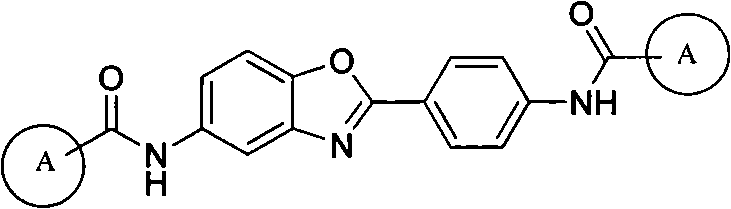
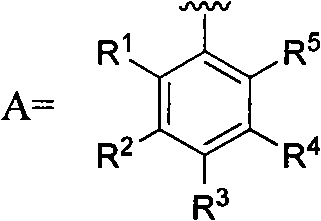
5-substituted-2-(4-substituted phenyl)benzoxazole derivatives and preparation method and application thereof
A technology of benzoxazoles and benzoxazoles, which is applied in the fields of organic compound synthesis and pharmaceutical applications, and can solve problems such as influenza virus mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation method of intermediate 5-nitro-2-(4-nitrophenyl) benzoxazole 5
[0046] Add 6.68g of p-nitrobenzoic acid and 30mL of thionyl chloride to a 250mL round bottom flask, reflux at 80°C for 2 hours, evaporate the solvent under reduced pressure to obtain p-nitrobenzoyl chloride, then add 3.08g of 2-amino- 4-Nitrophenol and 4.04g of triethylamine were dissolved in 50ml of dioxane, and were added dropwise to the above-mentioned acid chloride in an ice bath. After the dropwise addition was completed, they were reacted at 100° C. for 2 hours, and the reaction progress was monitored by TLC. After the reaction was completed, the solvent was evaporated under reduced pressure, an appropriate amount of water was added, suction filtered, and vacuum-dried at 90°C; (4-nitrobenzamide) phenyl-4-nitrobenzoate 4, yellow needle-like crystals, mp: 234-236°C;
[0047] Add 0.28g of intermediate 4, 0.2g of p-toluenesulfonic acid and 30mL of xylene into a 100mL round ...
Embodiment 2
[0050] Embodiment 2: the preparation method of intermediate 5-amino-2-(4-aminobenzene) benzoxazole 6
[0051] Add 0.89g of 5-nitro-2-(4-nitrophenyl)benzoxazole 5 and 30mL of N,N-dimethylformamide to a 250mL round bottom flask, heat to dissolve them all, and then add 10% Palladium carbon 0.09g, the air was removed, stirred for 24 hours under a hydrogen atmosphere, and the reaction progress was monitored by TLC. After the reaction was completed, a large amount of water was added to precipitate light pink needles, which were filtered by suction and recrystallized from methanol to obtain 60.55 g of the mother nucleus 5-amino-2-(4-aminobenzene) benzoxazole; the yield was 81.48%, mp: 230-231°C.
[0052] Product spectral analysis data
[0053] 1 H-NMR (DMSO-d 6 )δ: 7.78 (d, 2H, J=1.8Hz, Ar-H), 7.28 (d, 1H, J=9.0Hz, Ar-H), 6.76 (d, 1H, J=8.4Hz, Ar-H) , 6.66 (d, 2H, J=8.4Hz, Ar-H), 6.53-6.55 (dd, 1H, J 1 = 1.8Hz,J 2 =9.0Hz, Ar-H), 5.88(s, 2H, NH 2 ), 4.98 (s, 2H, NH 2 ); EI-MS...
Embodiment 3
[0054] Example 3: Preparation of 5-substituted-2-(4-substituted phenyl) benzoxazole derivatives 7
[0055] Add 0.0025mol of substituted benzoic acid and 30mL of thionyl chloride to a 50mL round bottom flask, reflux at 80°C for 2 hours, distill off the solvent under reduced pressure to obtain substituted benzoyl chloride, then dissolve in 10ml of dry N,N- in dimethylformamide. Dissolve 0.23g of 5-amino-2-(4-aminobenzene)benzoxazole 6 and 0.25g of triethylamine in 20mL of dry N,N-dimethylformamide, and add dropwise to Among the above acid chlorides, after the dropwise addition, the reaction was carried out at room temperature, and the reaction progress was monitored by TLC. After 2 hours of reaction, the solvent was distilled off under reduced pressure, excess water was added, crystals were precipitated, filtered by suction, and dried in vacuum at 90°C; recrystallization from N,N-dimethylformamide-ethanol gave the target compound 7a, the yield was 61.06 %, mp: >300°C.
[0056...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com