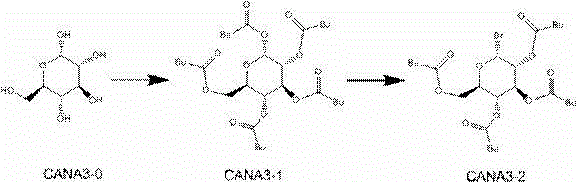
New synthesis process of canagliflozin
A new process and process technology, applied in the field of new synthetic process of canagliflozin, can solve the problems of harsh process conditions, high cost, low total yield, etc., and achieve cheap and easy raw materials, control production costs, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
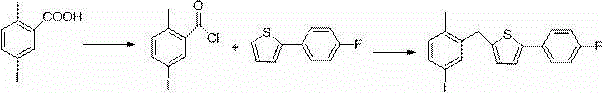
[0030] The new synthesis process of canagliflozin comprises the following steps:
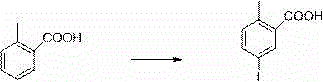
[0031] a. Using 2-methylbenzoic acid as the starting material, use self-made catalyst molecular sieve to support iron oxide, react with iodic acid and iodine with a molar multiple of 0.5 to generate intermediate-5-iodo-2-methylbenzoic acid, and post-treatment The process adopts cold filtration after cooling down, and the method of applying the mother liquor is as follows: the reaction temperature is lowered to 20 degrees Celsius, and a large amount of solids are precipitated. At this temperature, the product mixed with the catalyst is filtered, and the mother liquor is applied mechanically next time. After the filter cake is dissolved with ethyl acrylate, filter at room temperature, filter out the catalyst and apply mechanically for 5 times next time. After the filtrate is precipitated, the crude product is recrystallized with 2.5 times the weight of ethyl acrylate to obtain a white product....
Embodiment 2
[0038] The new synthesis process of canagliflozin comprises the following steps:
[0039] a. Using 2-methylbenzoic acid as the starting material, use self-made catalyst molecular sieve to support iron oxide, react with iodic acid and iodine with a molar multiple of 0.7 to generate intermediate-5-iodo-2-methylbenzoic acid, and post-treatment The process adopts cold filtration after cooling down, and the method of applying the mother liquor is as follows: the reaction temperature is lowered to 18 degrees Celsius, and a large amount of solids are precipitated. At this temperature, the product mixed with the catalyst is filtered, and the mother liquor is applied mechanically next time. After the filter cake is dissolved with ethyl acrylate, filter at room temperature, filter out the catalyst and use it mechanically next time, which can be applied 3 times. After the filtrate is precipitated, the crude product is recrystallized with 2.5 times the weight of ethyl acrylate to obta...
Embodiment 3
[0046] The new synthesis process of canagliflozin comprises the following steps:
[0047] a. With 2-methylbenzoic acid as the starting material, use self-made catalyst molecular sieve to support iron oxide, react with iodic acid and iodine with a molar ratio of 1.0 to generate intermediate-5-iodo-2-methylbenzoic acid, and post-treatment The process adopts cold filtration after cooling down, and the method of applying the mother liquor is as follows: the reaction temperature is lowered to 22 degrees Celsius, and a large amount of solids are precipitated. At this temperature, the product mixed with the catalyst is filtered. After the filter cake is dissolved with ethyl acrylate, filter at room temperature, filter out the catalyst and apply mechanically for the next time, which can be applied mechanically 4 times. After the filtrate is precipitated, the crude product is recrystallized with 2.5 times the weight of ethyl acrylate to obtain a white product. The product yield is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com