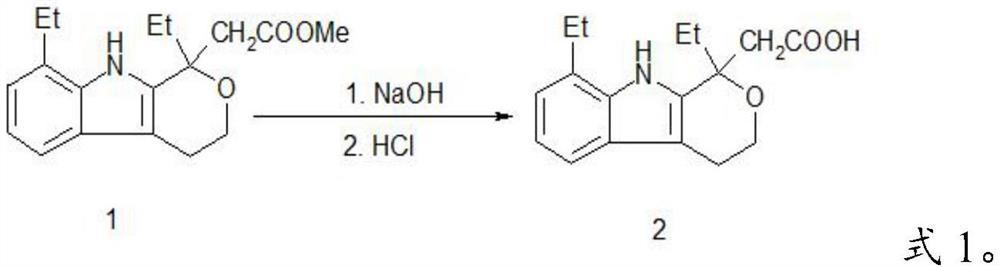
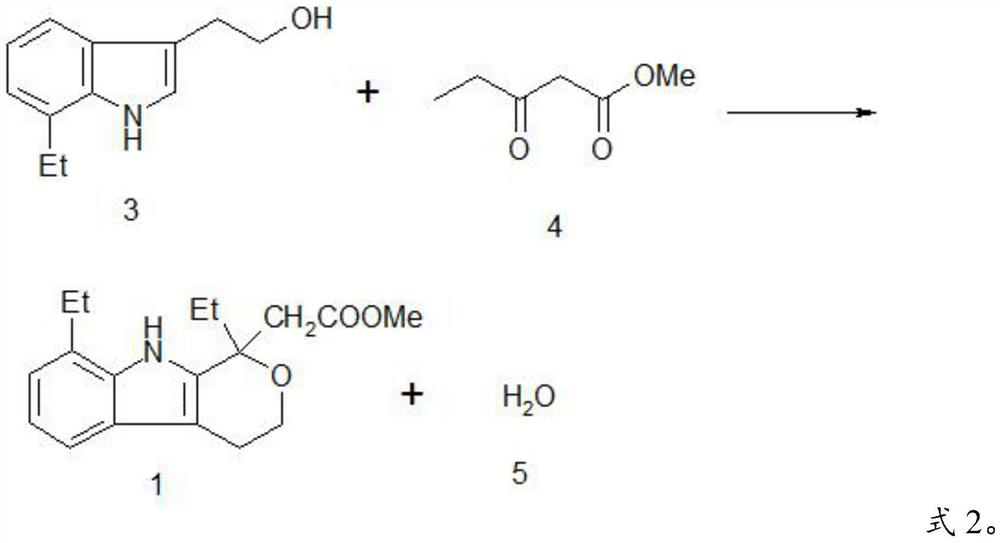
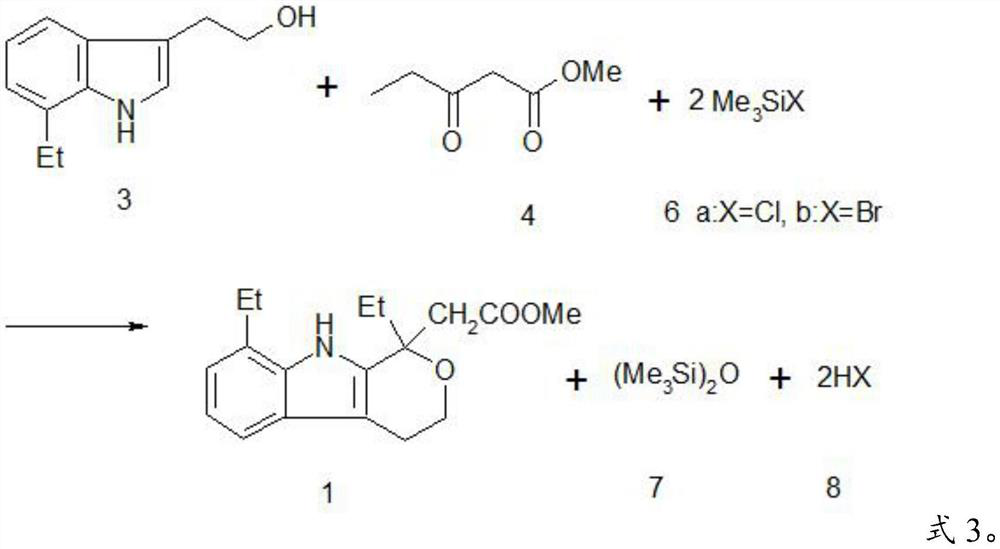
A kind of method preparing etodolac methyl ester
A technology of methyl etodolac and methyl oxopentanoate, which is applied in the field of preparing methyl etodolac, can solve the problems of affecting product appearance, harming concentrated sulfuric acid, and low yield, so as to achieve easy recycling, improve Product yield and effect of reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Trimethylchlorosilane as catalyst and dehydrating agent
[0052] At 18°C, in a four-necked flask equipped with a thermometer, a mechanically stirred drying tube and a constant pressure dropping funnel, 283.5 grams of methanol, 96.4 grams of 7-ethyltryptol (98.0% content, 0.5 mol of pure), 3- 71.5 grams (0.55 mol) of methyl oxopentanoate, add 59.7 grams (0.55 mol) of trimethylchlorosilane into the constant pressure dropping funnel, and slowly add it dropwise to the above reaction system, and control the temperature not to exceed 20°C , about 4 hours for the dropwise addition time, and reacted overnight (about 18 hours) at 22°C until the HPLC content of 7-ethyltryptol was less than 0.1%, cooled to 12°C, and incubated for 1 hour, filtered, 25 grams of cold methanol ( 10°C) for rinsing, and the mother liquor is to be treated.
[0053] The filtrate was washed with 150 g of 5% sodium bicarbonate solution and 50 g of water respectively, and dried to obtain a white solid produ...
Embodiment 2
[0059] Trimethylchlorosilane is a catalyst and dehydrating agent, and the mother liquor is applied mechanically
[0060] (1) The first batch of materials
[0061] At 18°C, in a four-necked flask equipped with a thermometer, a mechanically stirred drying tube and a constant pressure dropping funnel, 283.5 grams of methanol, 96.4 grams of 7-ethyltryptol (98.0% content, 0.5 mol of pure), 3- 71.5 grams (0.55 mol) of methyl oxopentanoate, add 59.7 grams (0.55 mol) of trimethylchlorosilane into the constant pressure dropping funnel, and slowly add it dropwise to the above reaction system, and control the temperature not to exceed 20°C , about 4 hours for the dropwise addition time, and reacted overnight (about 18 hours) at 22°C until the HPLC content of 7-ethyltryptol was less than 0.1%, cooled to 12°C, and incubated for 1 hour, filtered, 25 grams of cold methanol ( 10°C) for rinsing, and the mother liquor is to be treated.
[0062] The filtrate was washed with 150 g of 5% sodium ...
Embodiment 3
[0070] Bromotrimethylsilane as catalyst and dehydrating agent
[0071] At 18°C, in a four-necked flask equipped with a thermometer, a mechanically stirred drying tube and a constant pressure dropping funnel, 283.5 grams of methanol, 96.4 grams of 7-ethyltryptol (98.0% content, 0.5 mol of pure), 3- 71.5 grams (0.55 mol) of methyl oxopentanoate, add 82.5 grams (0.55 mol) of bromotrimethylsilane into the constant pressure dropping funnel, and slowly add it dropwise to the above reaction system, and control the temperature not to exceed 20°C , dropwise for about 4 hours, dropwise react overnight at 22°C (about 18 hours) until the HPLC content of 7-ethyltryptol is less than 0.1%, cool to 12°C, and keep warm for 1 hour, filter, and rinse with 25 grams of cold methanol Wash, the mother liquor is to be processed.
[0072] The filtrate was rinsed with 150 g of 5% sodium bicarbonate solution and dried to obtain 138.6 g of a white solid product, HPLC purity: 99.59%, yield: 92.1%, meltin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com