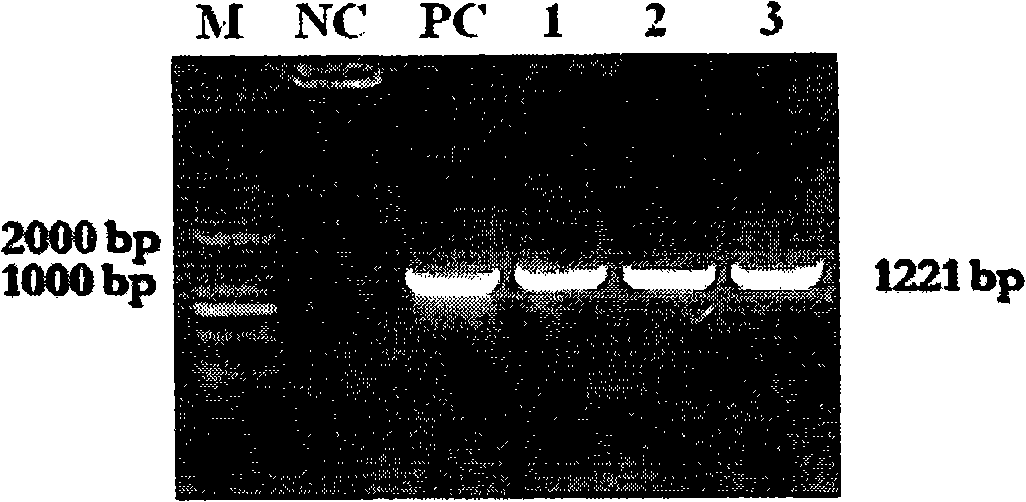
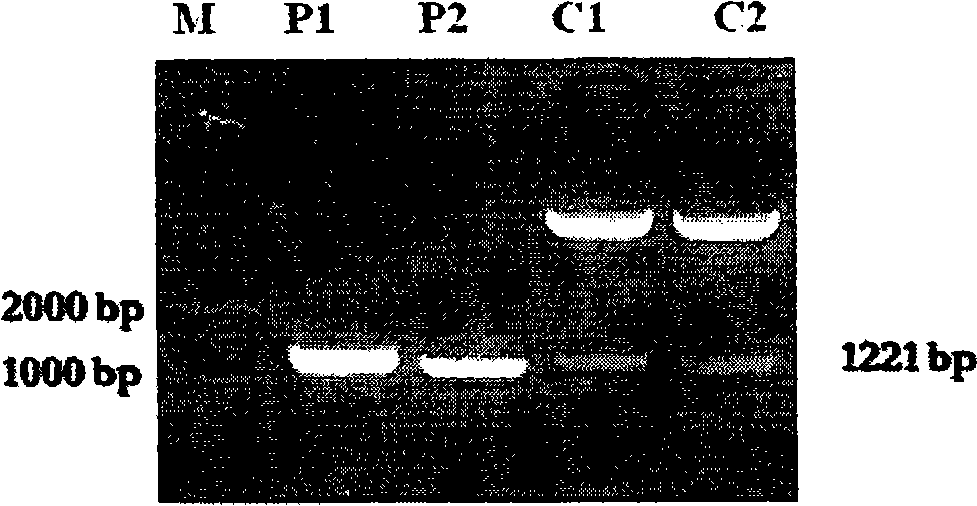
Recombinant mycobacterium smegmatis strain capable of expressing mycobacterium tuberculosis Ag 85B and ESAT-6 fusion protein and application thereof
A technology of Mycobacterium tuberculosis and Mycobacterium smegmatis, applied in bacteria, antibacterial drugs, bacterial antigen components, etc., can solve the problems of cell wall thickness, high nutritional requirements, slow growth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Construction of recombinant plasmid pDE-AEL:
[0020] According to the genome sequence of MTB H37Rv strain, 2 pairs of primers were designed. The primers for the ag85b sequence are:
[0021] P1: 5'-TAGGATCCATGACCGCGGGCGCGTTCTC-3', containing BamHI restriction site;
[0022] P2: 5'-GCGAAGCTTTCATGCGAACATCCCAGTGA-3', containing HindIII restriction site.
[0023] The primers for the esat-6 sequence are:
[0024] P1: 5'-GCATCGATGGTGGCTCAGGTGGCTCCGGTGGAGGCGGAAGCGGCGGTGGA GGA TC AACAGAGCAGCAGTGGAATTT-3', containing ClaI restriction site and 48bp linker sequence;
[0025] P2: 5'-GCGAAGCTTTCATGCGAACATCCCAGTGA-3', containing HindIII restriction site.
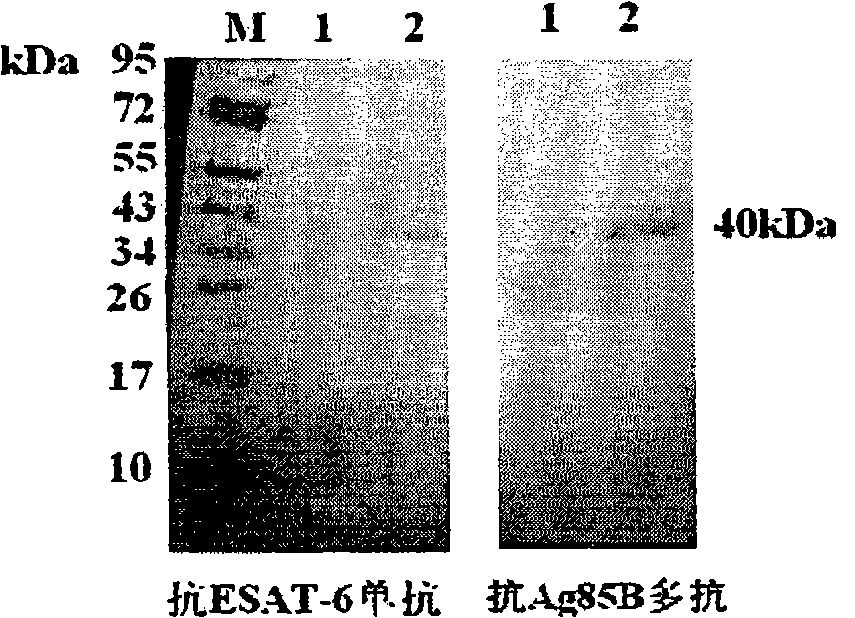
[0026] The PCR product of the target gene was cloned into the pGEM-T vector for sequencing. After the sequence was identified correctly, it was cloned into the pDE22 vector (mycobacterial secreted expression vector) according to their different restriction sites. The sequence determination showed that the fusion protein was suc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com