Novel Recombinant BCG Tuberculosis Vaccine Designed to Elicit Immune Responses to Mycobacterium Tuberculosis in all Physiological Stages of Infection and Disease
a technology of mtb and recombinant bcg, which is applied in the field of improved mycobacterium tuberculosis vaccines, can solve the problems of inability to prevent the establishment of latent tb or disease reactivation of infection in adolescents and adults, and no vaccines have been developed which confer immunity to infection by mtb and at the same time treat, so as to prevent the establishment of infection and prevent the reactivation of laten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selection of Suitable Antigens and Design of rBCG Based on this Selection
[0061]An initial group of Mtb proteins (189 antigens, see Table 1) was selected from all possible 3989 Mtb ORFs, according to the following selection procedure:
(1) Compilation of available data for all 3989 TB ORF products: Literature scan for global analyses (e.g. up / down regulation of expression under various conditions such as hypoxia, dormancy, interaction with macrophage, location and persistence in lung tissue; iron regulation; transcriptomics and proteomics profiles; mutation leading to attenuation of virulence; potential high immune response etc.).
(2) Establishing a subset of genes by cross matching the accumulated data: Selection of antigens that demonstrate positive evidence in any two independent studies originating from different criteria mentioned above.
(3) Iterative trimming of the above subset, aiming at selecting an initial group of candidates (Table 1, 189 antigens), based on the extent of effe...
example 2
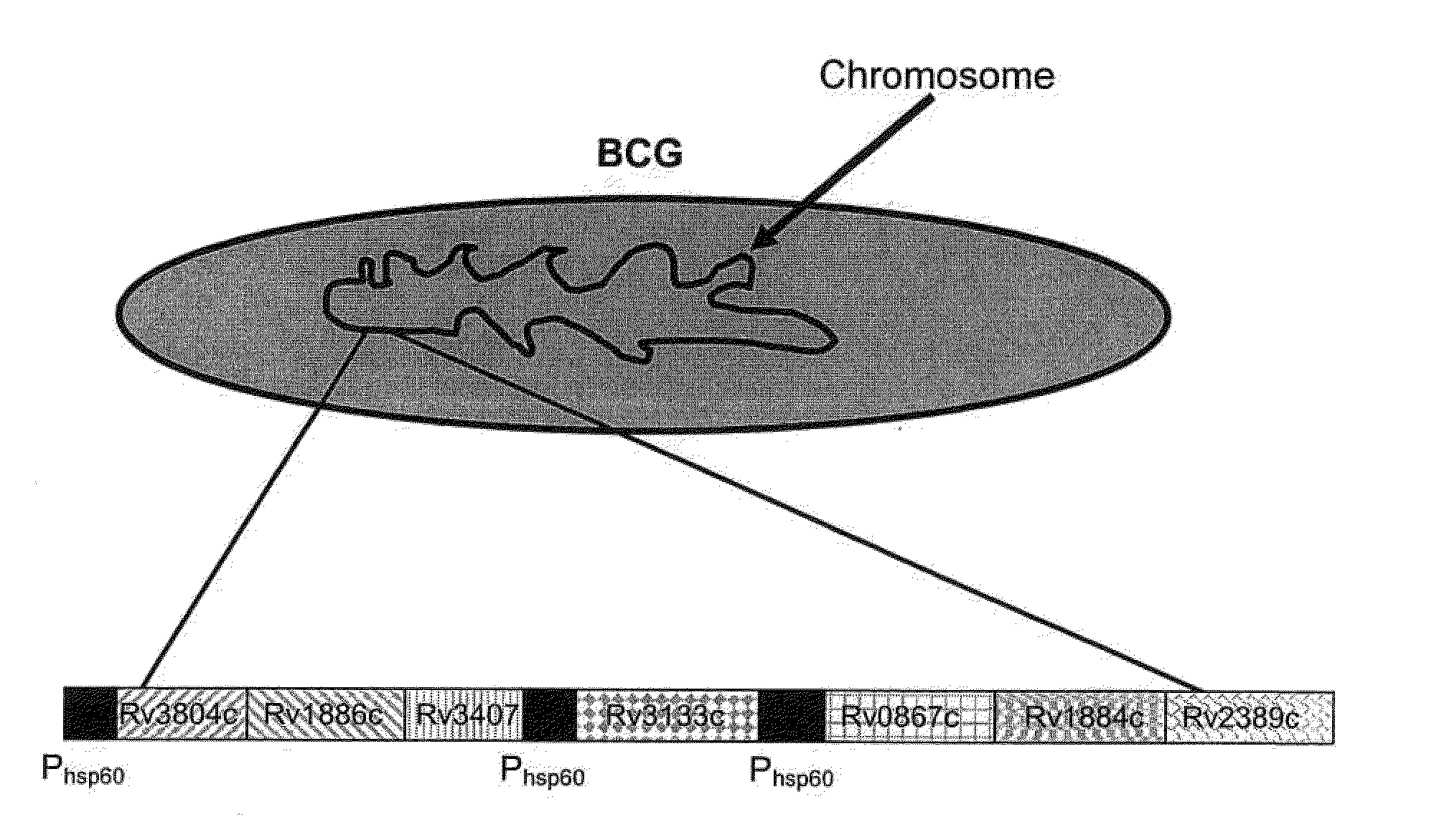
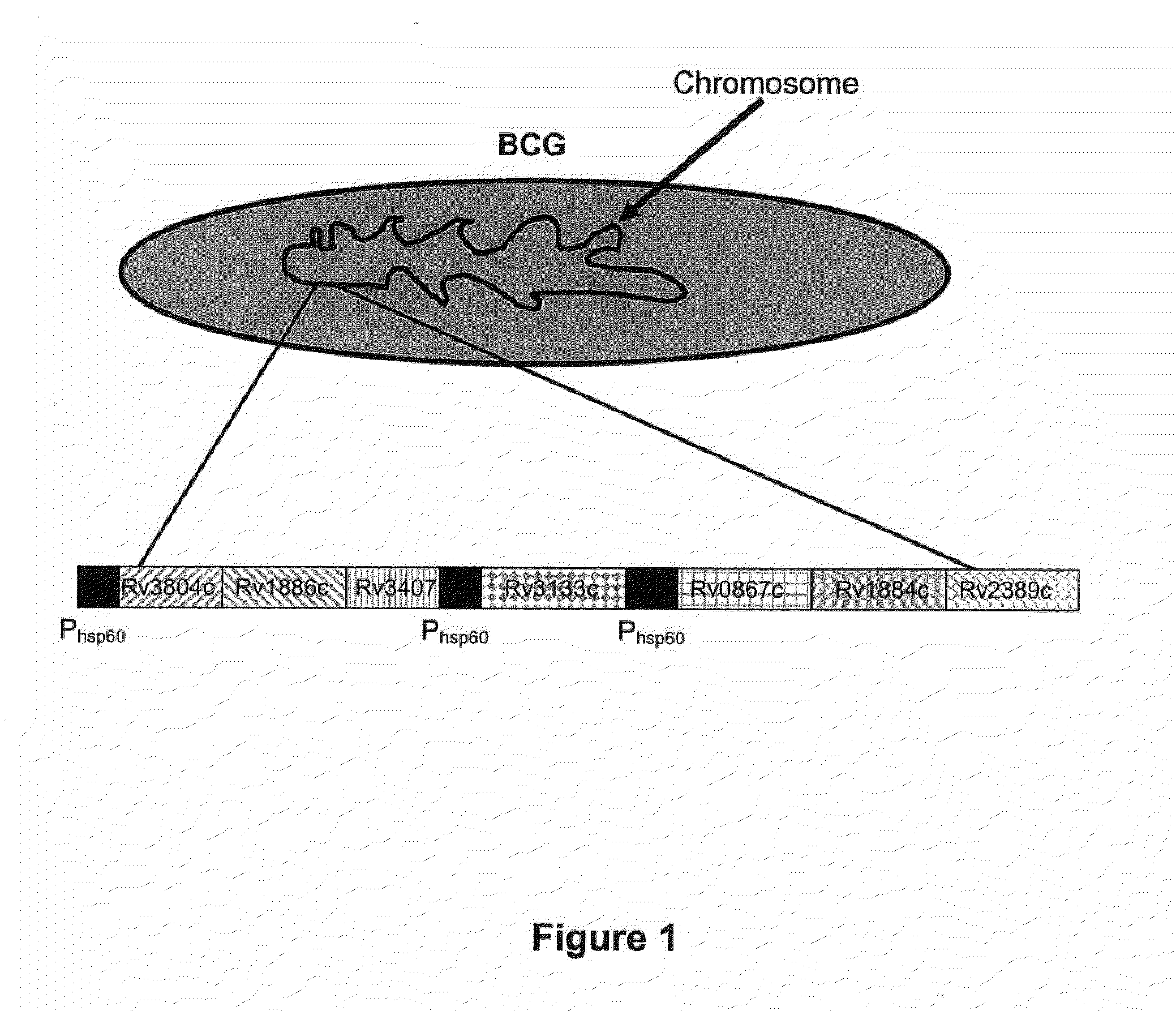
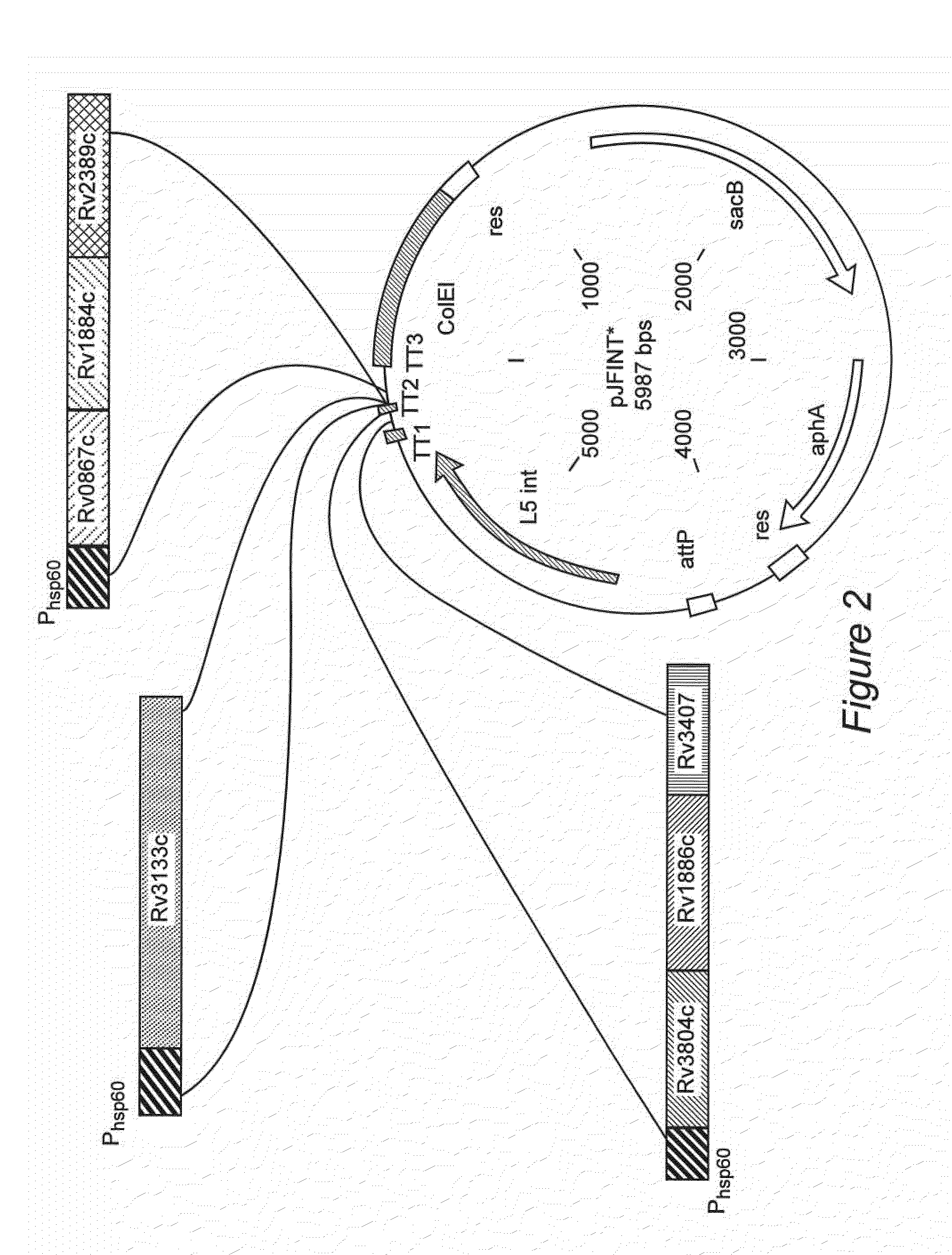
Construction of a Recombinant BCG Genetically Engineered to Express at Least One Classical Mtb Antigen, at Least One Mtb Resuscitation / Reactivation Antigen and in which the DosR Regulon is Upregulated
Materials and Methods: General
[0070]For the construction of an rBCG described in the following sections, restriction endonucleases (herein “REs”); New England Biolabs Beverly, Mass.), T4 DNA ligase (New England Biolabs, Beverly, Mass.) and Taq polymerase (Invitrogen, Carlsbad, Calif.) were used according to the manufacturers' protocols; Plasmid DNA was prepared using small-scale (Qiagen MiniprepR kit, Santa Clara, Calif.) or large-scale (Qiagen MaxiprepR kit, Santa Clara, Calif.) plasmids DNA purification kits according to the manufacturer's protocols (Qiagen, Santa Clara, Calif.); Nuclease-free, molecular biology grade Milli-Q water, Tris-HCl (pH 7.5), EDTA pH 8.0, 1M MgCl−2, 100% (v / v) ethanol, ultra-pure agarose, and agarose gel electrophoresis buffer were purchased from Invitrogen, ...
example 3
Demonstration of the Immunogenicity and Protection Elicited by Vaccination with AERAS-407 in Mice
[0081]To illustrate the immune responses elicited by AERAS-407 and to demonstrate its protective efficacy against tuberculosis, four groups of 10 C57 / BL6 mice are vaccinated subcutaneously with either 1) saline, 2) BCG, 3) BCG-PfoA or 4) AERAS-407. Mice in groups 2, 3, and 4 receive 5×105 cfu of BCG or recombinant BCG. After 10 weeks, five mice from each group are sacrificed for immune assays and the remaining animals are challenged with 100 cfu aerosolized M. tuberculosis Erdman.
[0082]To measure humoral immune responses, a peptide microarray chip has been designed. The chip is spotted with overlapping 50 amino acid peptides generated from the sequences of Rv1886c, Rv3804c, Rv3407c, Rv0867c, Rv1884c, Rv2389c, Rv3133c, and all BCG encoded DosR-regulated proteins which are at least 2 fold induced by the overexpression of DosR (i.e. DosR regulated genes for which transcription is at least 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com