Preparation for Rv2645 protein, and application thereof on aspects of tuberculosis diagnosis and reorganized BCG vaccines
A rv2645, protein technology, applied in the fields of molecular microbiology and infection immunology, can solve problems such as research difficulties, and achieve the effect of high application value, simple method and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The construction of each prokaryotic expression vector pET28a-RDs of embodiment 1RD10-14 district
[0054] 1.1 The design of primers for each RD gene, the specific primers required for construction are shown in Table 1:
[0055] Table 1. Primer sequences for each RD gene
[0056]
[0057]
[0058]
[0059]
[0060] Wherein, the underlined part is a restriction enzyme cutting site.
[0061] 1.2 Amplification and recovery of each RD gene
[0062] Using the genome of M.tb strain H37Rv (ATCC 25618) as a template, use the primers in the above table to PCR amplify each RD gene, and the reaction program varies according to the annealing temperature of each primer. Taking the amplification of the Rv2645 gene as an example, the PCR reaction program is: 95°C for 3min; 94°C for 20s, 58°C for 20s, 72°C for 20s, 30 cycles; 72°C for 3min; PCR products are detected by 1% agarose gel electrophoresis , Gel Purification Kit (Axygen) for recovery (operate according to kit p...
Embodiment 2
[0074] Expression and purification of the protein of interest in the recombinant plasmid of embodiment 2
[0075] 2.1 Expression of RD protein
[0076] Taking Rv2645 as an example, take 1 μL of pET28a-Rv2645 recombinant plasmid, add it to Escherichia coli expressing engineering strain BL21 competent, ice bath for 15 minutes, heat shock at 42°C for 90 seconds, ice bath again for 5 minutes, add 800 μL of LB liquid medium for shaking culture for 40 minutes, Take 100 μL coated kana-resistant LB solid plate (same as above). After overnight culture, pick positive clones and transfer them to 5 mL of LB liquid medium (kana concentration is 5 μg / mL) at 37°C, 200 rpm, and shake for 16 hours; Shake it in 250mL liquid medium, and add inducer IPTG when the absorbance value at OD600 is about 0.6, and the final concentration is 0.8mM. In a temperature-controlled shaker at 25°C, 200rpm, 20h; at the end of the induction culture, take 1mL of collected bacteria and 1mL of bacteria before induc...
Embodiment 3
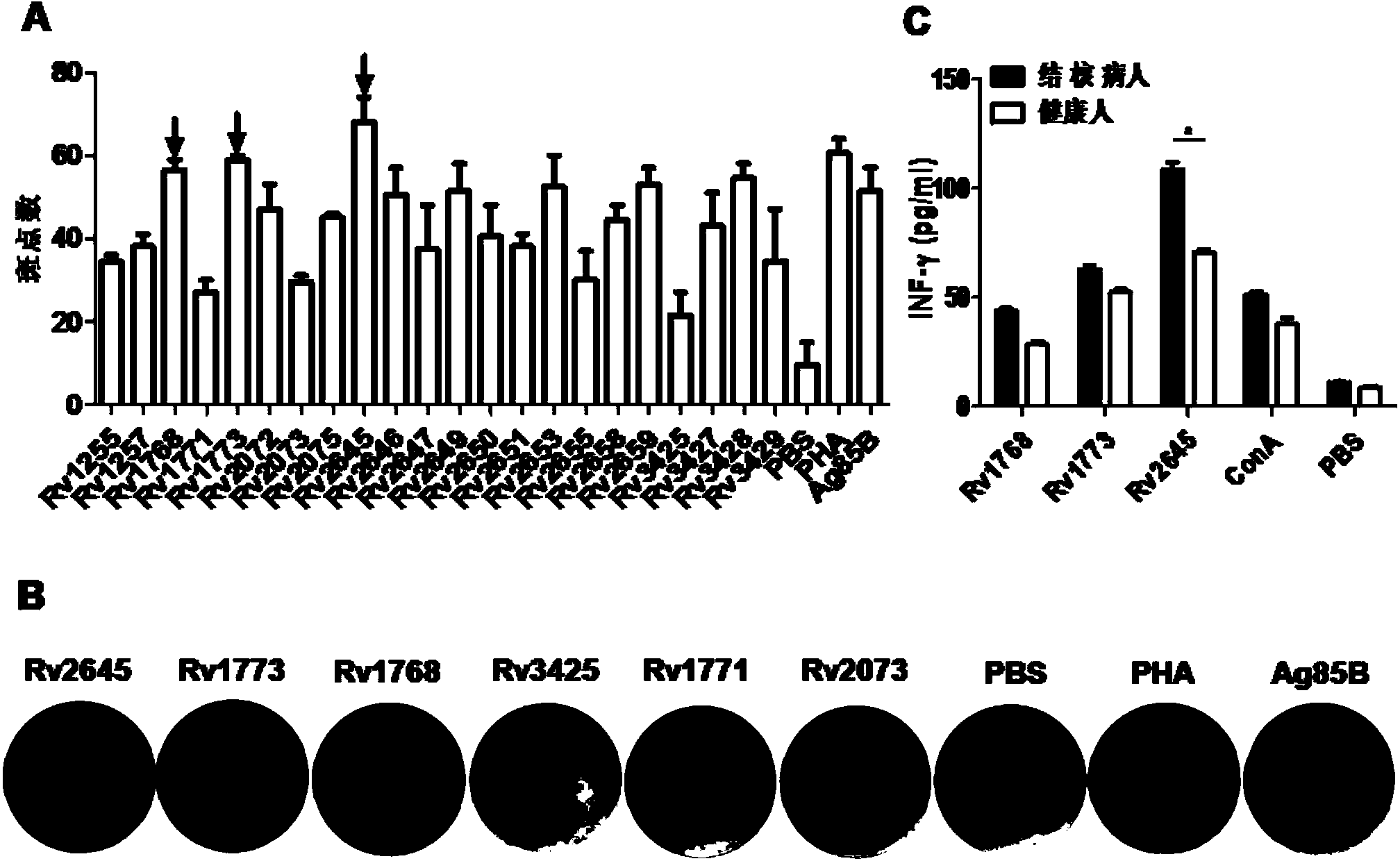
[0079] Example 3 Determination of the ability of each RD protein to stimulate IFN-γ release
[0080] 3.1 ELISPOT detection of IFN-γ release level in preimmunized mice
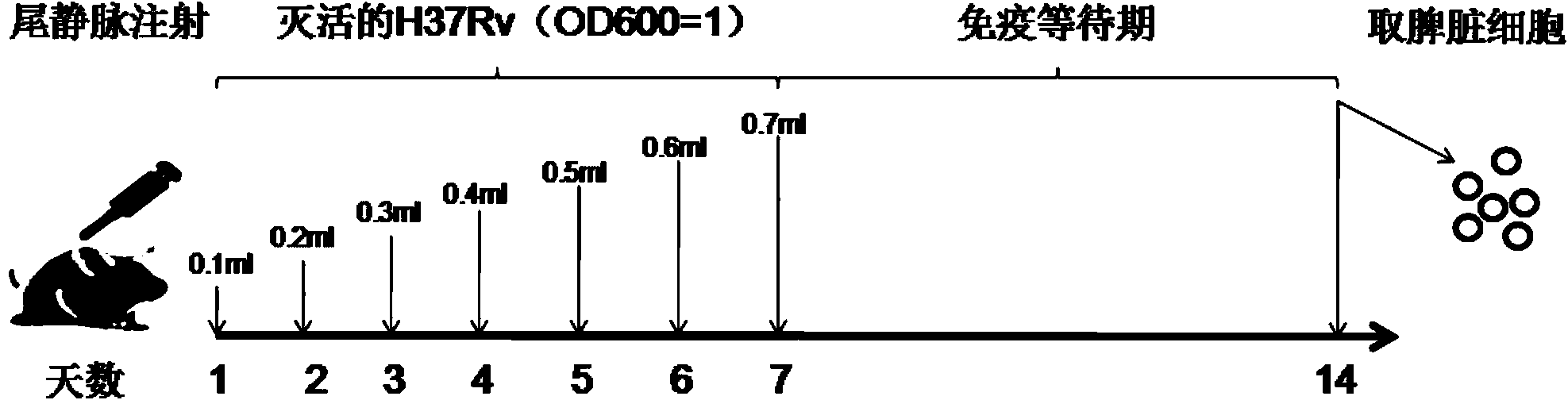
[0081] The expressed and purified RD proteins were quantified by the Bradford method. BALB / c mice (specific mouse immunization scheme as shown in figure 1 (shown) was executed to obtain the spleen, and the mouse splenocytes were obtained after the erythrocytes were broken with AKT (erythrocyte lysate).
[0082] According to the instructions of the Mouse IFN-gamma ELISPOT pre-coating kit (for Daktronics). Spread 5×10 per hole 5 splenocytes, 5 μg of stimulator RD protein was added to each well, and cultured in a cell culture incubator for 48 hours. After the experiment, the 96-well plate was sent to Daktronics to read the number of spots.
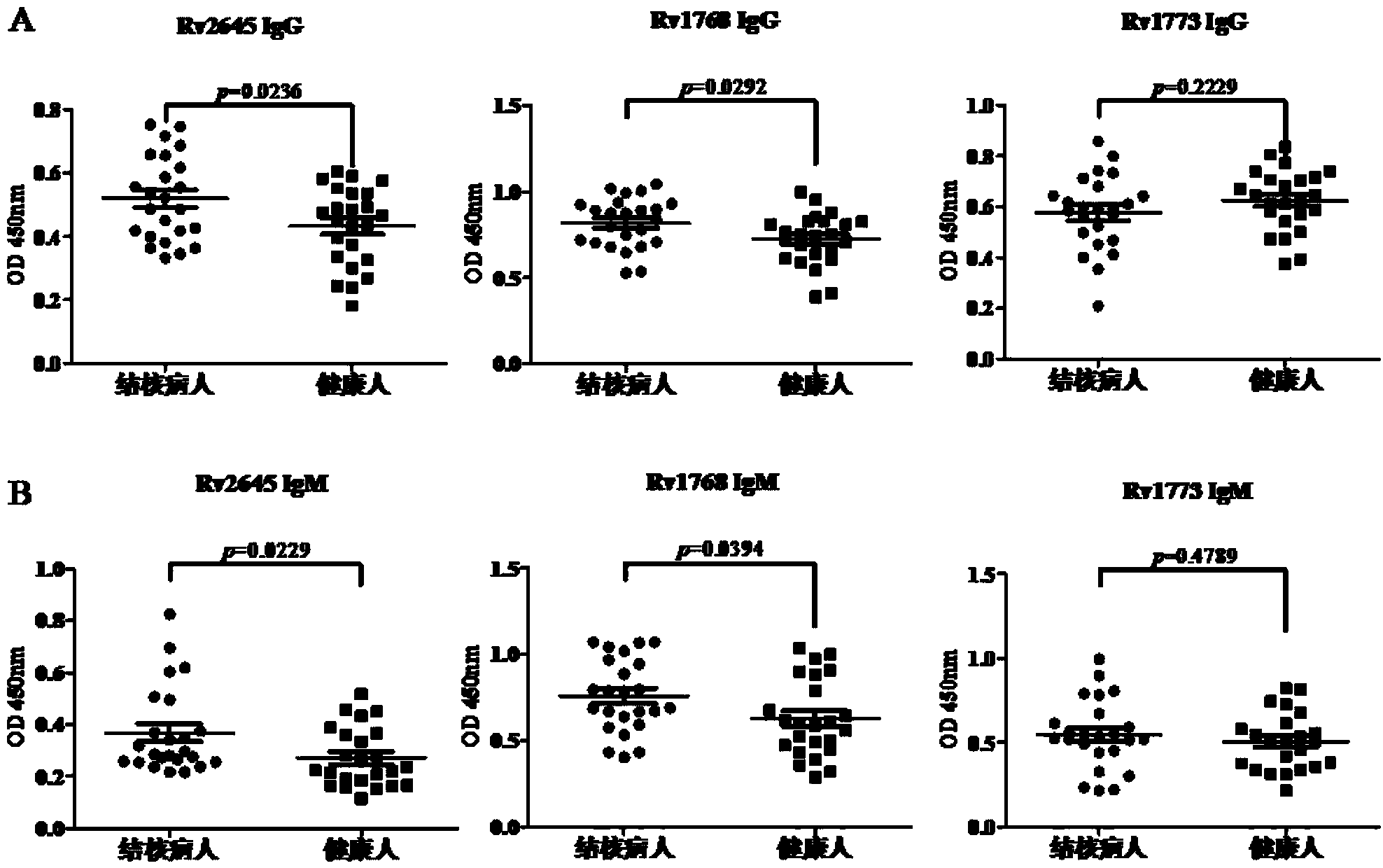
[0083] 3.2 ELISA detection of IFN-γ release level of human PBMC
[0084] Wuhan Medical Assistance Center obtained whole blood from tuberculosis patients and separated PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com