Anti-herpes simplex virus I-form medicament composition and uses thereof
A technology of herpes simplex virus and composition, which is applied in the directions of drug combination, antiviral agent, medical preparation containing active ingredients, etc., can solve the problems such as no dammarane-type tetracyclic triterpenoids and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
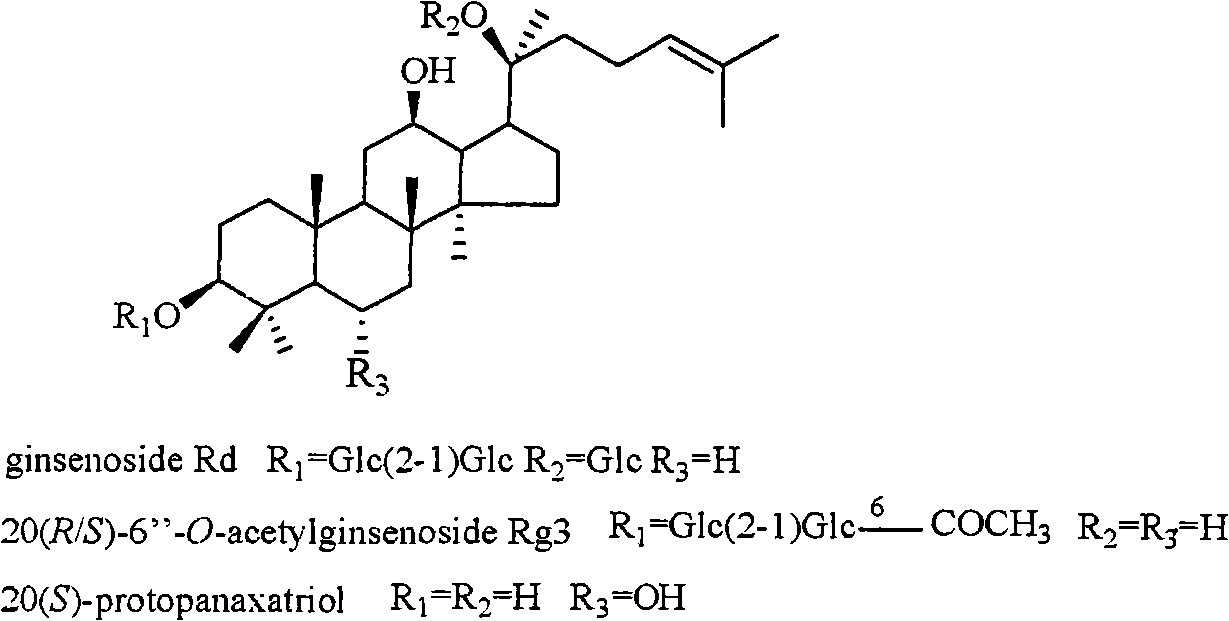
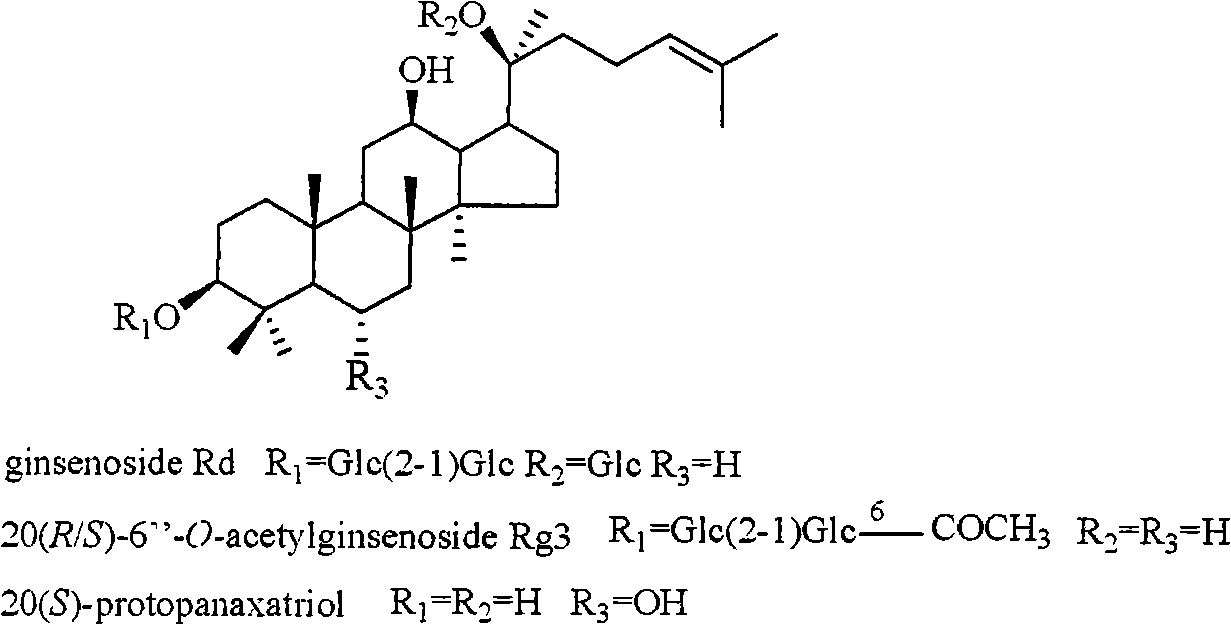
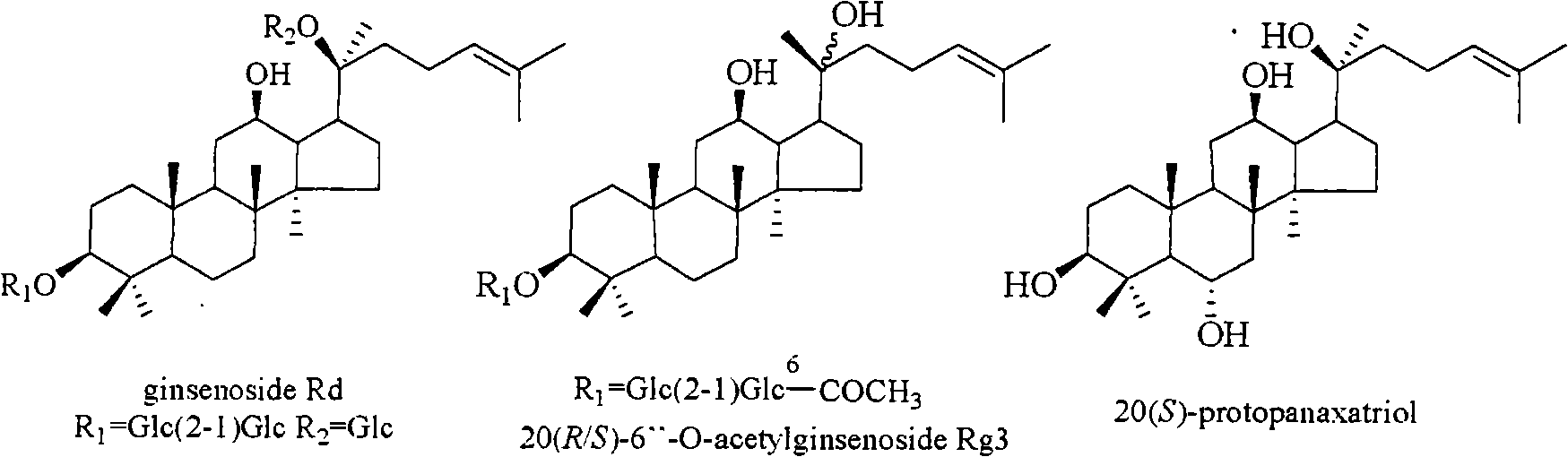
[0028] Extraction, separation and structure identification of 3 active compounds:
[0029] 5kg of Panax notoginseng was purchased in Yunnan. After being crushed into granules, it was wrapped with double gauze and placed in a pressure cooker. The temperature was controlled at a high temperature of 120°C. After being steamed for 12 hours, cooked Panax notoginseng was obtained. The cooked Panax notoginseng was extracted with industrial ethanol under reflux (5L×4), and the solvent was recovered under reduced pressure and concentrated to obtain 778 g of total extract. The extract was dissolved in water, column chromatography was performed with D101 macroporous adsorption resin (90×8.5cm), and the effluent was first eluted with water until the Molish reaction was negative, and then eluted with industrial methanol to obtain 440 g of crude saponin.
[0030] The crude saponin was mixed with 500 g of silica gel (200-300 mesh), and subjected to silica gel (200-300 mesh) dry column chromatogr...
Embodiment 2
[0041] Toxicity test of ginsenoside Rd(1) on Vero cells:
[0042] After the Vero cells are trypsinized, add 100μL / well to a 96-well culture plate. When the cells grow into a monolayer, discard the growth medium. Add 100μL of sample-containing maintenance solution to each well. Set four replicate wells for each dilution. Set up a normal cell control group with 5% CO 2 Incubate in an incubator at 37°C, observe the changes of the cells every day, and calculate the maximum non-toxic concentration TC of the drug 0 .
[0043] Results The maximum non-toxic concentration of ginsenoside Rd on Vero cells TC 0 At 200μg / mL, there is no obvious toxic effect.
Embodiment 3
[0045] The pharmacodynamic experiment of ginsenoside Rd(1) against HSV-1 virus:
[0046] Vero cells were cultured to a single layer in a 96-well plate, the growth medium was discarded, the sample maintenance solution was diluted to seven concentrations (within the maximum non-toxic range of the sample), and 50 μL of the sample maintenance solution and the virus dilution solution were added to each well. Set 4 multiple holes for each concentration, and set up positive control group, virus control group and normal cell control group at the same time, set 5% CO 2 Incubate in an incubator at 37°C and observe the condition of cytopathic (CPE) every day. When the cytopathic effect of the virus control group reaches 75% and above and the normal cell control group is normal, observe and record the CPE of each well, use the MTT method to detect the inhibitory rate of the sample against the virus, and calculate the half inhibitory concentration of the sample against HSV-1 (IC 50 ).
[0047]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com