Vaccine containing codon-optimized mycobacterium tuberculosis fusion protein AH
A Mycobacterium tuberculosis, codon optimization technology, applied in the field of molecular biology, can solve problems such as poor protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]Example 1 Preparation method of recombinant protein AH
[0022]1.1AH construction and induction expression
[0023](1) The original gene sequence of AH fusion expressed, such as SEQ ID NO.1:
[0024]ATGCAGCTTGTTGACAGGGTTCGTGGCGCCGTCACGGGTATGTCGCGTCGACTCGTGGTCGGGGCCGTCGGCGCGGCCCTAGTGTCGGGTCTGGTCGGCGCCGTCGGTGGCACGGCGACCGCGGGGGCATTTTCCCGGCCGGGCTTGCCGGTGGAGTACCTGCAGGTGCCGTCGCCGTCGATGGGCCGTGACATCAAGGTCCAATTCCAAAGTGGTGGTGCCAACTCGCCCGCCCTGTACCTGCTCGACGGCCTGCGCGCGCAGGACGACTTCAGCGGCTGGGACATCAACACCCCGGCGTTCGAGTGGTACGACCAGTCGGGCCTGTCGGTGGTCATGCCGGTGGGTGGCCAGTCAAGCTTCTACTCCGACTGGTACCAGCCCGCCTGCGGCAAGGCCGGTTGCCAGACTTACAAGTGGGAGACCTTCCTGACCAGCGAGCTGCCGGGGTGGCTGCAGGCCAACAGGCACGTCAAGCCCACCGGAAGCGCCGTCGTCGGTCTTTCGATGGCTGCTTCTTCGGCGCTGACGCTGGCGATCTATCACCCCCAGCAGTTCGTCTACGCGGGAGCGATGTCGGGCCTGTTGGACCCCTCCCAGGCGATGGGTCCCACCCTGATCGGCCTGGCGATGGGTGACGCTGGCGGCTACAAGGCCTCCGACATGTGGGGCCCGAAGGAGGACCCGGCGTGGCAGCGCAACGACCCGCTGTTGAACGTCGGGAAGCTGATCGCCAACAACACCCGCGTCTGGGTGTACTGCGGCAACGGCAAGCCGTCGGATCTGGGTGGCAACAACCTG...
Embodiment 2
[0031]Example 2 Evaluation of immunity effects of subunit vaccine AH
[0032]2.1AH immunogenicity and protective evaluation
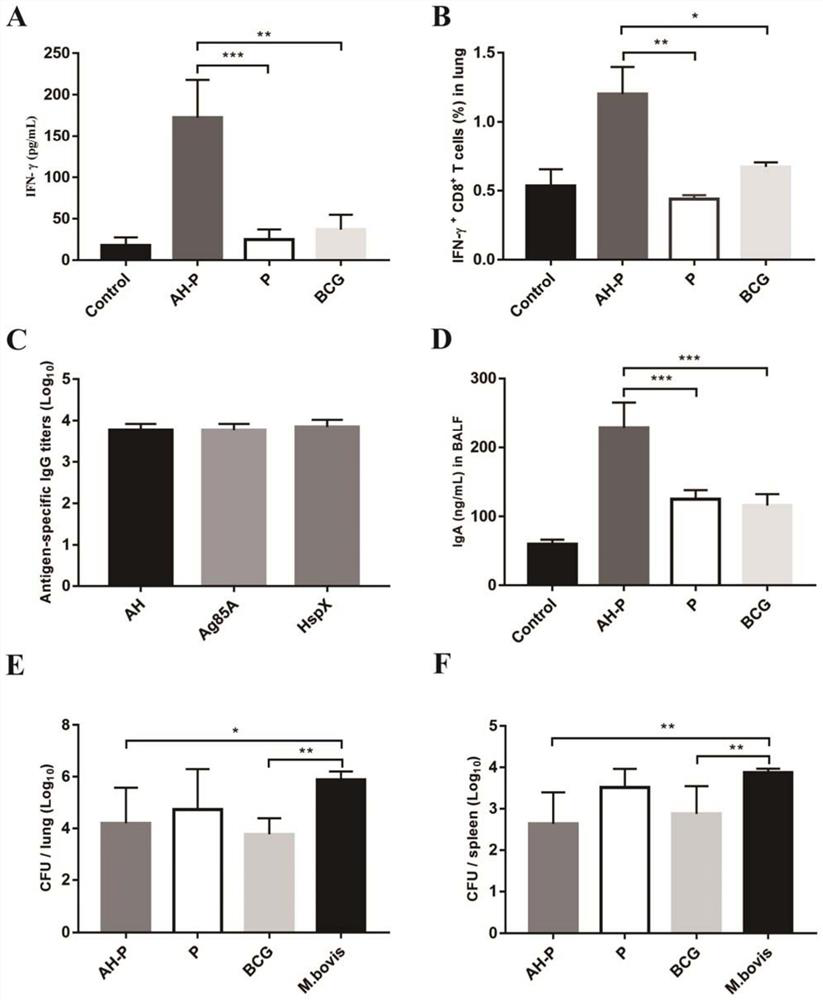
[0033]50 6-8 weeks of female C57BL / 6 mice were randomly divided into 5 groups, 10 in each group, a normal control group (CONTROL), BCG control group (BCG), polymycoplasm and AH immunization group (AH- P), polymeric adjuvant control group (P), attacking model group (M.BOVIS). The intranasal immunotic dose of polymethics and AH is 20 μg / only, a total of 3 weeks per interval. BCG control group mouse skin immunization BCG during the second immunization (105CFU / only), 3 mice were selected for 3 mice for 3 weeks after 3 weeks after the last immunization. Spleen cells were separated, and the spleen cells were stimulated with antigen AH 24 h. After 24 h, the cell supernatant IFN-γ level was determined, and the lung tissue separation lymphocytes were used to detect the number of T cells generated by IFN-γ, and the bronchial alveolar lavewater (BALF) was used for IgA l...
Embodiment 3
[0036]Example 3. Experimental results
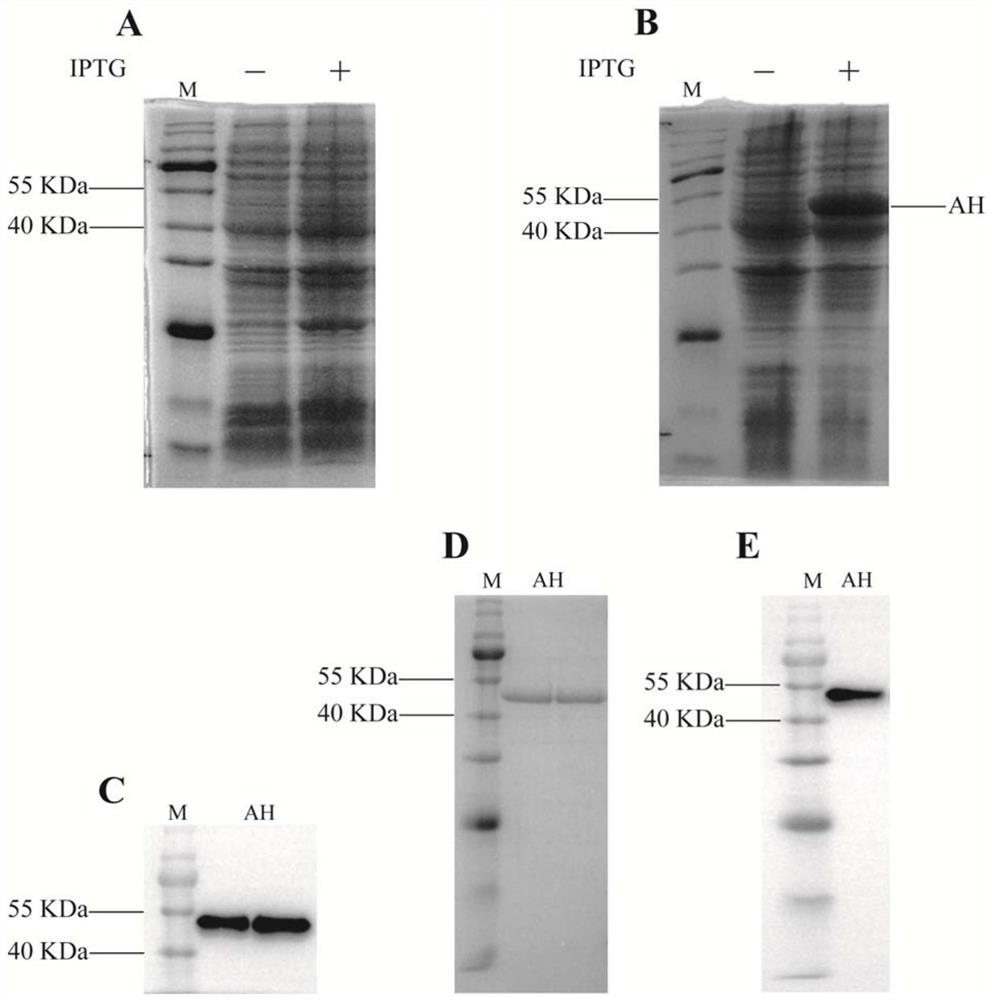
[0037]The AH original sequence constructed with overlapping PCR is connected to the expression vector PET-30A (+), and transformed into BL21 (DE3), recombinant expression of the original sequence induced expression, bacterial protein via SDS-PAGE gel electrophoresis After the Kakas Bright blue staining, the results showed that the protein expected size position (about 54 kDa) protein strip had no significant difference before the induced bacteria protein was induced.figure 1 A). However, after the recombinant expression of the AH sequence containing a codon-optimized AH sequence, the significant strip is visible in the position of the protein expected size (figure 1 B), use anti-HIS tag antibody for WesternBlot identification, a single strip appears on the NC film, in line with the expected size (figure 1 C). SDS-PAGE gel electrophoresis and Caucas were dyed, and the purification of the purified AH purity was greater than 90%.figure 1 D) and use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com