Rubiaceae-type cyclopeptide, its pharmaceutical composition and application
A Rubiaceae, type technology, applied in the application field of Rubiaceae type cyclic peptides to prepare anti-herpes simplex virus type I (HSV-1) drugs, can solve the problem of relatively expensive aminopropyl bonds and silica gel materials, heavy methanol The controllability and reproducibility of crystallization technology is not good, and it is not commonly used.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Rubiaceae type cyclic peptide compounds RA-V (1), RA-I (2), RA-XXIV (3), RA-XII (4), rubiyunnanin D (5), rubiyunnanin C (6), rubiyunnanin E (7 ), rubiyunnanin F (8), rubiyunnanin G (9), rubiyunnanin H (10) and RY-II (11) preparation methods and structural identification:
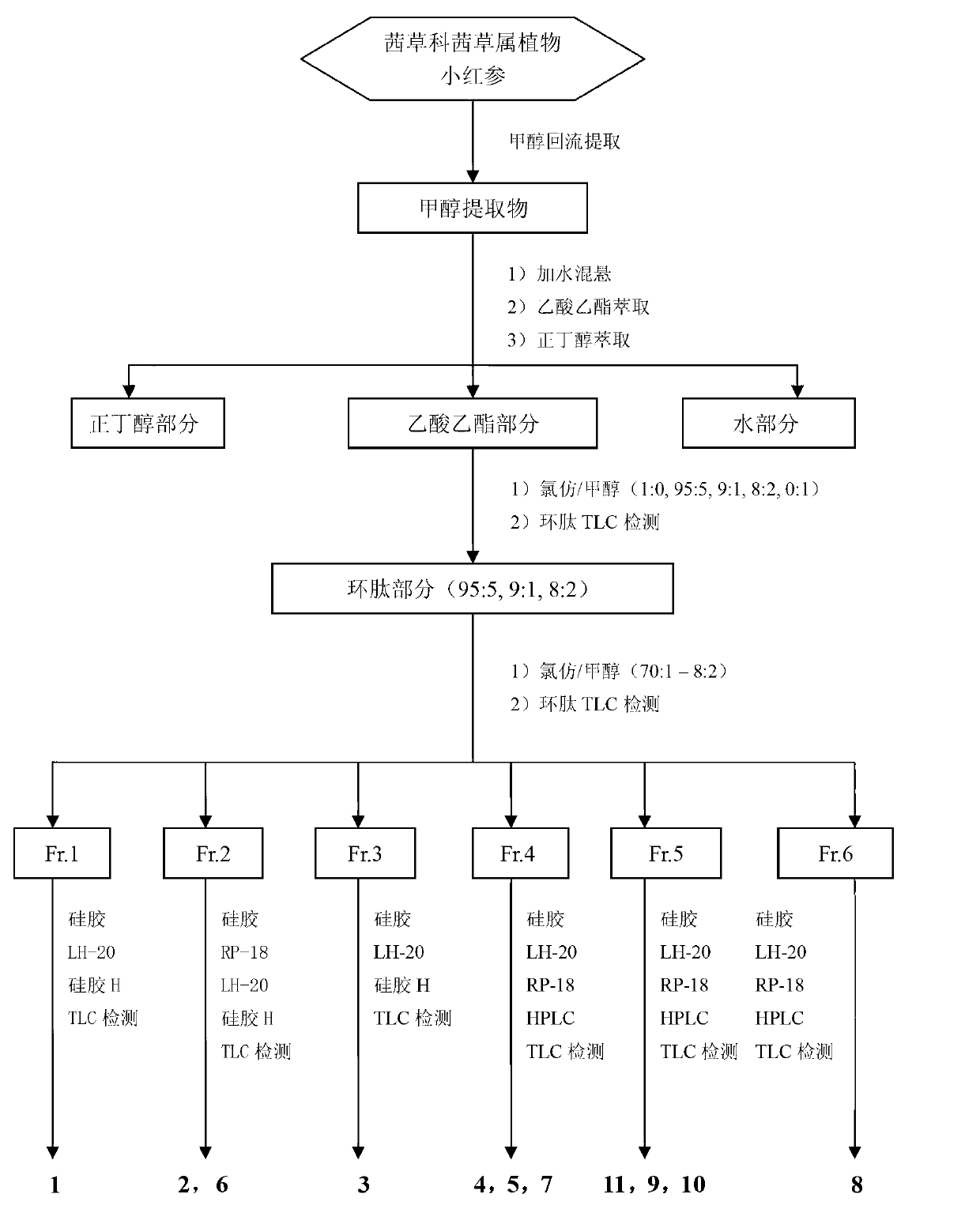
[0035] Take 100kg of the rhizome of red ginseng, dry and pulverize it, and extract it with methanol reflux for 3 times (100L×3 times), the time is 3h, 3h, 2h, and the extract is concentrated under reduced pressure to obtain 21kg of methanol extract. Add water to the methanol extract and suspend it, then fully extract it with ethyl acetate and n-butanol successively, extract three times in equal volumes (30L x 3 times), recover the solvent to obtain 6.4kg of ethyl acetate, 8kg of n-butanol and water 5kg. The ethyl acetate part was subjected to silica gel column chromatography, eluted with chloroform / methanol gradient (100:0, 95:5, 9:1, 8:2, 0:100), thin layer chromatography, combined with cyclic pepti...
Embodiment 2
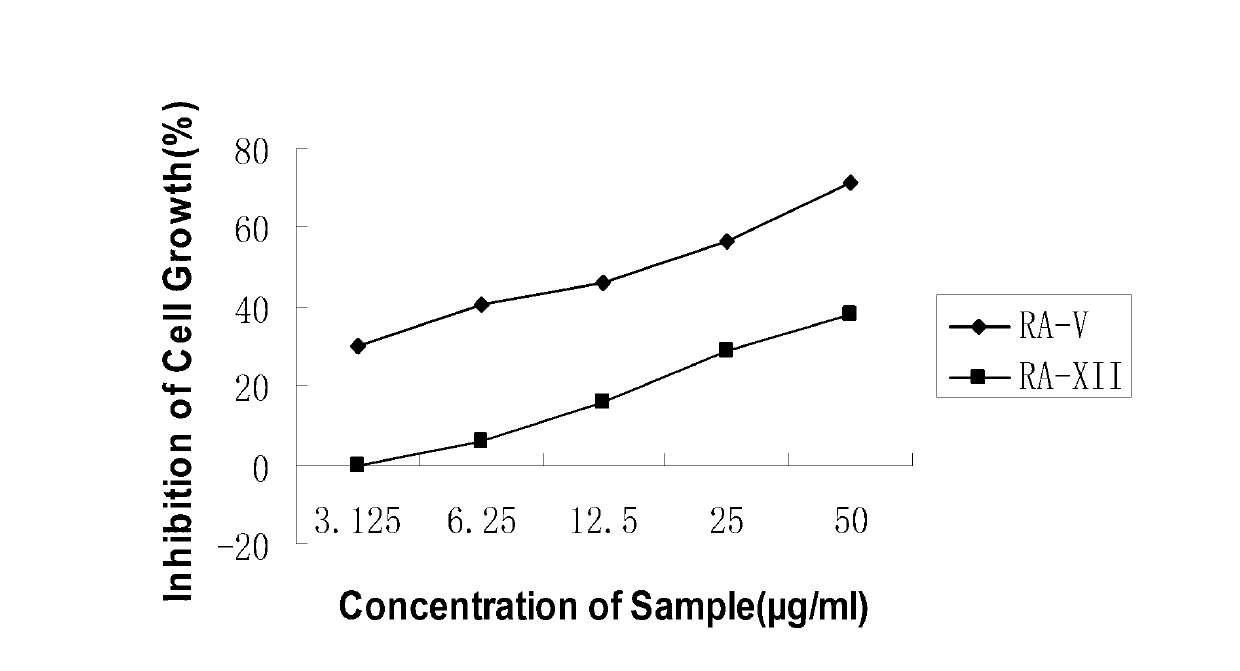
[0054] Rubiaceae type cyclic peptide compounds of the present invention RA-V (1), RA-I (2), RA-XXIV (3), RA-XII (4), rubiyunnanin D (5), rubiyunnanin C (6), rubiyunnanin E (7), rubiyunnaninF (8), rubiyunnanin G (9), rubiyunnanin H (10) and RY-II (11) can significantly inhibit the LPS and IFN-γ-induced mouse peritoneal macrophage cell line RAW264.7 model NO generated. The experimental principles, methods and results are as follows:
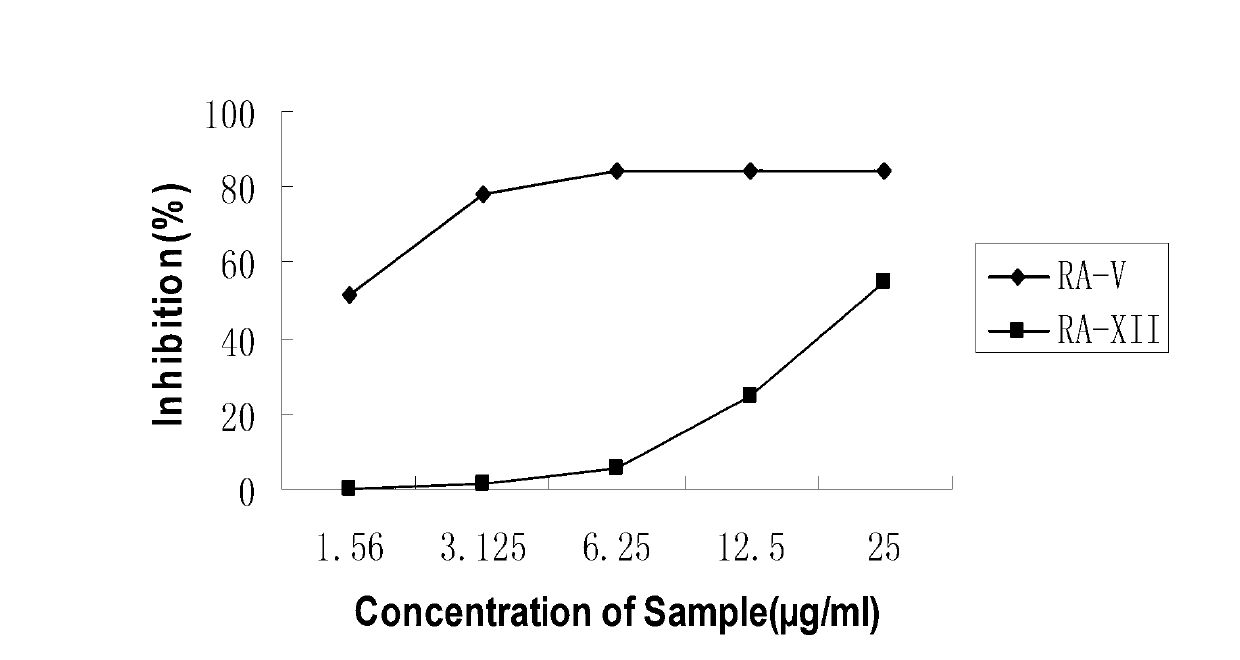
[0055] Experimental principle: The gene encoding nitric oxide synthase (Nitric Oxide Synthase, NOS) is one of the direct target genes of the NF-κB signaling pathway. When cells are stimulated by microbial endotoxins and inflammatory mediators, the NF-κB signaling pathway will be activated , so as to generate a large amount of inducible NOS. Nitric oxide synthase catalyzes its substrate L-arginine to generate NO and L-citrulline. NO exists in the form of nitrite ions in aqueous solution. In this experiment, LPS and IFN-γ were used to stimulate RAW...
Embodiment 3
[0062] Rubiaceae type cyclic peptide compounds of the present invention RA-V (1), RA-I (2), RA-XXIV (3), RA-XII (4), rubiyunnanin D (5), rubiyunnanin C (6), rubiyunnanin E (7), rubiyunnanin F (8), rubiyunnanin G (9), rubiyunnanin H (10) and RY-II (11) used the luciferase reporter gene method to detect the inhibitory activity of NF-κB pathway, and further confirmed the role of cyclic peptide molecules in the NF-κB pathway. The experimental principles, methods and results are as follows:
[0063] Experimental principle: NF-κB nuclear transcription factor has a conserved DNA-binding sequence, which regulates the expression of downstream genes by binding to it. The pNF-κB-Luc reporter plasmid (Clontech) contains the firefly luciferase reporter gene, whose expression is regulated by a synthetic promoter, which contains the basic promoter element (TATA box), and the NF-κB transcription factor DNA binding sequence, so the expression of the firefly luciferase reporter gene is regula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com