Preparation and application of recombinant protein CFP-10 nanoparticles
A CFP-10, recombinant protein technology, applied in the directions of non-active ingredients medical preparations, bacterial antigen components, capsule delivery, etc., can solve the problems of missing RD1 region and limited protective efficacy in adults, to improve IgA levels, promote BCG immune effect, the effect of reducing tissue load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation method of recombinant protein CFP-10 nano vaccine
[0027] Preparation of nanoparticles by double emulsion method: according to the CFP10 gene sequence of M. The plasmid was transformed into E.coli BL21(DE3) competent cells for induced expression, and the expressed protein was purified and freeze-dried. Dissolve the purified and lyophilized protein in PBS solution to form an internal aqueous phase, inject the internal aqueous phase into the organic phase, and ultrasonically form colostrum, inject the colostrum into 1% PVA solution, ultrasonically form double emulsion, and Pour milk into 0.5% PVA solution, stir for 3-4 hours, and volatile oil phase. The recombinant protein CFP-10 nanoparticles can be obtained by centrifugal washing and freeze-drying. The specific operation steps are as follows:
[0028] (1) configuration of polylactic acid-glycolic acid copolymer PLGA solution;
[0029] Dissolve polylactic acid-glycolic acid copolymer PL...
Embodiment 2
[0038] Example 2: Physical Characterization and Morphological Observation of Nanoparticles
[0039] The samples prepared with the ratio of internal water phase and oil phase at 1:9 were characterized, and the particle size and potential were measured using a Malvern Zetasizer nanometer particle size potentiometer. The prepared CFP10 nanoparticles have an average particle size of about 247nm and a potential of -28.8mV. After being measured by an ultraviolet spectrophotometer, the encapsulation efficiency of the nanoparticles is calculated to be 80.53%. The results of scanning electron microscopy showed that the size of CFP10 nanoparticles was relatively uniform, and it was spherical with a smooth surface ( figure 1 ).
Embodiment 3
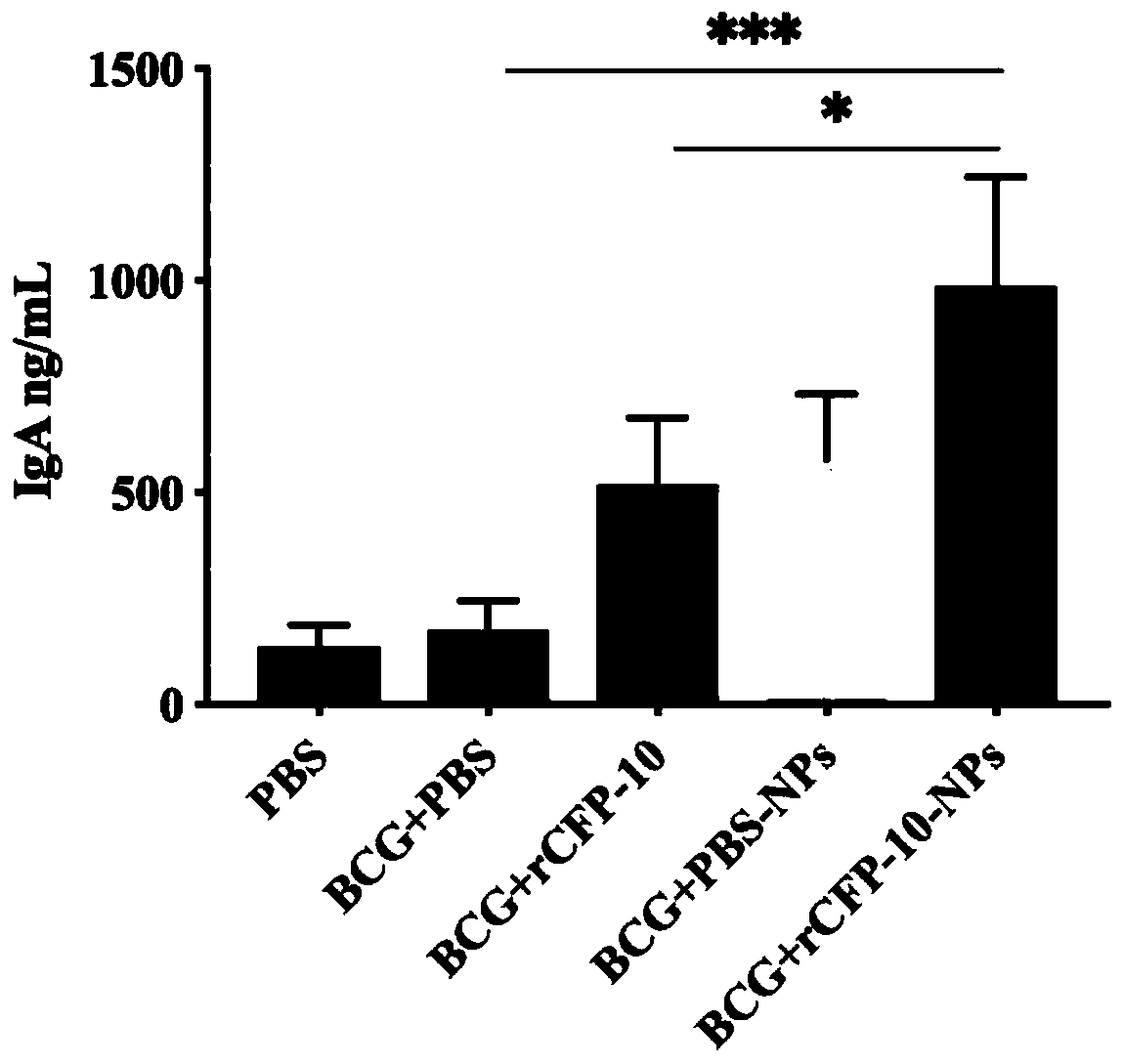
[0040] Example 3: Evaluation of Recombinant CFP-10 Protein Nanoparticles Enhanced BCG Immune Effect
[0041] The experimental animals were randomly divided into 5 groups, 9 mice in each group, divided into PBS (no immunization but challenge), BCG control group (BCG+PBS), BCG+rCFP-10, BCG+PBS-NPs (BCG+blank nano microparticles), BCG+rCFP-10-NPs (BCG+recombinant protein nanoparticles). Use BCG for the first free (10 6 CFU / monkey), and 4 weeks later, the first booster immunization with nanoparticles (rCFP-10 protein dose was 50 μg / bird) was carried out by nasal drops, with an interval of 2 weeks, a total of 3 times. Four weeks after the third immunization, 3 mice were randomly selected from each group to detect relevant immune indicators. The rest were challenged with the NTSE-2 strain (1000 CFU / bird) by nasal drops, and samples were taken for follow-up detection after 4 weeks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com