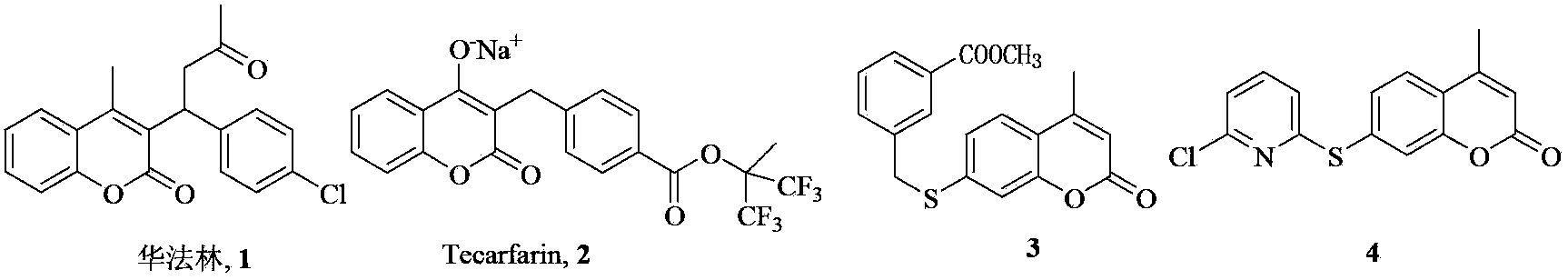
7-oxy, thio or imino substituted coumarin, and derivatives and applications thereof
A technology of coumarin and nitrogen substitution, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, medical preparations containing active ingredients, etc., can solve the problem of rare and few clinical drugs with anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesize compound 7-oxygen, sulfur or nitrogen substituted coumarin and derivatives thereof of formula 8 or 12 according to general formula one or general formula two,
[0028] 1. According to general formula 1, compound (6) is obtained by reflux reaction of commercially available 7-hydroxyl, mercapto or amino substituted coumarin (5) with N-oxide-2,6-dichloropyridine in pyridine; Phosphorous chloride and chloroform solvent reflux reduction to obtain 7-(6-chloropyridyl)-7-oxo, sulfur and azacoumarin compound (7); at the same time, compound (5) was treated with potassium carbonate and acetone at room temperature React with various alkyl, substituted aryl and substituted heterocyclic halides to obtain different substituted 7-oxo, sulfur and aza coumarin compounds (8),
[0029] General formula one:
[0030]
[0031] 2. According to the general formula 2, the starting material (9) was synthesized (refer to the reference (European Journal of Medicinal Chemistry, 2011, ...
Embodiment 2
[0035] According to the general formula 1, a compound 13 in compound type 6 was synthesized
[0036]
[0037] 7-Mercapto-4-methyl-chromen-2-one (5,300mg, 1.56mmol) and 2,6-dichloropyridine N-oxide (256mg, 1.56mmol) were refluxed in pyridine (6mL) solvent for 2 hour; then add 2 equivalents of hydrochloric acid (50mL), filter and distill off the solvent under reduced pressure, separate with a chromatographic column (eluent is chloroform / ethyl acetate=99 / 1) to obtain 240mg of product 13, yield 48%, melting point It is 236-237°C. 1 H NMR (CDCl 3 ):δ2.50(3H,s,4-CH 3),6.41(1H,s,3-H),6.55(1H,dd,J1=8.1Hz,J2=1.8Hz,6-H),7.01(1H,t,J=8.4Hz,H of pyridine), 7.28,7.54(each 1H,dd,J1=8.4Hz,J2=1.8Hz,2Hof pyridine),7.62(1H,d,J=1.8Hz,8-H),7.73(1H,d,J=8.1Hz, 5-H). 13 C NMR δ18.83,58.42,116.76,120.27,122.30,124.00,125.77,126.45,131.11,133.28,141.80,151.75,154.05,154.91,159.97. ESI MS m / z 320(M + +1).
Embodiment 3
[0039] According to general formula 1, a compound 4 in compound type 7 is synthesized,
[0040]
[0041] Compound 13 (500mg, 1.56mmol) was dissolved in phosphorus trichloride (PCl 3 , 2mL) and chloroform (CHCl 3 , 20mL), after reflux reaction for 1.5 hours, add ice water (200mL) to dilute the reaction solution, extract with dichloromethane (30mL×4), combine the organic phases and dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and then chromatograph Column separation (eluent: chloroform / methanol=20 / 1) yielded 237 mg of product 4 with a yield of 50% and a melting point of 138-140°C. 1 H NMR (CDCl 3 )δ2.46(3H,s,4-CH 3 ),6.33(1H,s,3-H),7.01,7.13,7.52(each 1H,d,J=7.8Hz,3H ofpyridine),7.45(1H,d,J=8.4Hz,6-H),7.48 (1H,s,8-H),7.62(1H,d,J=8.4Hz,5-H). 13 C NMR δ18.94,116.55,121.00,122.05,122.26,122.31,126.24,129.81,136.78,140.25,152.41,152.80,154.75,160.35,161.25.ESI MS m / z 304(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com