmol), and triethylamine (0.5 mmol) in dichloromethane (20 mL) is stirred at room temperature overnight. The reaction mixture is washed with water. The organic layer is dried over anhydrous magnesium sulfate and concentrated in vacuo, and the residue is purified by flash column chromatography (eluting with 4% ethyl acetate in n-hexane) to give anhydride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com