Echinococcus granulosus recombinant BCG vaccine and preparation method thereof
A technology of Echinococcus granulosus and Echinococcus granulosus, which is applied in the field of vaccine preparation, can solve the problems of large damage, recurrence, and adverse reactions, and achieve the effects of enhancing immunity, less immunization times, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1 material
[0027] The Escherichia coli-mycobacterium shuttle plasmid pMV361 and BCG used in the present invention and E.coli DH5α bacterial strain can be obtained through commercial channels, and pMV361 and BCG and E.coli DH5α bacterial strain are purchased from Biovector Science lab Co., Ltd. (China plasmid vector Strain Cell Line Gene Collection Center).
[0028] 2 methods
[0029] 2.1 Design and synthesis of primers
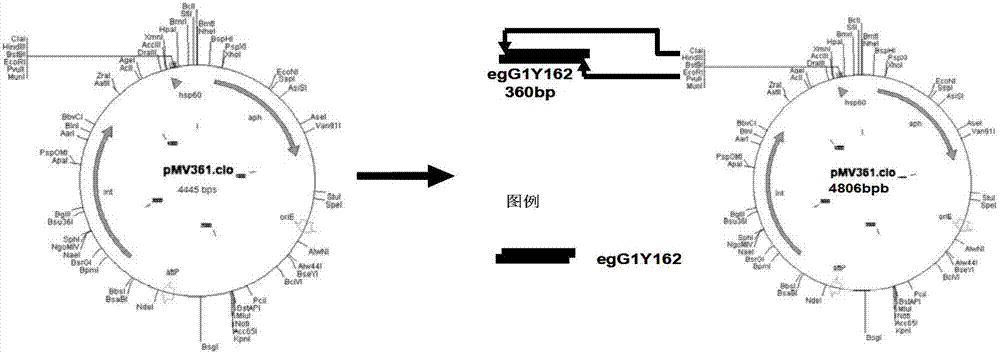
[0030] The upstream and downstream primers were designed according to the gene sequence of Echinococcus granulosus egG1Y162 (GenBank accession numbers are AB458258 and AB458259) and the plasmid map of PMV361, and EcoRI and HindⅢ restriction endonuclease sites were introduced.
[0031] Upstream primer: 5′-CC GAATTCATGGTACTTCGATTCTGT-3′, the underlined part is the EcoRI site;
[0032] Downstream primer: 5′-CC AAGCTT AGTAAGTAATAGGAGCCCA-3′, the underlined part is HindⅢ site.
[0033] 2.2 PCR amplification of target gene egG1Y162
[0034] Using Ec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com