A kind of enterotoxin gene lt knockout Escherichia coli and its construction method
A technology of Escherichia coli and construction method, which is applied in the field of gene knockout, can solve the problems of low efficiency of bacterial knockout, high degree of dependence, leaving FRT marks, etc., and achieves the effect of convenient and seamless knockout and slowing growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
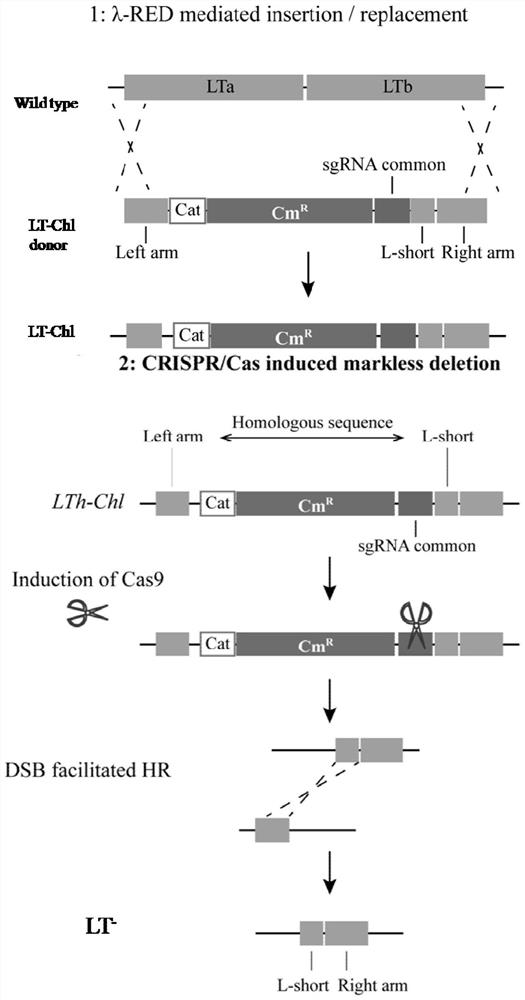
[0044] Example 1 Construction of LT gene homologous recombination donor LT donar
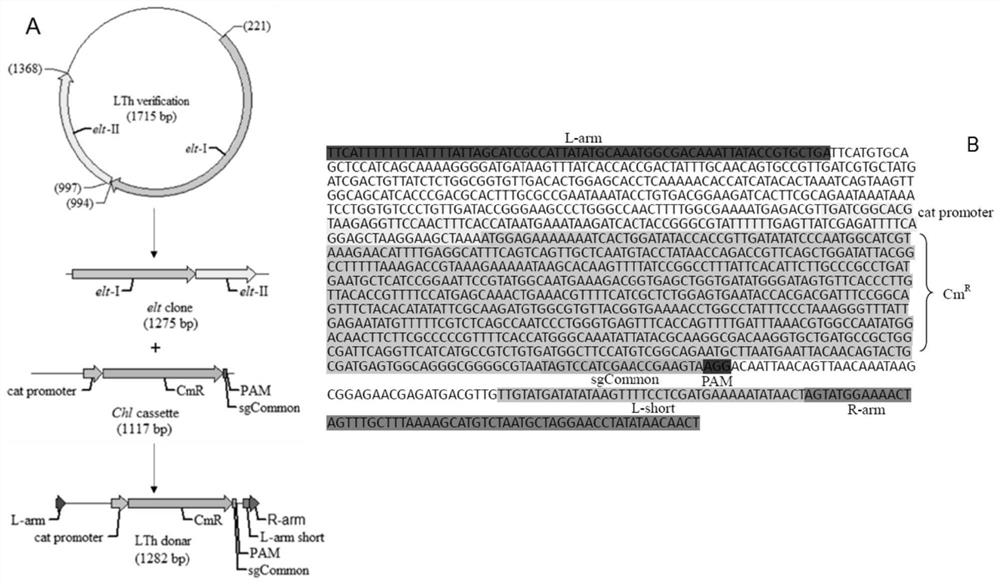
[0045] The full-length LT gene was cloned with the primers LT-F / LT-R in Table 1, connected to the TA-cloning vector, and transformed into competent cells. Select a single clone, sequence the positive clones verified and screened by PCR, and compare them to the NCBI database to obtain the full length of the LT gene and its upstream and downstream sequences. According to the LT gene and its upstream and downstream sequences, the 5'-end homology arm sequence L-arm short and L-arm, and the 3'-end homology arm sequence R-arm were designed. With DNA fragment Cat-chl cassette as the replacement gene of LT, the donor LThdonar (L-arm+Cat-chl cassette+L-arm short+R-arm) of synthetic LT gene (see image 3 ). The base sequence of LTh donar is as follows:
[0046] TTCATTTTTTTTATTTTATTAGCATCGCCATTATATGCAAATGGCGACAAATTATACCGTGCTGATTCATGT GCAGCTCCATCAGCAAAAGGGGATGATAAGTTTATCACCACCGACTATTTGCAACAGTGCCGTTGATCG ...
Embodiment 2
[0054] Embodiment 2 LT donar conversion
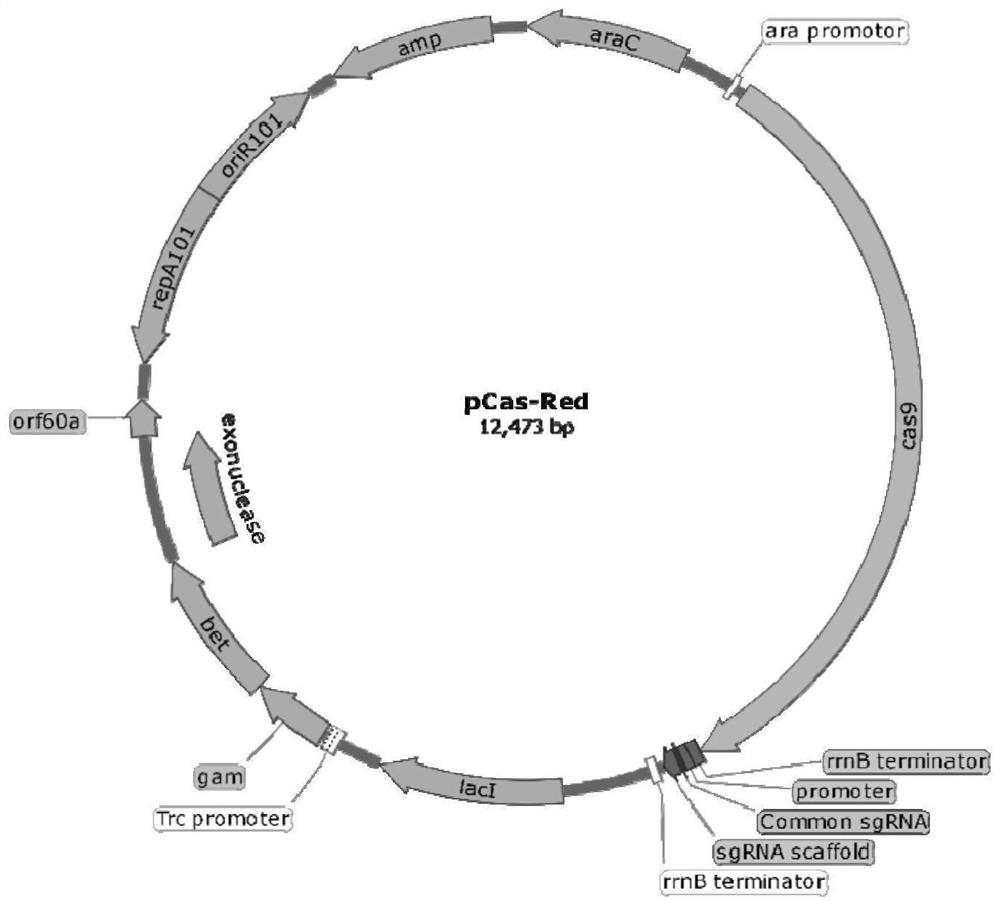
[0055] Prepare electrotransfection competent cells containing the pCas-Red plasmid, inoculate the ETEC K88 positive clone containing the pCas-Red plasmid (see Figure 2) into 50 mL of LB liquid medium containing Amp and 1% glycerol, at 30°C, 200r· min -1 , after culturing for 2h, add 0.1mmol·L -1 IPTG induces the expression of λ-RED protein, grows at 30°C until OD600=0.6, and other steps are the same as before. Take 50 μL of prepared competent cells containing pCas-Red plasmid, add LT donar, transfer to the electroporation cuvette, mix well, and perform electroporation under the same conditions as before. The cells were recovered at 30°C for 2h, spread on LB medium containing Amp, chloramphenicol and 1% glycerol, and cultured at 30°C for 12h. Single clones were picked, and the insertion of the LTh donar gene was verified by PCR using the primers LT donor-F and LT donor-R in Table 1.
Embodiment 3
[0056] Example 3 Screening of LT gene knockout strains
[0057]The correct clone that had been inserted into the LT donar gene was inoculated in a medium containing Amp, IPTG (final concentration 0.1 mmol·L -1 ) and L-arabinose (final concentration 0.2%) in LB medium to induce CRISPR / Cas9 system and λ-RED protein expression. 30℃, 200r·min -1 , cultured for 6h, spread the cells on LB plates containing Amp, and cultured at 30°C for 12h. (dilute at least 10 -4 times). Clones were identified by PCR with primers LT donor-F / LT donor-R. At 42°C, the pCas-Red plasmid is sensitive to temperature. Escherichia coli may lose the pCas-Red plasmid when the temperature is higher than 37°C. Cultivate and screen the LT gene-positive clones to the logarithmic growth phase, and dilute the culture by at least 10 -4 Double and spread on LB plates without any antibiotics, and incubate at 37°C for 12h. Perform PCR verification with the primers Cas9-F / Cas9-R in Table 1, screen the positive clon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com