ELISA reagent kit for screening, diagnosis and treatment effect forecast of nasopharyngeal carcinoma
A diagnostic reagent, EB virus technology, applied in the medical field, can solve problems such as poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
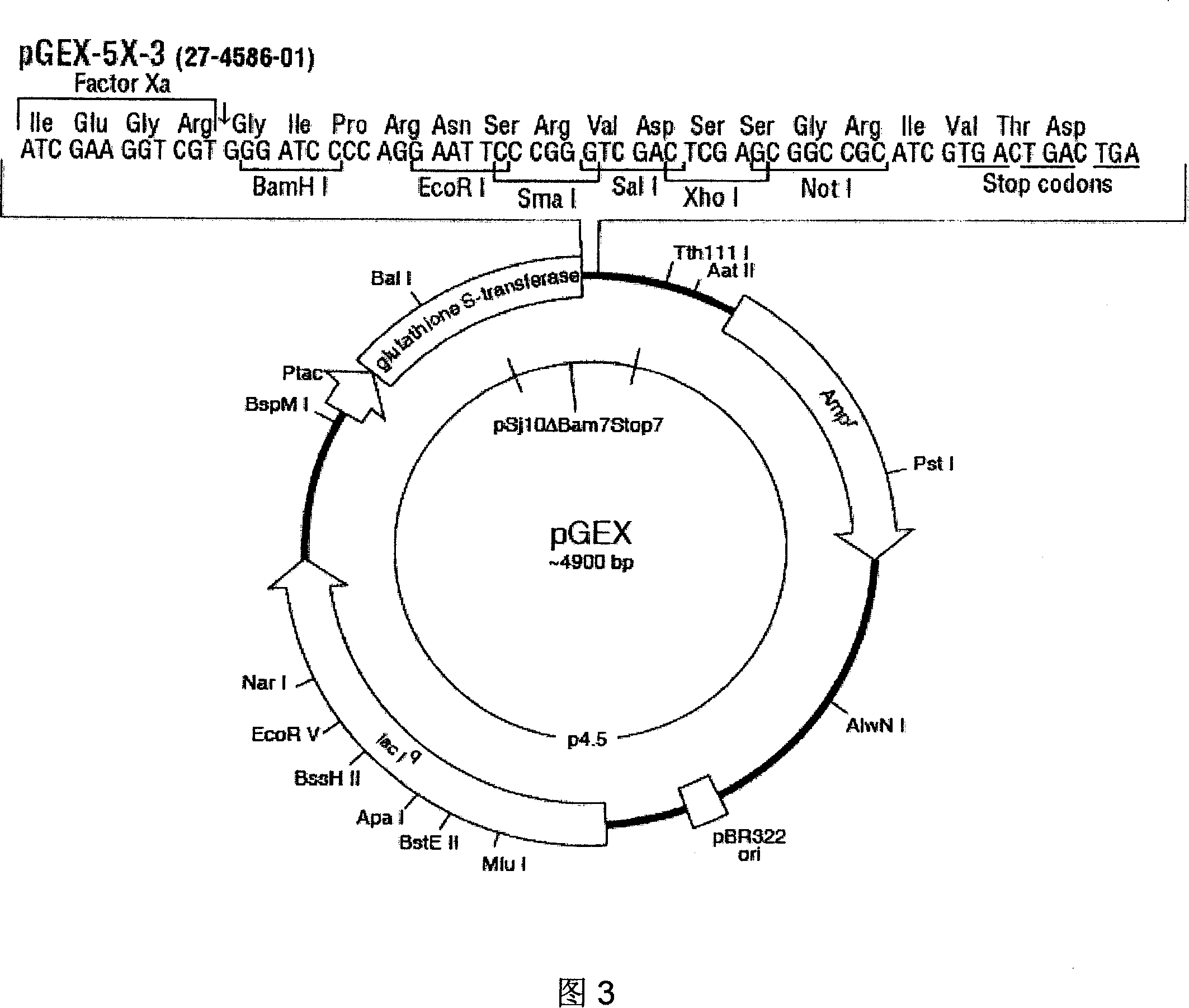
[0098] Example 1. Obtaining of recombinant plasmids pGEX-R185 and pGEX-R150
[0099] 1. Acquisition of BRLF1 gene
[0100] (1) Design and synthesis of primers: The PCR primer sequences designed according to the known BRLF1 sequence (GenBank No. gi: 94734074) are:
[0101] F: 5'-CCGGAATTCATGAGGCCTAAAAAGGATGGCTT-3';
[0102] R: 5′-TGCTCTAGACTAAAATAAGCTGGTGTCAAAAATAG-3′
[0103] (2) RT-PCR amplification:
[0104] B95-8 cells (ATCC Number: CRL- 10624 TM ) into the lytic phase (Feng.P, Chan.SH, Soo.MY, etc. Antibody response to Epstein-Barr virus Rta protein in patients with nasopharyngeal carcinoma: a new serologic parameter for diagnosis. Cancer 2001 Oct 1; 92( 7): 1872-80.), using RT-PCR method to obtain the cDNA of BRLF1 gene, the specific method is as follows:
[0105] PCR reaction tubes, tips, and amplification water were routinely treated with DEPC.
[0106] Two-step RT-PCR kit from TaKaRa Company ( Item number in the catalog TAK_RR019A), using the total RNA of B95-8...
Embodiment 2
[0178] Example 2, Preparation and Purification of GST-R150 and GST-R185 Proteins
[0179] 1 Induced expression and identification of recombinant fusion protein
[0180] After the above pGEX-R185 and pGEX-R150 recombinant plasmids were transformed into Escherichia coli BL21(DE3) (commercially purchased Promega L1191), single clones were screened on the LB plate, and a small amount of single clones were selected and cultured overnight at 37°C at 150rpm overnight, at a ratio of 1:500 For expansion, 100mg / ml AMP was added at 1:1000, 37°C, 180rpm (desktop high-speed refrigerated centrifuge (Eppendorf Company)), and cultured for 4 hours (full temperature shaking incubator (Harbin Donglian Factory, HZQ-F)). 1mmol / L IPTG (TaKaRa Company) was added to the medium at a ratio of 1:1000, and induced for 4 hours. The bacterial cells before induction, 1h, 2h, 3h, and 4h after induction were centrifuged at 13,000rpm to obtain a precipitate, added to denatured Loading Buffer (commercially ava...
Embodiment 3
[0203] Embodiment 3 application ELISA method detects the antibody of Epstein-Barr virus BRLF1 gene expression antigen in serum
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com