Applications of Bacteroides fragilis in prevention and/or treatment of meningitis
A technology of Bacteroides fragilis and meningitis, applied in the fields of microorganisms, health care products, food and daily chemical products, and pharmaceuticals, to achieve the effect of no drug resistance and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
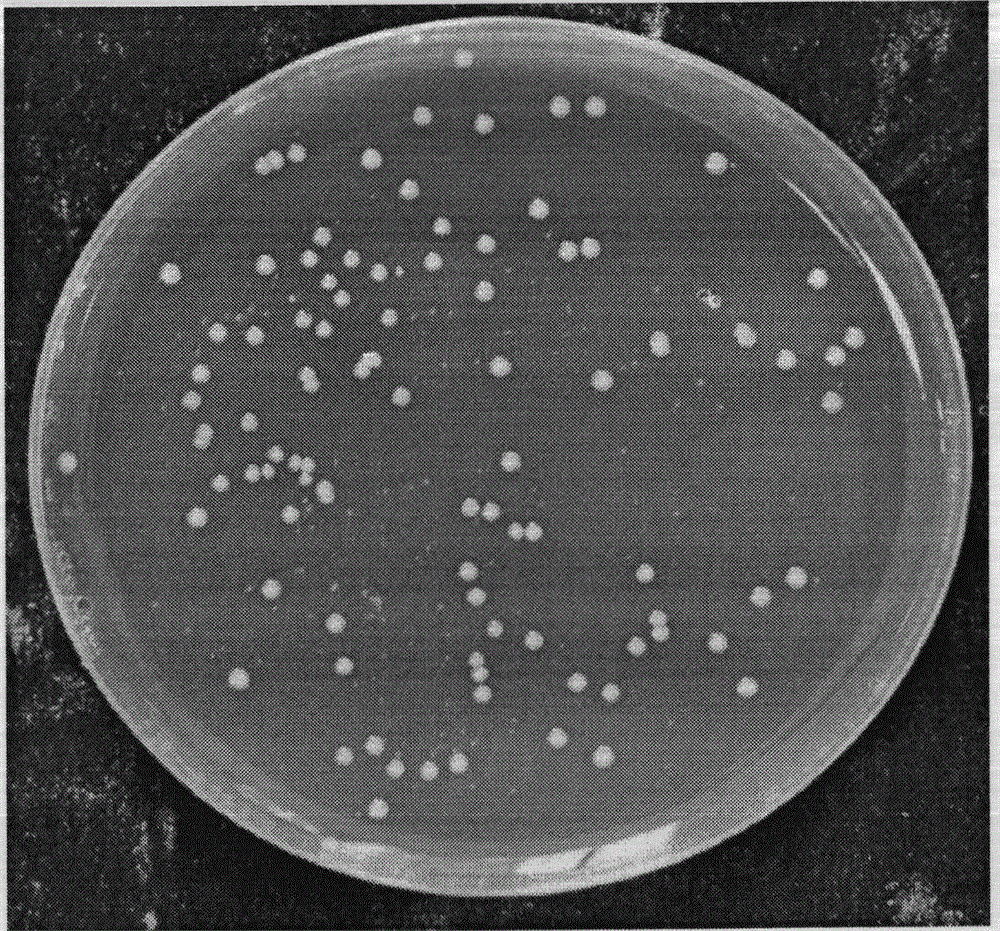
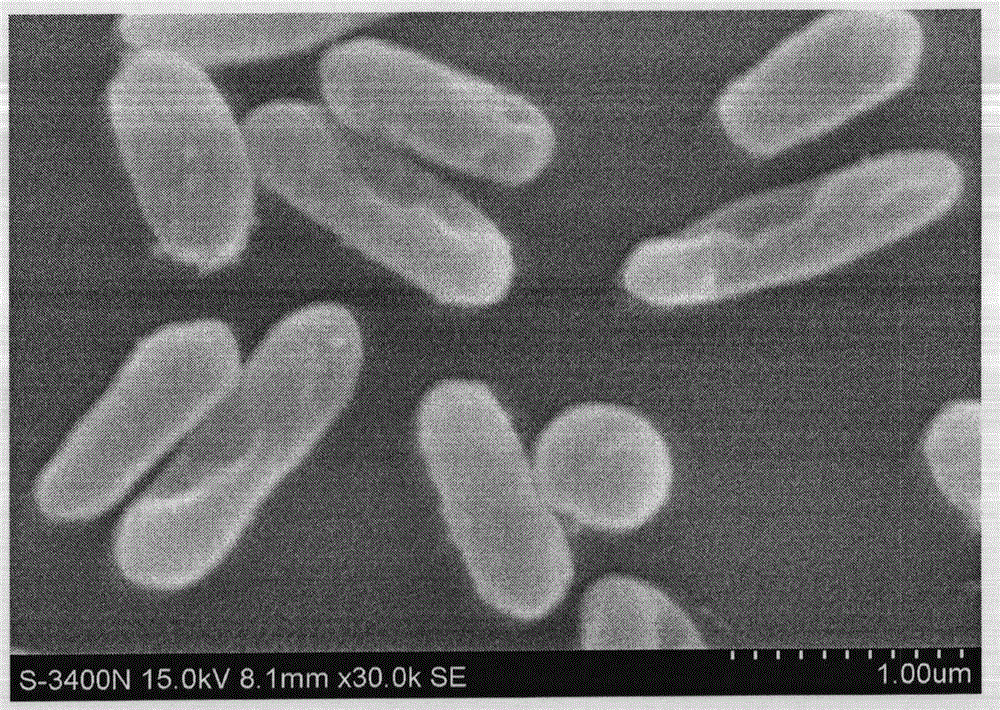
[0047] Isolation and Purification of Bacteroides fragilis ZY-312
[0048] Reagents and Instruments
[0049] (1) Medium A: Bacteroides-bile-escin (BBE) agar (Qingdao Haibo Biotechnology Co., Ltd., product number: HB7028) was added with an improved formula, and the specific components are shown in Table 1:
[0050] Table I
[0051]
[0052]
[0053] (2) Medium B: Bacteroides-bile-escin (BBE) agar (Qingdao Haibo Biotechnology Co., Ltd., product number: HB7028) was added with an improved formula, and the specific ingredients are as follows in Table 2:
[0054] Table II
[0055]
[0056] (3) Medium C: Fetal bovine serum was added to Brinell Broth (Qingdao Haibo Biotechnology Co., Ltd., product number: HB0241), and the addition amount was 5% (v / v) (Zhejiang Tianhang Biotechnology Co., Ltd., Brand: Sijiqing, Item No.: HB0205).
[0057] (4) Experimental equipment
[0058] 2.5L sealed culture tank (Mitsubishi Gas Chemical Co., Ltd., C-31)
[0059] Constant temperature in...
Embodiment 2
[0097] Identification of Bacteroides fragilis ZY-312
[0098] The polymerase chain reaction (PCR) primer (synthesized by Yingwei Jieji (Shanghai) Trading Co., Ltd.) sequence is as follows:
[0099] Primer pair 1:
[0100] Forward primer: 5'-ACGCTTGCACCCCTCCGTATTA-3'
[0101] Reverse primer: 5'-GCTTAGAGTTTGATCCTGGCTCAG-3'
[0102] Primer pair 2:
[0103] Forward primer: 5'-TGGGTGGTTGCTGCCTGGACACA-3'
[0104] Reverse primer: 5'-CATCCGGGTATGGATATGAA-3'
[0105] Primer pair 3:
[0106] Forward primer: 5'-GATGCTCCAGTTACAGCTTCCATTG-3'
[0107] Reverse primer: 5'-CGCCCAGTATATGACCTAGTTCGTG-3'
[0108] bft gene primer pair:
[0109] Forward primer: 5'-GACGGTGTATGTGATTTGTCTGAGAGA-3'
[0110] Reverse primer: 5'-ATCCCTAAGATTTATTATCCCAAGTA-3'
[0111] 1. PCR identification (PCR is the polymerase chain reaction, which is a commonly used method for rapidly amplifying genes)
[0112] (1) 16S rRNA sequence determination
[0113] The above strains were inoculated on medium A, and cu...
Embodiment 3
[0134] Tolerance of Bacteroides fragilis ZY-312 to Gastric Acid
[0135] 1. Artificial gastric juice preparation (according to the 2010 "Chinese Pharmacopoeia" artificial gastric juice preparation method)
[0136] Dissolve 23.4mL of concentrated HCl in 100mL of purified water to obtain dilute hydrochloric acid. Take 8.2mL dilute hydrochloric acid, add 400mL purified water and 5g pepsin ( Porcine source, 1:15000), dilute to 500mL. Stir overnight at 37°C with magnetic force to obtain artificial gastric juice.
[0137] 2. Cell preparation
[0138] Collect the bacterial solution, centrifuge, discard the supernatant, resuspend in normal saline, centrifuge again, discard the supernatant, and keep the bacteria for later use.
[0139] 3. Add artificial gastric juice to determine the number of viable bacteria
[0140] Add artificial gastric juice to the bacteria, resuspend, and measure the number of viable bacteria at 0, 1, 2, and 3 hours respectively.
[0141] The 10-fold seria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com