Treble fluorescent quantitation PCR reagent kit for detecting sea, seb and sec genes of staphylococcus aureus enterotoxins
A fluorescent quantitative and enterotoxin technology, applied in the field of bioengineering, can solve the problems of cumbersome procedures, unfavorable detection of a large number of samples, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Design and optimization of primers and probes
[0037] Sequence source: multiple sequences of S. aureus enterotoxin genes sea, seb, and sec were searched through the NCBI gene bank. Through sequence comparison, the following three sequences were finally selected for the design of primers and probes: the accession number of the representative strain of sea It is EF520720.1, the accession number of the seb representative strain is KX168632.1; the accession number of the sec representative strain is KX168615.1;
[0038] sea extraction GenBank: EF520720.1.
[0039] atgaaaaaaacagcatttatactacttttattcattgccctaacgtggacaacaagtccacttgtaaatggtagcgagaaaagcgaagaaataaatgaaaaagatttgcgaaaaaagtctgaattgcagggaacagctttaggcaatcttaaacaaatctattattacaatgaaaaagctaaaactgaaaataaagagagtcacgatcaatttttacagcatactatattgtttaaaggcttttttacaaatcattcatggtataacgatttattagtagattttgattcaaaggatattgttgataaatataaagggaaaaaagtagacttatatggtgcttattatggttatcaatgtgcgggtggtacaccaaacaaaacagcttgcatgtatggtggtgtaacgtta...
Embodiment 2
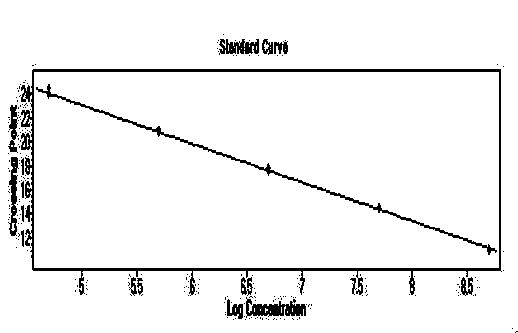
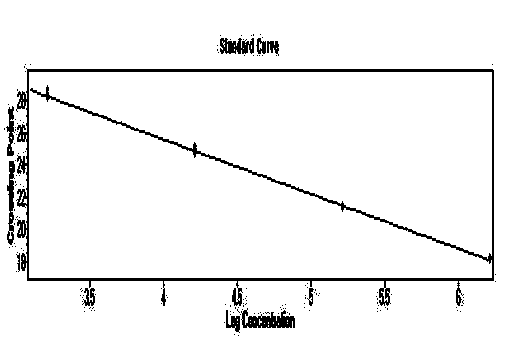
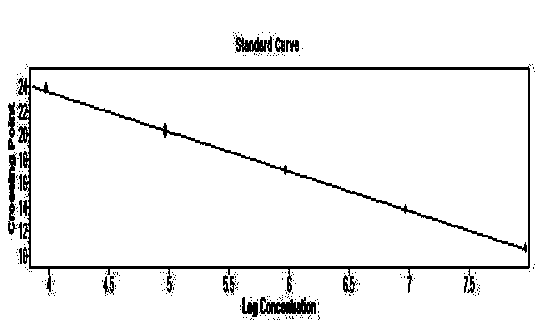
[0065] Embodiment 2 standard curve test
[0066] The positive plasmids in the kit were taken, and their concentrations were determined to be 39.0ng / μL, 62.7ng / μL, and 36.2ng / μL, respectively. Use DEPC water to perform 10-fold gradient dilutions, and dilute 6 gradients in total, and perform qPCR detection for each dilution to obtain the slope and correlation coefficient R 2 value.
[0067] Experimental results: For the standard curve of the plasmid, see Figure 1-3 , the slopes are -3.2678, -3.4117, -3.3128 respectively, according to the formula E (amplification efficiency) = 10 -1 / 斜率 , converting the amplification efficiency into a percentage ((E-1)×100%) is 98.6%, between 90% and 110%, indicating that the amplification efficiency of this method is good; its R 2 The values are 0.9992, 0.9982, 0.9998, respectively, between 0.99 and 1, indicating that the method has high reliability.
Embodiment 3
[0068] Embodiment 3 specificity test
[0069] The 14 standard strains in Table 3 were amplified and detected using two sets of enterotoxin triple qPCR systems, and the results showed that except for the positive strains (Staphylococcus aureus enterotoxin A ATCC 13565, Staphylococcus aureus Staphylococcus enterotoxin C ATCC 19095), other strains (Staphylococcus aureus CMCC26003, Staphylococcus aureus ATCC 29213, Micrococcus luteus CMCC 28001, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, pneumoniae Lebsiella ATCC700603, Salmonella paratyphi beta CMCC 50094, Candida albicans CMCC98001, Bacillus pumilus CMCC 63202, Bacillus subtilis CMCC 63501, Enterococcus faecalis ATCC 29212) had no amplification curves, and the designed primer probes had good specificity .
[0070] Table 3 Specific test strains
[0071]
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com