Preparation method of adefovir
A technology of ethyl and adenine, which is applied in the field of preparation of adefovir, can solve problems such as long time and unsuitable for industrial production, and achieve the effects of reducing the hazards of workers, reducing reaction time, and shortening working hours
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
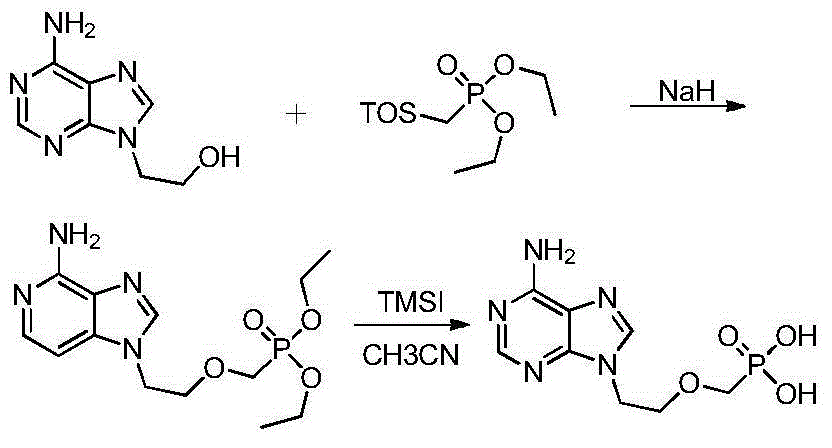
[0033] Example 1: A preparation method of adefovir
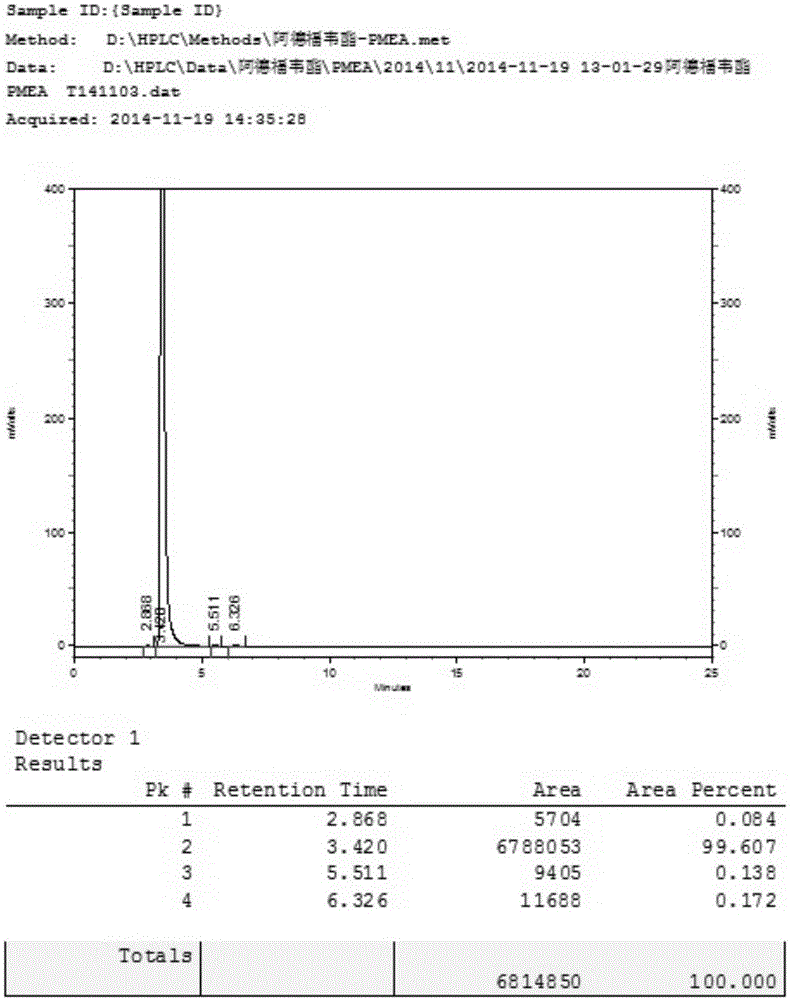
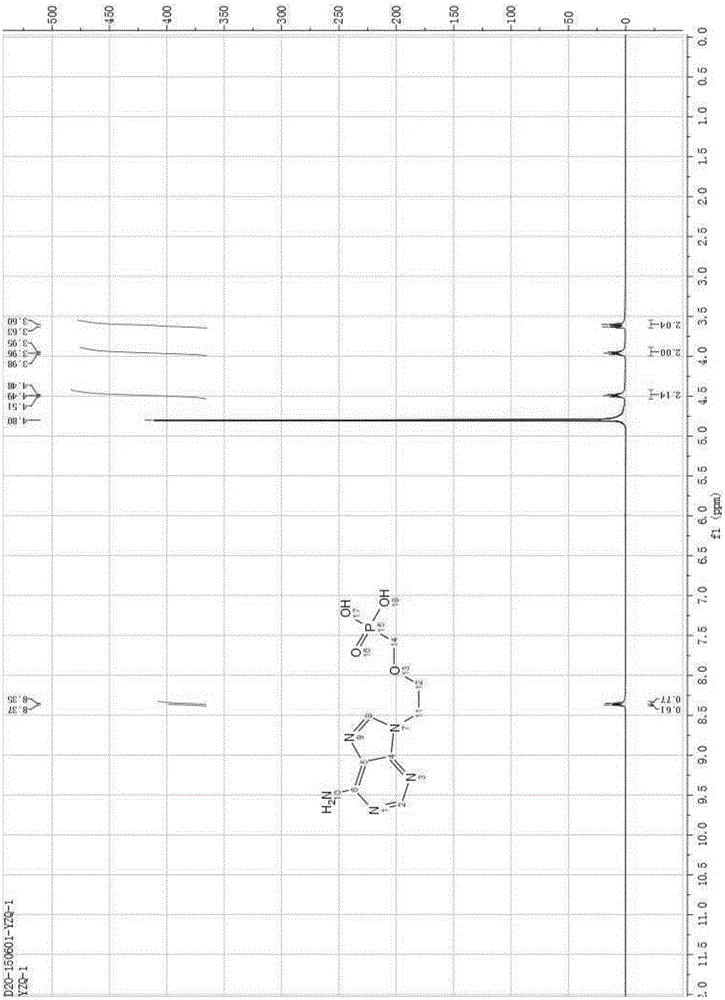
[0034] 20 g of 9-[2-(diethoxyphosphoryl methoxy) ethyl] adenine was added to 100 g of HBr aqueous solution with a concentration of 48%, stirred and heated to 90° C., and reacted for 5 hours. TLC detects that the reaction is complete and stops the reaction. The solution was cooled to room temperature and added water, keeping the temperature below 45°C, and neutralized with 50% sodium hydroxide to pH ≈ 2.5. A large amount of white solid precipitated, and stirring was continued for 4 hours, filtered and dried to obtain white PMEA. Purity determined by HPLC: 99.0% yield: 83%. The nuclear magnetic data of the product are as follows: 1HNMR(D 2 O)δ: 8.37(s,1H,2-H),8.35(s,1H,8-H), 4.49(t,2H,J=6.0Hz,1'-H), 3.86(t,2H,J =6.0Hz,2'-H),3.51(d,2H,J=9.0Hz,OCH 2 P).
Embodiment 2
[0035] Example 2: A preparation method of adefovir
[0036] 20 g of 9-[2-(diethoxyphosphoryl methoxy) ethyl] adenine was added to 100 g of HBr aqueous solution with a concentration of 48%, stirred and heated to 80° C., and reacted for 8 hours. TLC detects that the reaction is complete and stops the reaction. The solution was cooled to room temperature and added water, keeping the temperature below 45°C, and neutralized with 50% sodium hydroxide to pH ≈ 2.5. A large amount of white solid precipitated, and stirring was continued for 4 hours, filtered and dried to obtain white PMEA. Purity determined by HPLC: 98.7% yield: 81%.
Embodiment 3
[0037] Example 3: A preparation method of adefovir
[0038] 20 g of 9-[2-(diethoxyphosphoryl methoxy) ethyl] adenine was added to 80 g of HBr aqueous solution with a concentration of 48%, stirred and heated to 90° C., and reacted for 5 hours. TLC detects that the reaction is complete and stops the reaction. The solution was cooled to room temperature and added water, keeping the temperature below 45°C, and neutralized with 50% sodium hydroxide to pH ≈ 2.5. A large amount of white solid precipitated, and stirring was continued for 4 hours, filtered and dried to obtain white PMEA. Purity determined by HPLC: 99.4% yield: 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com