Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
A technology of herpes simplex virus and carrier, applied in the application field of gene medicine, which can solve the problems of short production cycle, high oncolytic activity, and insufficient oncolytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The present invention also provides a method for preparing a recombinant type II herpes simplex virus vector, wherein the method comprises the following steps:
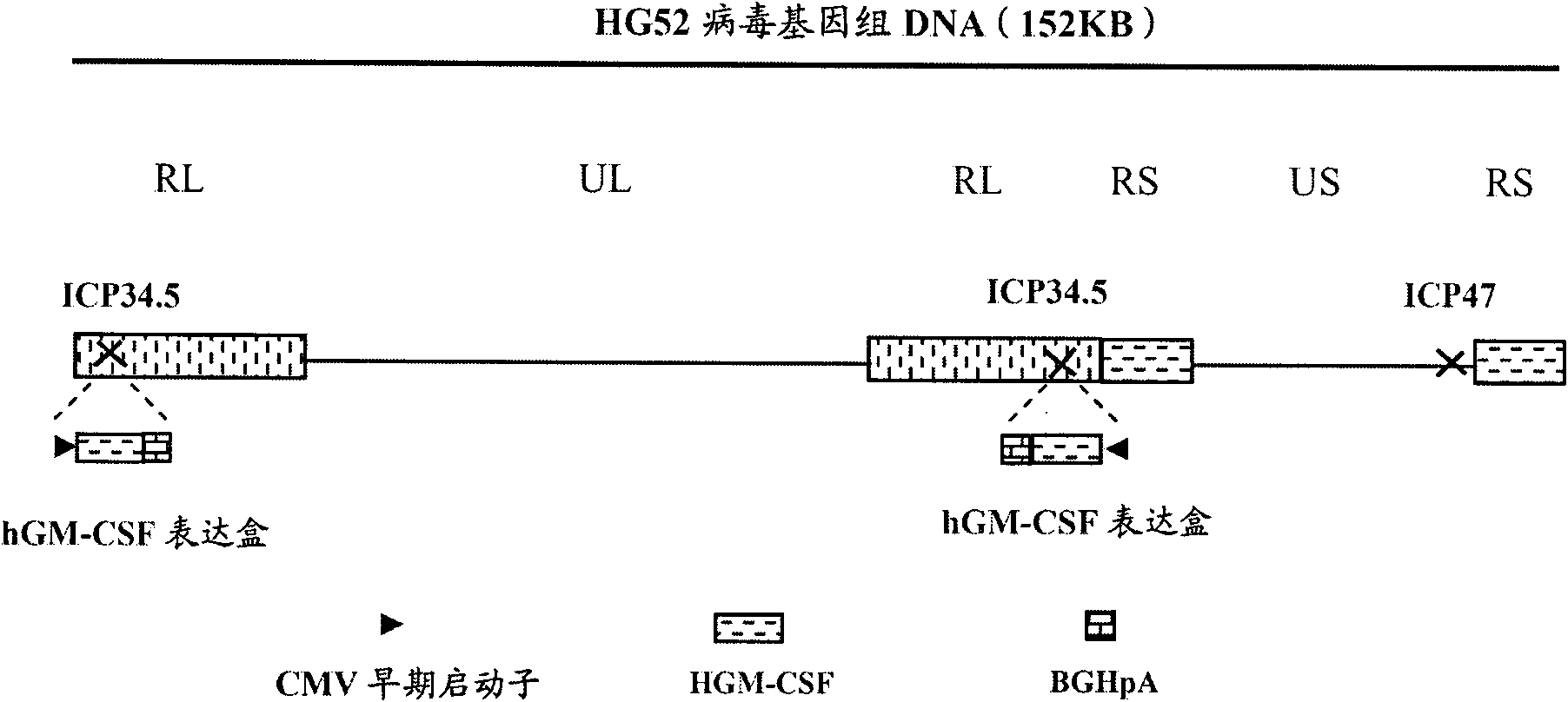
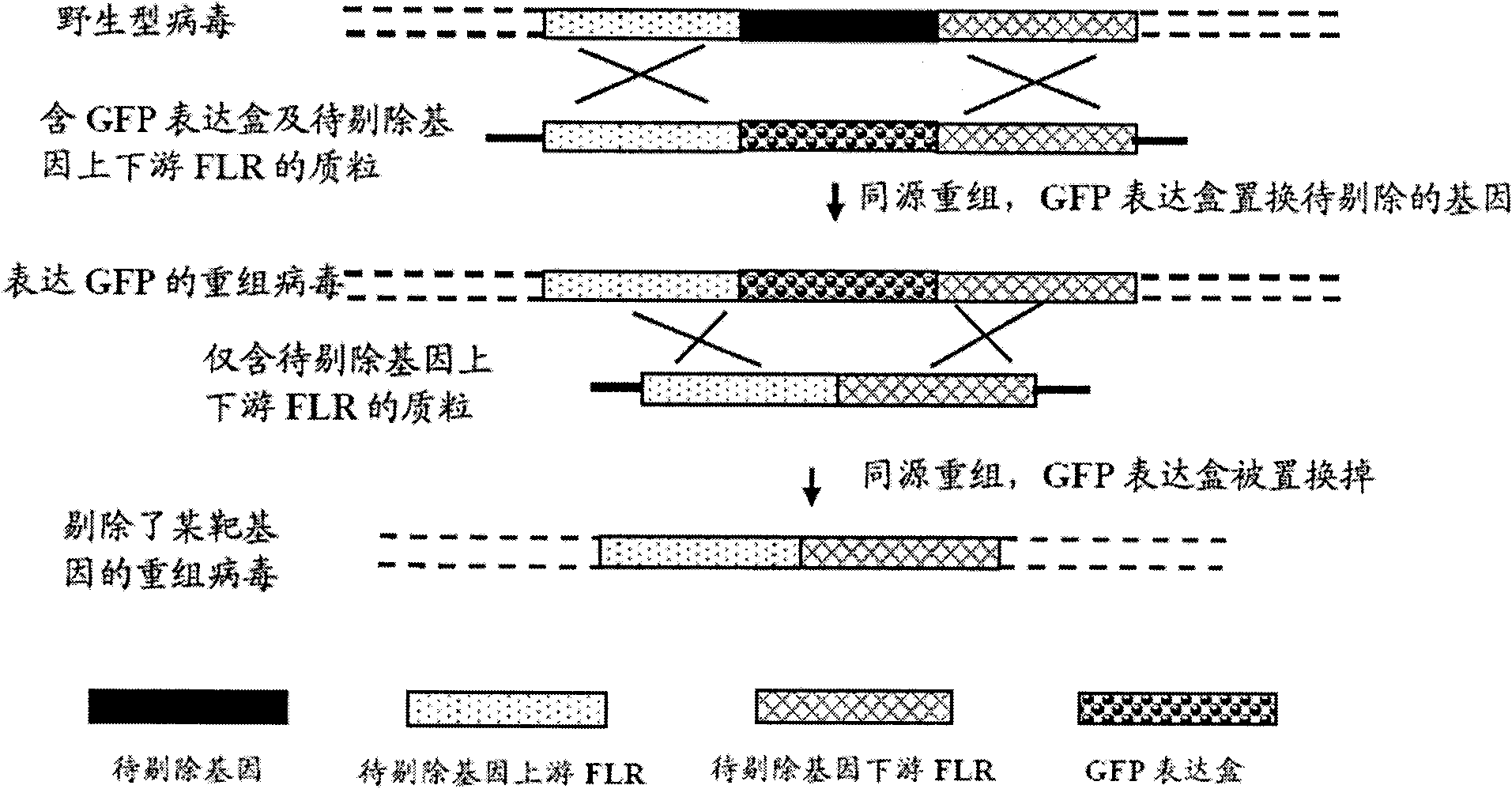
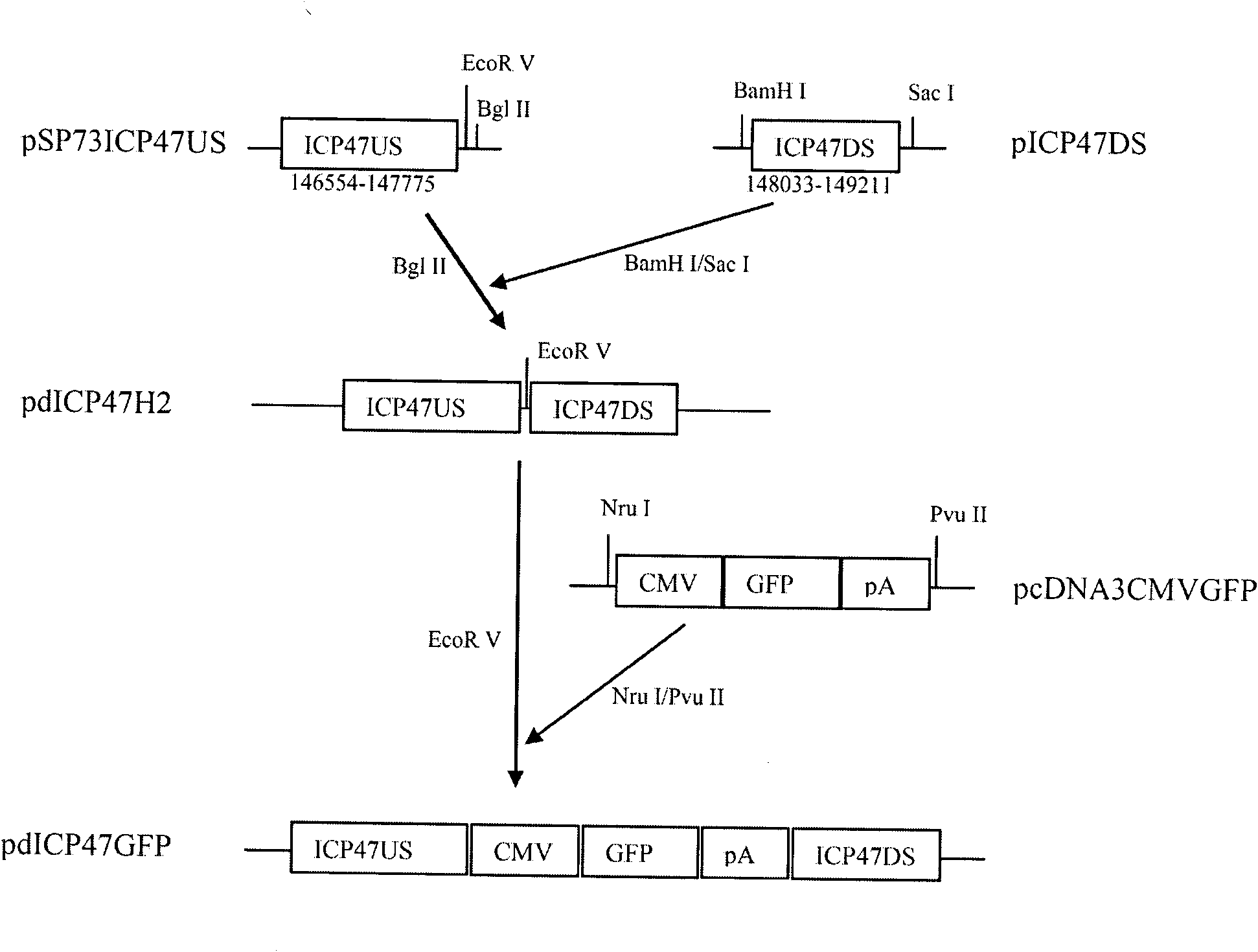
[0086] (1) Delete the ICP47 gene from the wild type II herpes simplex virus HG52 strain to construct HG52dICP47 recombinant type II herpes simplex virus:
[0087] a. extracting the full-length viral DNA of type II herpes simplex virus HG52 strain;
[0088] b. Construct the plasmid pdICP47H2 containing the upstream flanking region sequence and the downstream flanking region sequence of the ICP47 gene:
[0089] b1. Using the primers shown in Table 1, using the full-length viral DNA obtained in step a as a template, PCR amplifies the upstream flanking region sequence and the downstream flanking region sequence of the ICP47 gene;
[0090] Table 1
[0091]
[0092] b2. Insert the upstream flanking region sequence of the PCR product amplified in step b1 into the SmaI site of the pSP73 plasmid to obtain the pSP73IC...
Embodiment 1
[0203] Delete the ICP47 gene from the wild-type HSV-2 (HG52) genome to construct HG52dICP47 .
[0204] (1) DNA of purified HG52 wild-type virus
[0205] HG52 virus was cultured with BHK cells and DNAzol TM Genomic DNA Isolation Kit (Helena Biosciences Cat. No. DN127200) was used to purify viral DNA. BHK cells were grown in a 175 cm square culture flask, and the culture medium was DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin. The culture conditions were 37°C, 5% carbon dioxide. When the cells were grown to 90% saturation, the virus was inoculated. Continue to incubate for 24-48 hours. When more than 90% of the cells have cytopathic changes, remove the culture medium and add 10ml of DNAzol. Use a 10ml pipette to suck and blow 5 times, and transfer the cell lysate to a 50ml Falcon test tube, add 5ml of 100% ethanol, and shake the test tube gently in a circular motion to fully analyze the viral DNA. Pick the DNA into another test tube with a tip...
Embodiment 2
[0271] This example illustrates the pharmaceutical composition of the invention.
[0272] The preparation of the injection will prepare a group of phosphate buffer solution according to the dosage shown in Table 3, and sterilize at 121°C for 20 minutes. Under sterile conditions, filter the herpes simplex virus stock solution of known titer in Example 1 with a microporous membrane of 0.45 microns to remove cell debris, collect the filtrate into a sterile centrifuge tube, and centrifuge at 8000 rpm for 1 hour, discard Remove the supernatant, and according to the virus titers shown in Table 3, disperse the obtained virus precipitate in the above-mentioned high-temperature sterilized phosphate buffer solution to obtain the injection solution of the present invention.
[0273] table 3
[0274] drug
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com