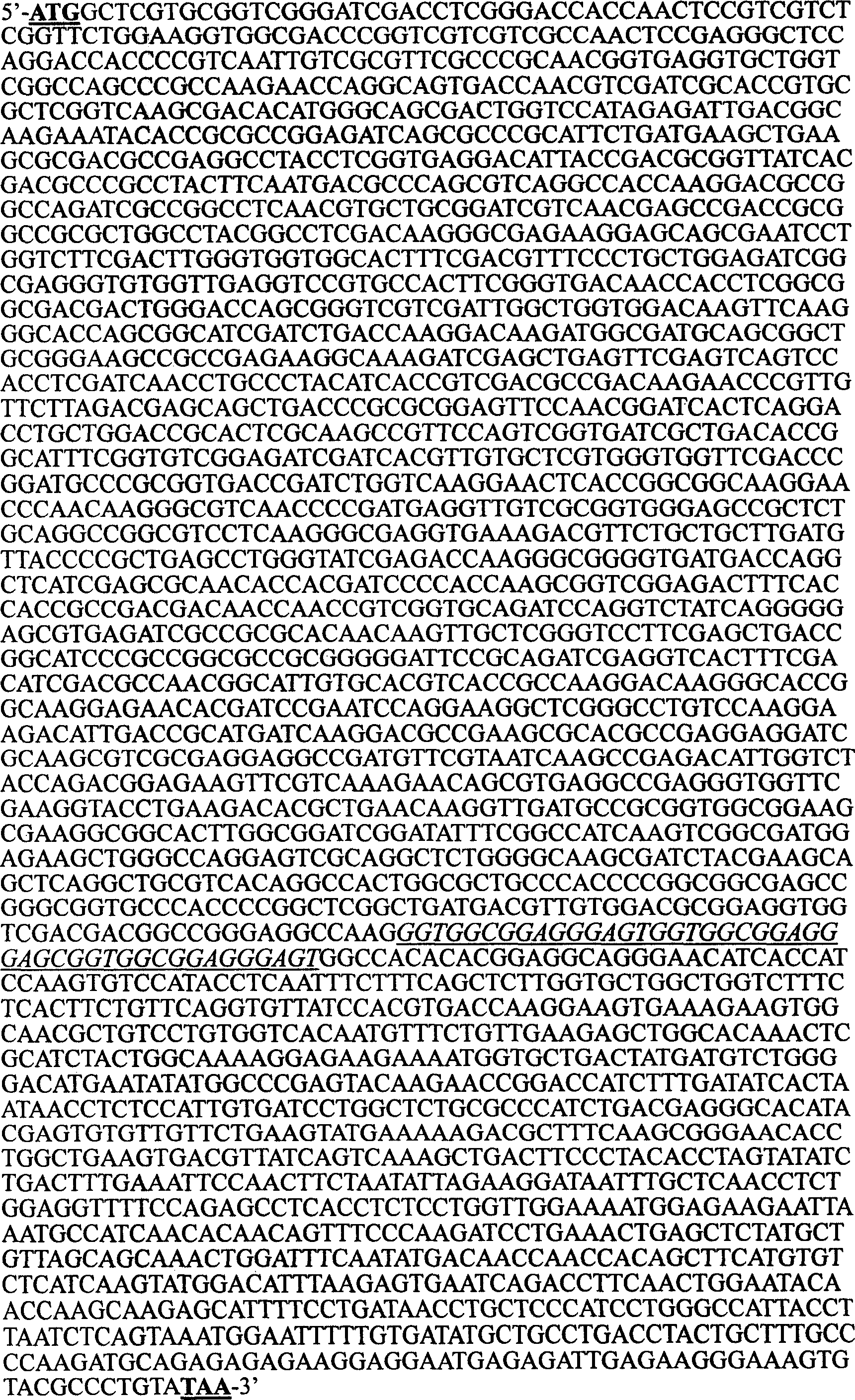Mosaic type DNA vaccine in use for preventing tuberculosis and immunological therapy
A DNA vaccine and chimeric technology, applied in gene therapy, application, drug combination and other directions, can solve the problems of unable to improve the protection level of immune population and poor adult effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
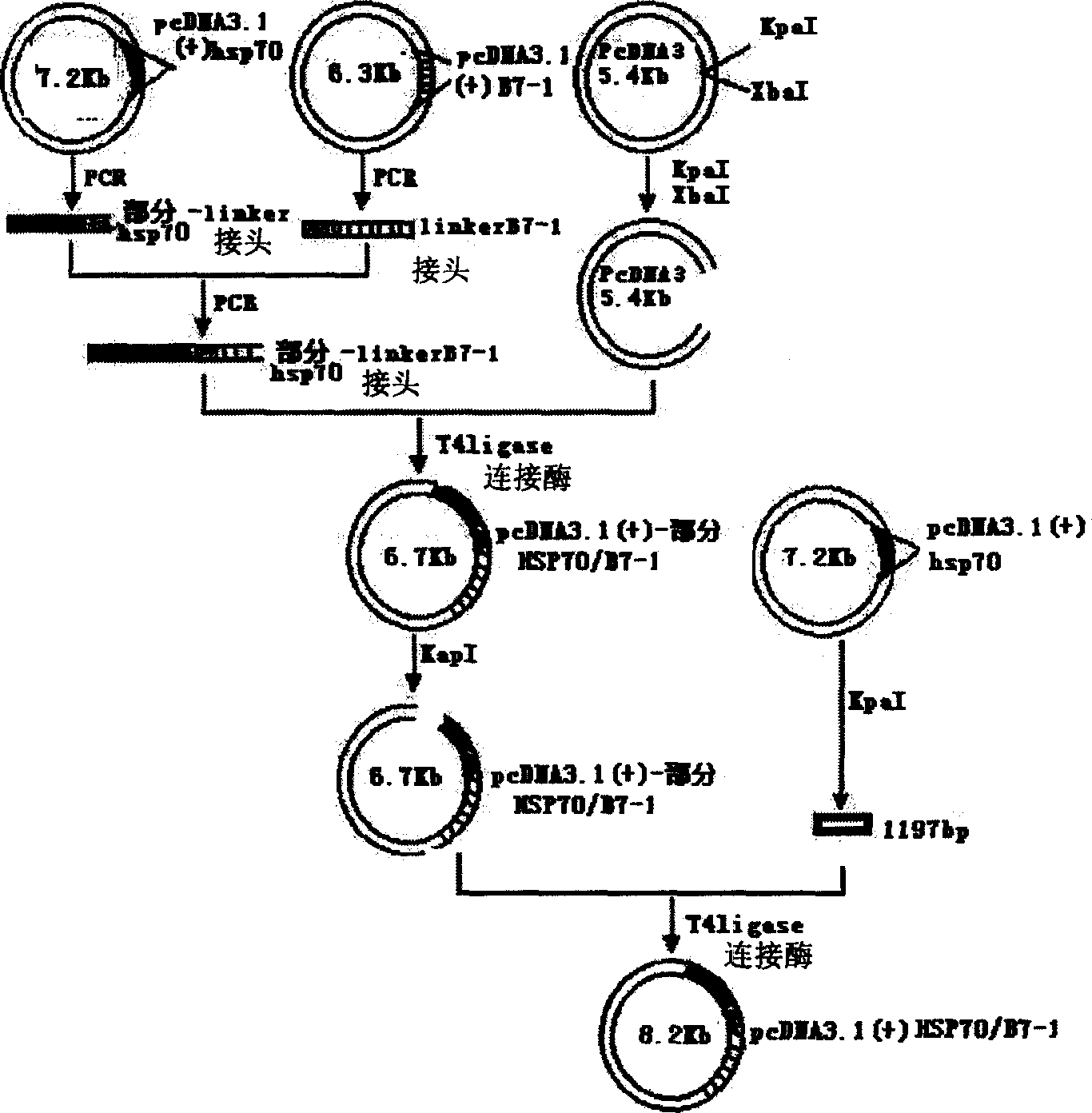
[0038] [Example 1] Construction of pcDNA3.1(+)-hsp70 / B7-1 chimeric plasmid.
[0039] see figure 1 with figure 2 :
[0040] main experimental materials
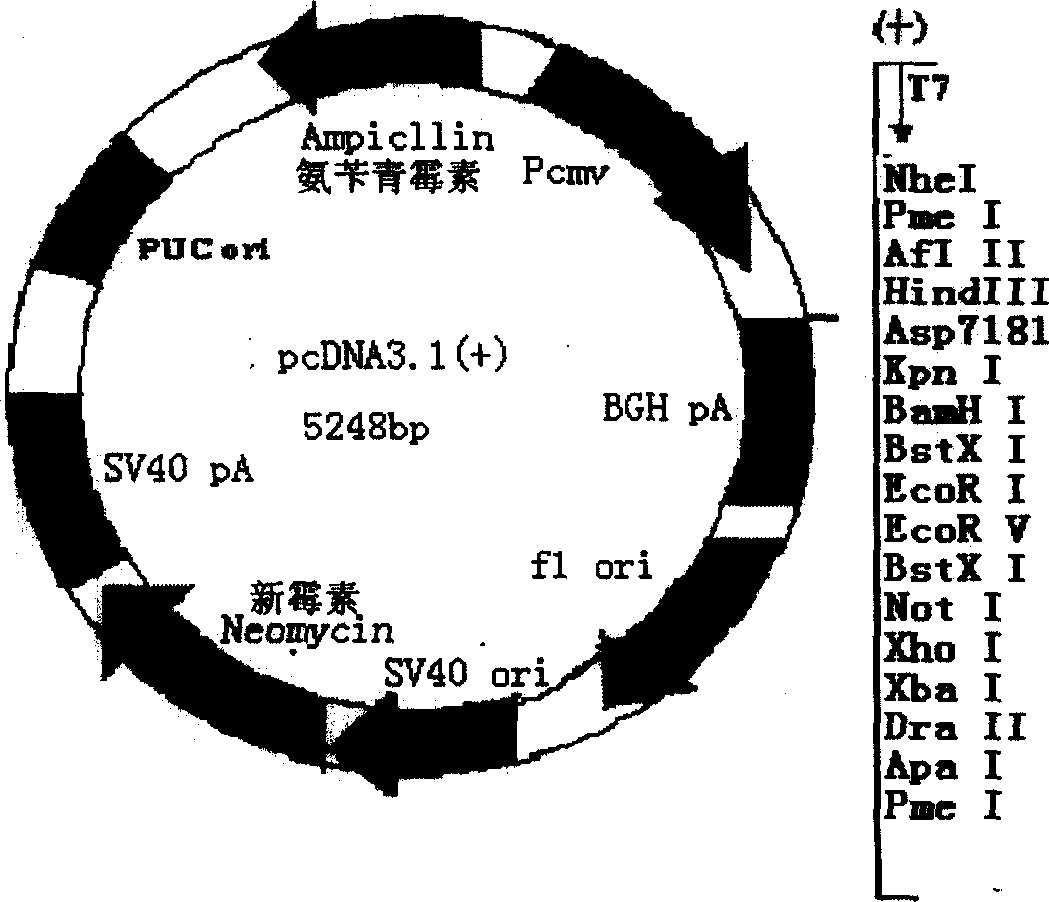
[0041] 1. Plasmids and bacterial strains: pcDNA3.1(+)-B7-1 (including human B7-1 coding gene and regulatory genes on both sides) and plasmid pcDNA3.1(+) were purchased from Invitrogen, and engineering bacteria Dh5α was purchased from Shanghai Sangong bioengineering company.
[0042]2. Design of oligonucleotide primers: According to the published gene sequence of human tuberculosis H37Rv strain hsp70 (Mckenzie.KR, Adams E, Britton WJ, et al.Sequence and immunogenicity of the 70-kDa heatshock protein of Mycobacterium leprae.J .Immune.1991;147.:312-319.) and human B7-1 sequence (GenBank accession number NM005191) design.
[0043] 1. Recombinant pcDNA3.1(+)-hsp70 plasmid primer:
[0044] P1 (SEQ ID NO.4): 5'ggagccATGGCTCGTGCGGTCGGGATC 3' (containing BamHI site and initiation codon ATG)
[0045] P2 (SEQ ID NO. 5): 5' gaattc...
Embodiment 2
[0111] [Example 2] Expression of pcDNA3.1(+)hsp70 / B7-1 chimeric DNA in eukaryotic cells
[0112] The pcDNA3.1(+)hsp70 / B7-1 chimeric DNA was encapsulated and transfected into eukaryotic cells (COS.7) with liposomes, and the mRNA was extracted for RT-PCR at 24.48.72 hours, and the product was subjected to agarose electrophoresis. See the target zone ( Image 6 ).
Embodiment 3
[0113] [Example 3] pcDNA3.1 (+) hsp70 / B7-1 chimeric DNA vaccine immune prevention effect research on mice
[0114] Animal prevention experiment: DNA vaccine inoculation: experimental animals 6-8 weeks old, clean grade healthy mouse C57BL / 6N (purchased from Animal Experiment Center of Chongqing Medical University). Eight rats in each group, half male and half male, were divided into experimental group and control group. The experimental group was injected with 100 μg of pcDNA.3.1(+), pcDNA.3.1(+)-hsp70 and pcDNA.3.1(+)-hsp70 / B7-1 respectively. (0.5ml), BCG was injected according to body weight; the control group was injected with normal saline 0.5ml. The injection site is the tibialis anterior muscle on both sides, and the dose is divided into half on each side. Inoculation 3 weeks and 6 weeks each according to the original plan booster vaccination once. Three weeks after the third immunization, each mouse was injected with H37RV tail vein for 10 4 -10 5 CFU.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com