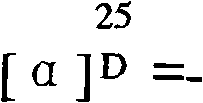Cyclo-pentapeptide with antineoplastic activity
A technology of cyclic pentapeptides and anti-tumor drugs, which is applied in the field of cyclic pentapeptides, can solve the problems of extracting cyclic peptides that have not yet been seen, and achieve the effects of strong anti-tumor activity, low production cost, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 cyclic pentapeptide
[0028] The preparation method of cyclic pentapeptide, its concrete steps are as follows:
[0029] (1) Soak and extract Filamentous Lactobacillus in ethanol at a temperature of 20°C, and concentrate the soaked extract under reduced pressure after 45 days;
[0030] (2) After the concentrated extract was extracted with ethyl acetate solution, concentrated under reduced pressure at 20°C to obtain the crude extract;
[0031] (3) The above crude extract is subjected to silica gel chromatography to obtain the crude cyclopentapeptide, and then recrystallized from methanol to obtain the cyclopentapeptide monomer.
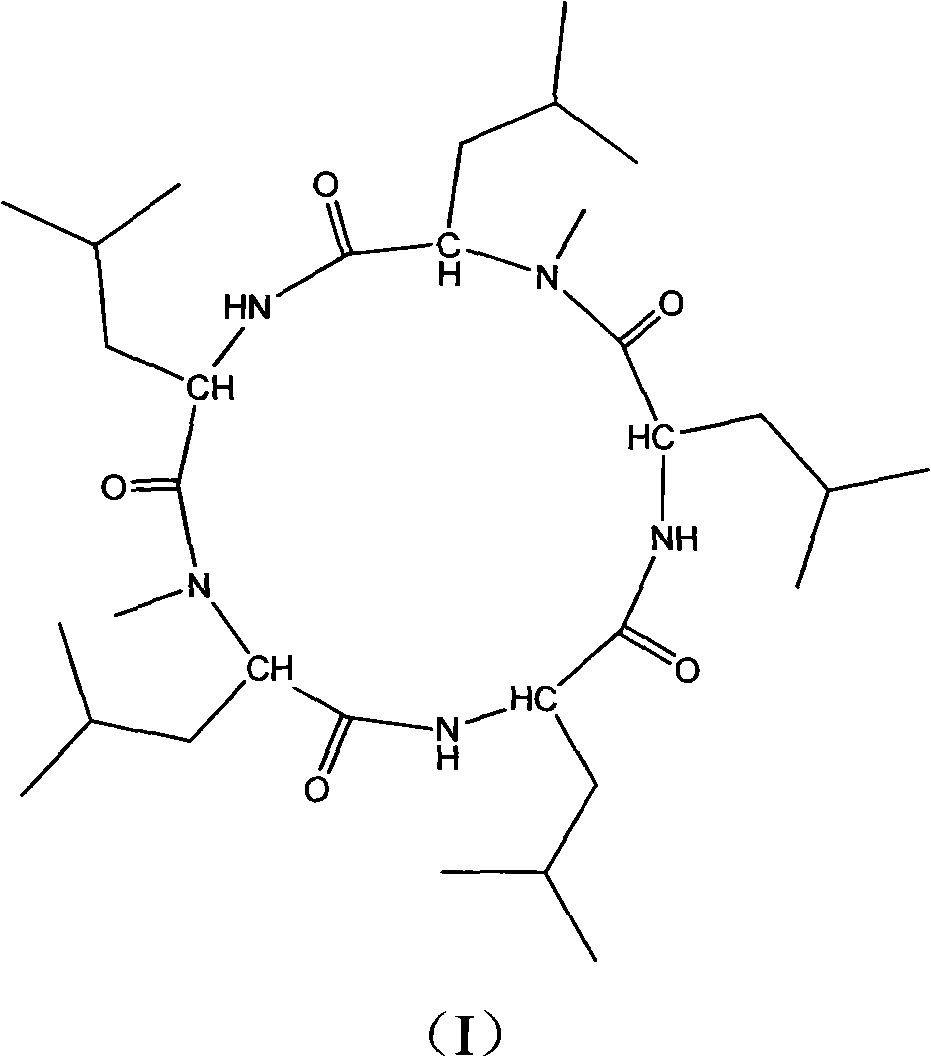
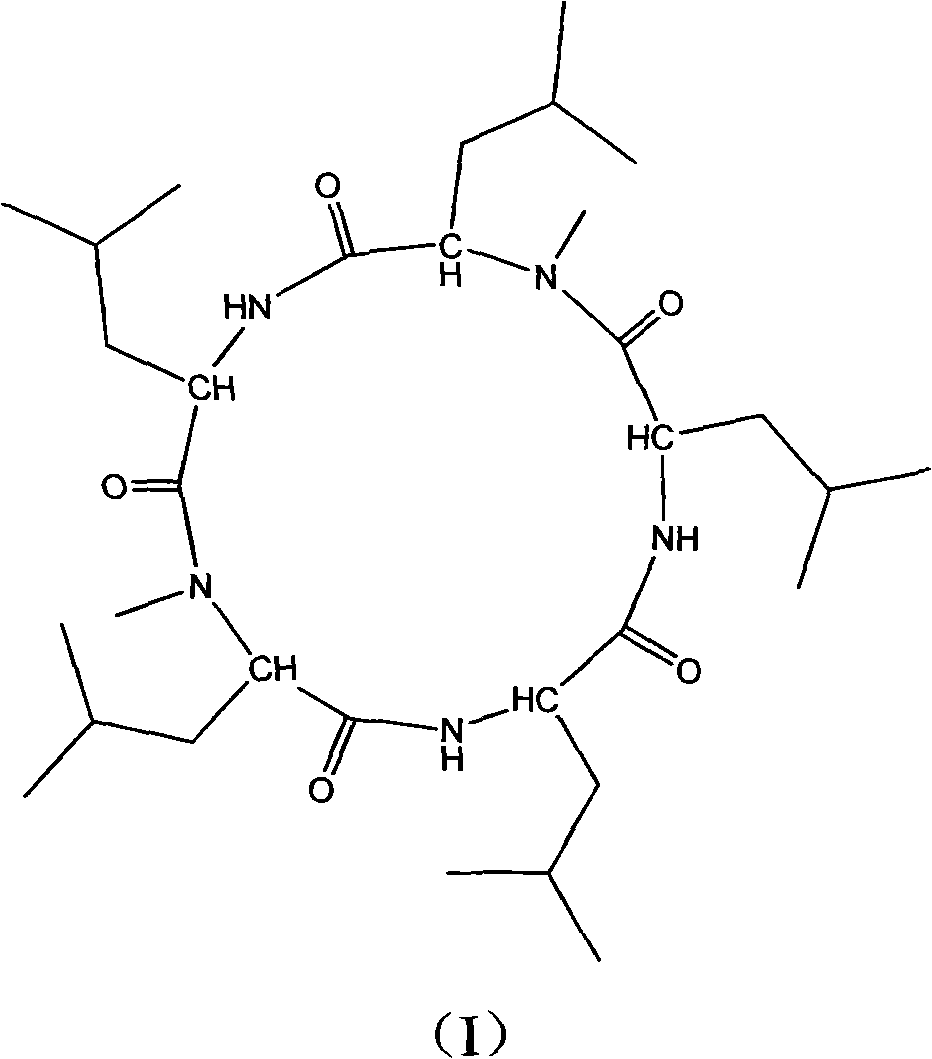
[0032] The cyclic pentapeptide is a white needle-like crystal with a molecular formula of C 32 h 59 N 5 o 5 ,[M+H] + m / z: 594.4, mp: 208-210°C, [ α ] D 25 = - 130 ...
Embodiment 2
[0033] Embodiment 2 in vitro antitumor activity test
[0034] Adopt tetramethyl azolium salt microenzyme reaction colorimetry (MTT method), the cyclic pentapeptide (I) that embodiment 1 obtains and human liver cancer cell line HepG2, human breast cancer cell line MCF-7, human liver cancer cell line Strain BEL-7402, human colon cancer cell line LOVO, human nasopharyngeal carcinoma cell line CNE and human lung cancer cell line PC84045 were treated for 72 hours respectively. The results are shown in Table 2.
[0035] Table 2 cyclic pentapeptide (I) to the half maximal effective concentration (IC) of tumor cells 50 )
[0036]
[0037] Example 3 Effect test of cyclic pentapeptide on cycle of BEL-7402 liver cancer cells
[0038] Adopt propidium iodide (PI) staining method, after cyclic pentapeptide of different concentration (the cyclopentapeptide that embodiment 1 prepares) acts on BEL-7402 liver cancer cell 48 hours, flow cytometer measures cell cycle result as shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com