Tsukamurella-tyrosinosolvens and application thereof in catalysis preparation of (S) -alpha - ethyl -2-oxo-1-pyrrolidine acetic acid prepared by catalysis
A technology resistant to T. tyrosinii and T. tyrosinii, applied in biochemical equipment and methods, bacteria, methods based on microorganisms, etc., can solve the problem of large amount of active Raney nickel and high cost of raw materials , Raw materials and reagents are expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the isolation of bacterial strain
[0062] Add 1g of soil sample to 10mL of 0.85% normal saline, shake well to make a uniform suspension; draw 0.5mL of soil suspension and inoculate it into a 250mL Erlenmeyer flask containing 50mL of enrichment medium, shake the flask at 30°C and 200rpm Cultivate for 4 to 5 days. After the culture medium becomes turbid, take 1 mL of the culture medium and transfer it to a fresh enrichment medium, continue to cultivate for 4 to 5 days, and repeat the enrichment culture for 3 to 4 times. The enrichment solution was serially diluted and applied to the separation plate, and a single colony was obtained after multiple separations.
[0063] The enrichment medium uses racemic α-ethyl-2-oxo-1-pyrrolidine ethyl ester as the sole carbon source, and its formula is as follows: racemic α-ethyl-2-oxo-1-pyrrolidine ethyl ester Ester 10mmol / L, (NH 4 ) 2 SO 4 2g / L, K 2 HPO 4 2g / L, KH 2 PO 4 1g / L, NaCl 0.5g / L, MgSO 4 ·7H 2 O 0.5g / ...
Embodiment 2
[0065] Embodiment 2: the acquisition of wet fungus cells
[0066] Seed medium formula: glucose 15g / L, yeast powder 12.1g / L, NH 4 Cl 9.5g / L, KH 2 PO 4 1g / L, K 2 HPO 4 2g / L, MgSO 40.6g / L, NaCl 0.6g / L, pH 7.0;
[0067] Fermentation medium formula: glucose 15g / L, yeast powder 12.1g / L, NH 4 Cl 9.5g / L, KH 2 PO 4 1g / L, K 2 HPO 4 2g / L, MgSO 4 0.6g / L, NaCl 0.6g / L, pH 7.0;
[0068] Pick a ring of bacteria from the mature slant and insert it into a 250mL shake flask with 50mL seed medium, cultivate it at 30°C and 200rpm for 24 hours to obtain a seed solution, and then transfer the seed solution to In a 250mL shake flask containing 100mL fermentation medium, culture at 30°C and 200rpm for 36 hours. After the cultivation, the fermentation broth was centrifuged and washed once with a phosphate buffer solution of pH 8.0, and the wet bacterial cells were collected for future use.
Embodiment 3
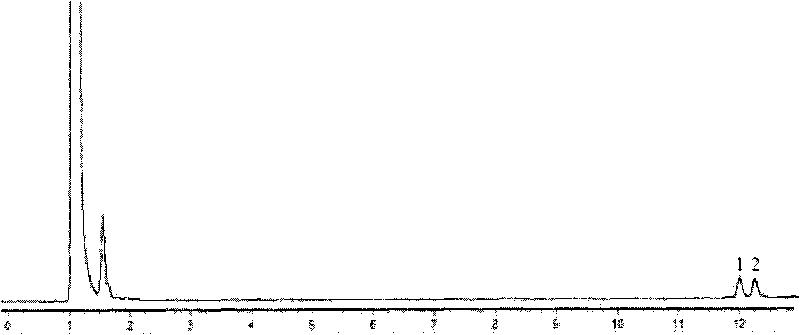
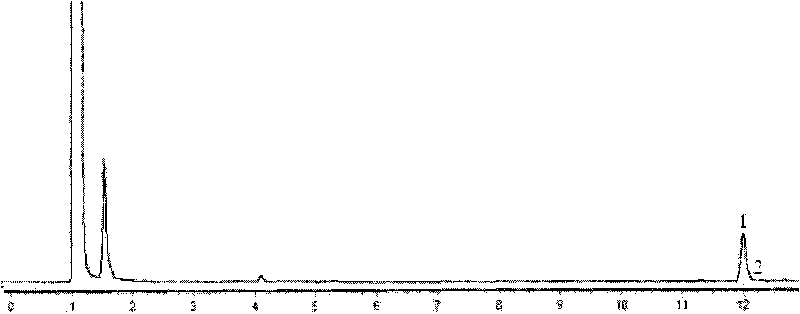
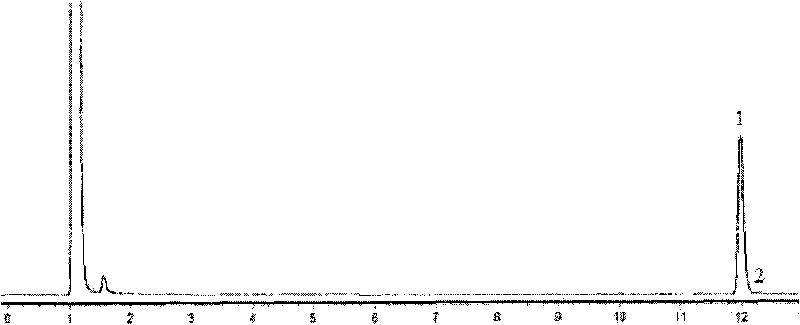
[0070] The wet thallus obtained in Example 2 was suspended in phosphate buffer (0.1mol / L, pH 6); 10.4g / L of racemate α-ethyl-2-oxo-1-pyrrolidine was added The ester was used as a substrate, and the cell concentration (by dry weight) was 20 g / L, and placed in a shaker at 30° C. at 200 rpm for 24 hours. After the reaction, the reaction solution was centrifuged, and the supernatant was taken, and the yield and optical purity of the product (S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid were measured by the aforementioned detection method, and the yield was 37.7%. Optical purity 64% e.e.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com