Vibrio cholerae multiplex fluorescence PCR detection kit as well as preparation and application thereof
A detection kit, Vibrio cholerae technology, applied in the field of biotechnology detection, can solve the problems of poor accuracy, cross-contamination, low efficiency, etc., and achieve the effect of simple operation, high sensitivity, and improved detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 kit
[0044] The primers and probes for Vibrio cholerae hemolysin gene detection, the primers and probes for Vibrio cholerae O1 antigen gene detection, the primers and probes for Vibrio cholerae O139 group antigen gene detection, the primers and probes for Vibrio cholerae enterotoxin gene detection were synthesized respectively. needle, its nucleotide sequence is shown in Table 1 below:
[0045] Table 1
[0046]
[0047] The above-mentioned sets of primer pairs and probes can be packaged individually, or can be combined to make a multiple fluorescent PCR detection mixture. In the multiplex fluorescent PCR detection mixture, the amounts of the above-mentioned primers and probes can be conventional amounts known to those skilled in the art.
[0048] That is to say, the kit of the present invention may contain the aforementioned independently packaged sets of primer pairs and probes, or may contain a configured multiplex fluorescent PCR...
Embodiment 2
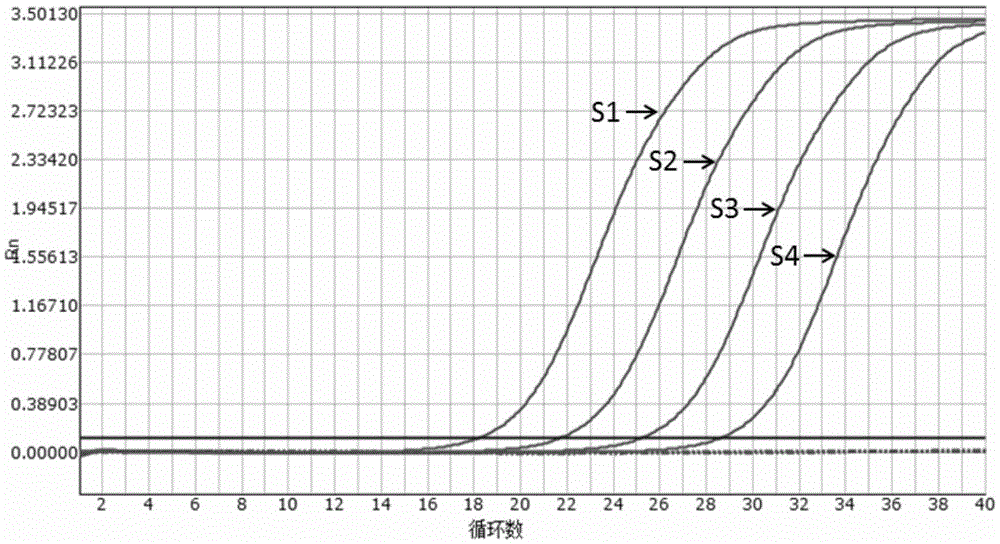
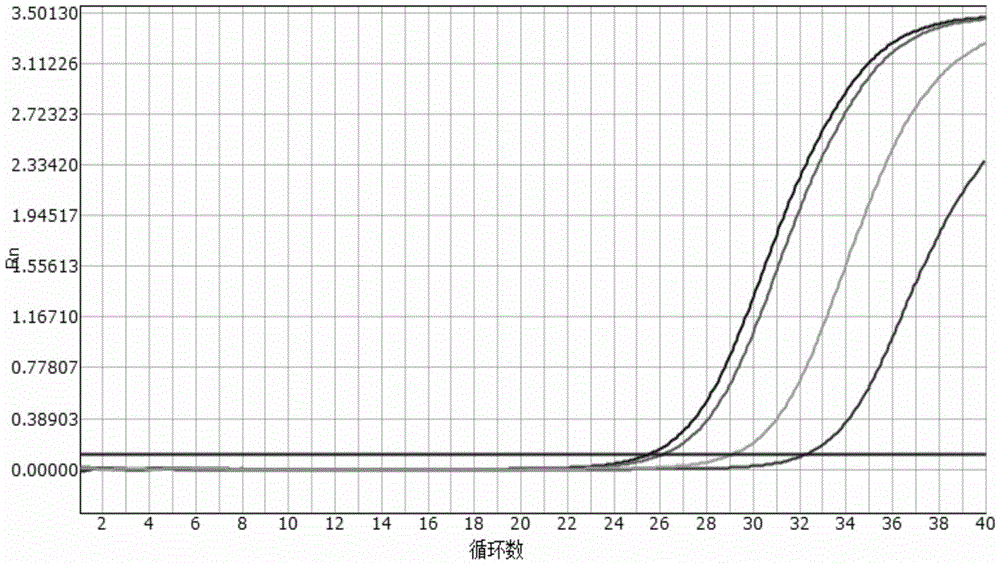
[0050] Sensitivity evaluation of embodiment 2 kit
[0051] Experiment purpose: determine the detection limit (minimum detection concentration) of the present invention (embodiment 1) multiple fluorescent PCR kit
[0052] experimental method:
[0053] 1. Preparation of positive control substance:
[0054] The pMD8 plasmid containing Vibrio cholerae hemolysin, Vibrio cholerae O1, O139 group-specific antigens and specific amplified fragments of enterotoxin gene was constructed as a positive control. The pMD8 plasmid is connected with Vibrio cholerae hemolysin gene detection primers, Vibrio cholerae O1 group antigen gene detection primers, Vibrio cholerae O139 group antigen gene detection primers, and Vibrio cholerae enterotoxin gene detection primers. The augmenter fragment can be jointly recognized by the above-mentioned 4 kinds of primers and probes. It was verified by sequencing that the positive plasmid was successfully constructed.
[0055] Wherein, the nucleotide sequen...
Embodiment 3
[0081] Example 3 Kit Test Result Specificity Evaluation
[0082] Experimental purpose: Select the nucleic acid extract of Vibrio cholerae sample that has been verified by Sanger sequencing to test the specificity and reliability of multiple fluorescent PCR detection results.
[0083] experimental method:
[0084] 1. Sample processing: select nucleic acid extraction solutions of different types of samples from the specimen bank, thaw at room temperature and take 4ul for subsequent experiments.
[0085] Feces sample: Pick the feces the size of a grain of rice, put them into a centrifuge tube that has been pre-added with 0.5ml of normal saline, shake and mix, centrifuge at 13000rpm for 2 minutes, and remove the supernatant; add 100ul of the nucleic acid extractor provided in the kit to the precipitate extract, mix well. Boiling water bath for 10 minutes, centrifugation at 13000rpm for 5 minutes, the supernatant is the sample nucleic acid extraction solution, which can be direct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com