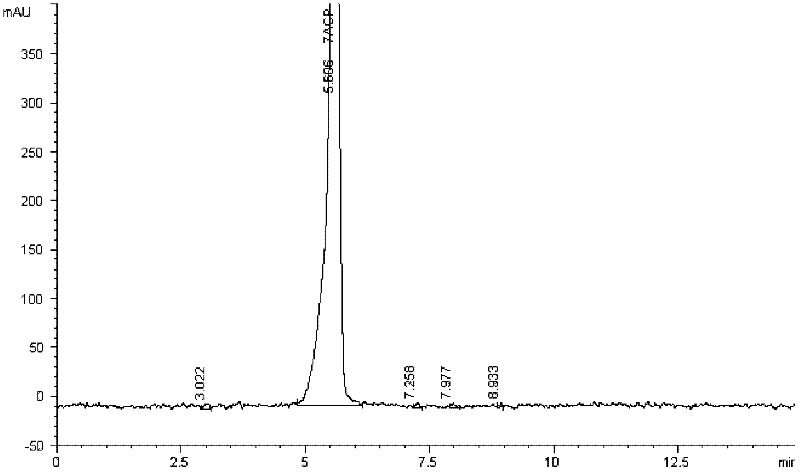
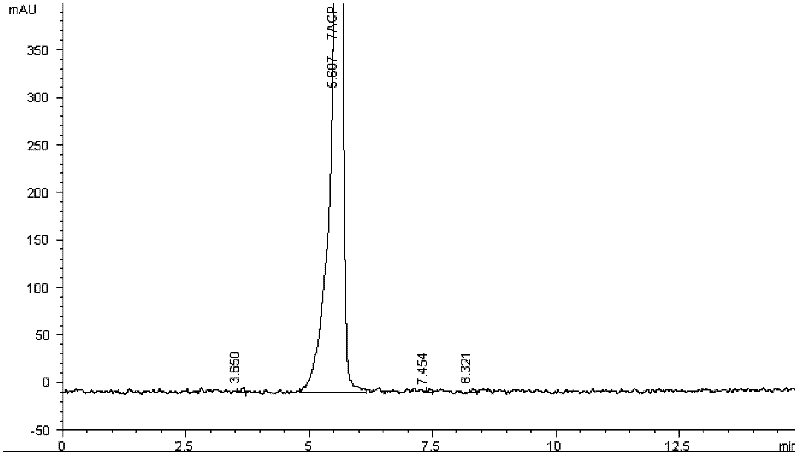
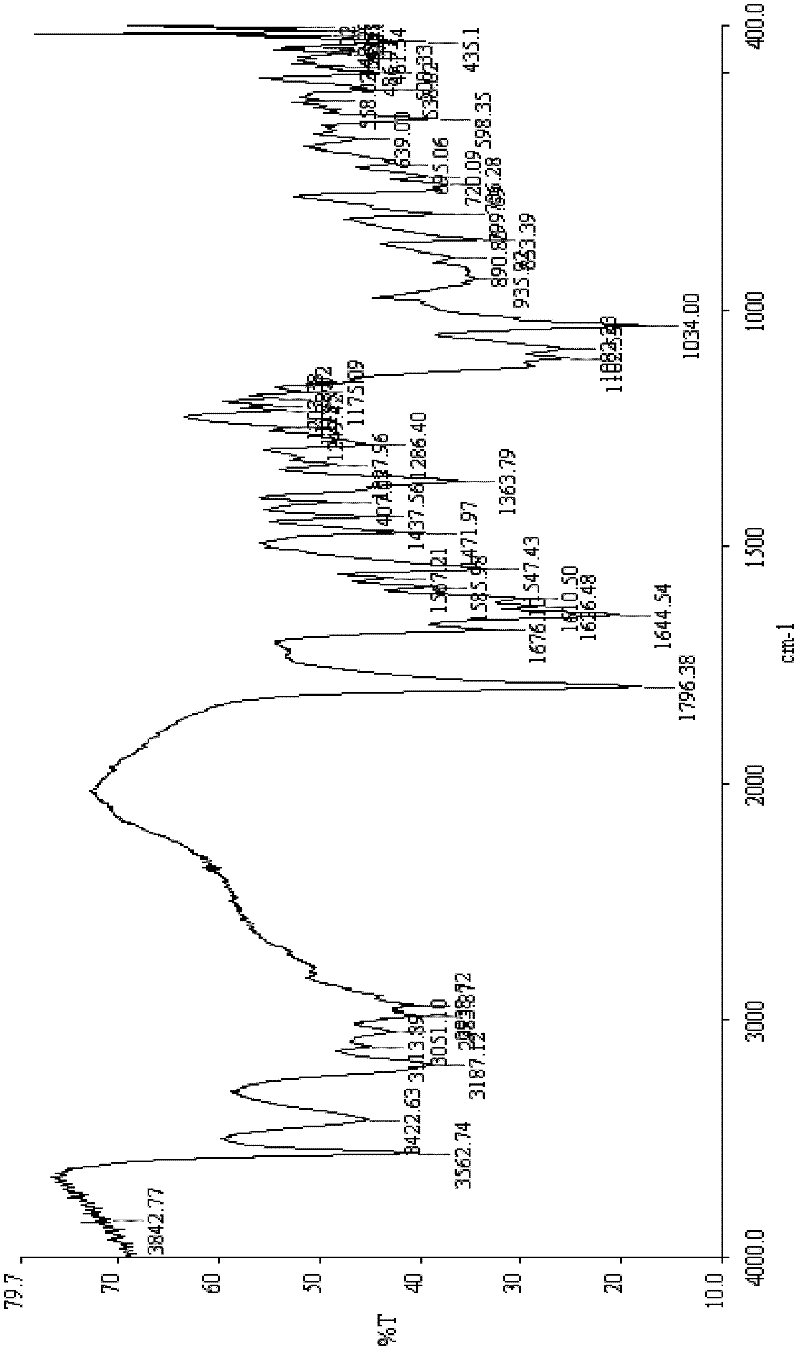
Patents
Literature
34 results about "CEFPIROME SULFATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
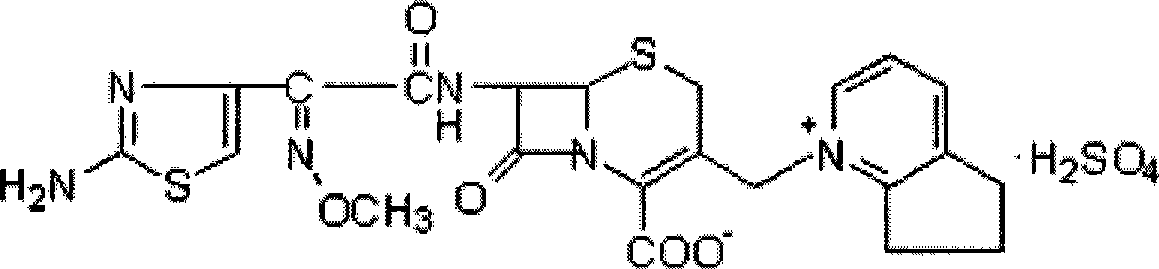
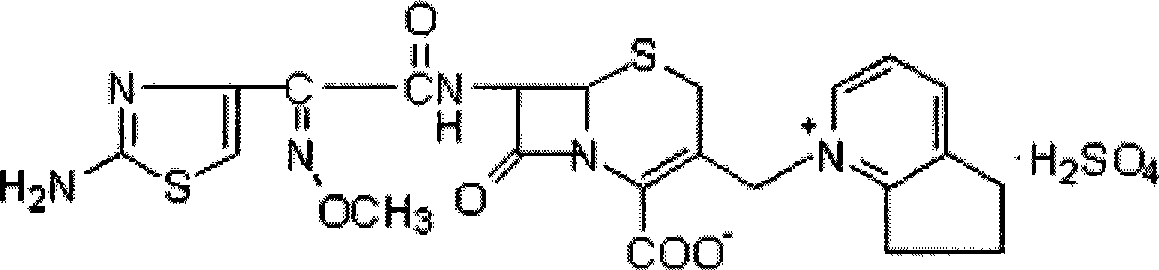
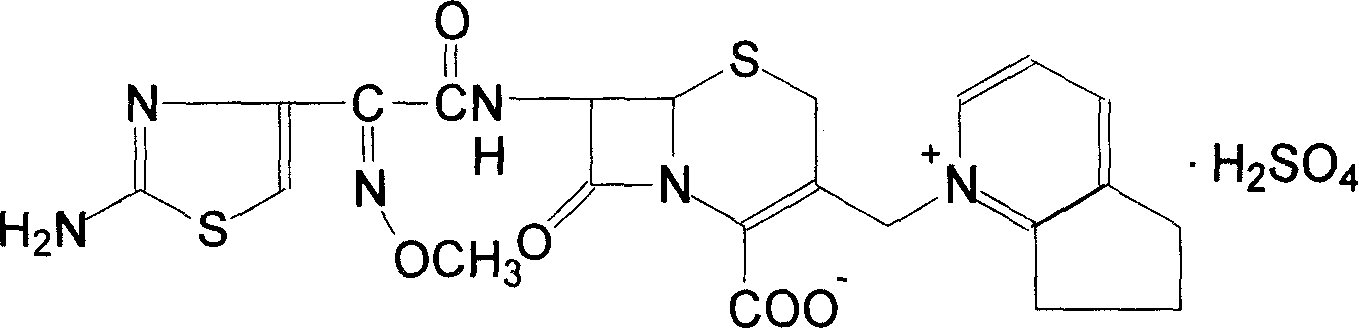
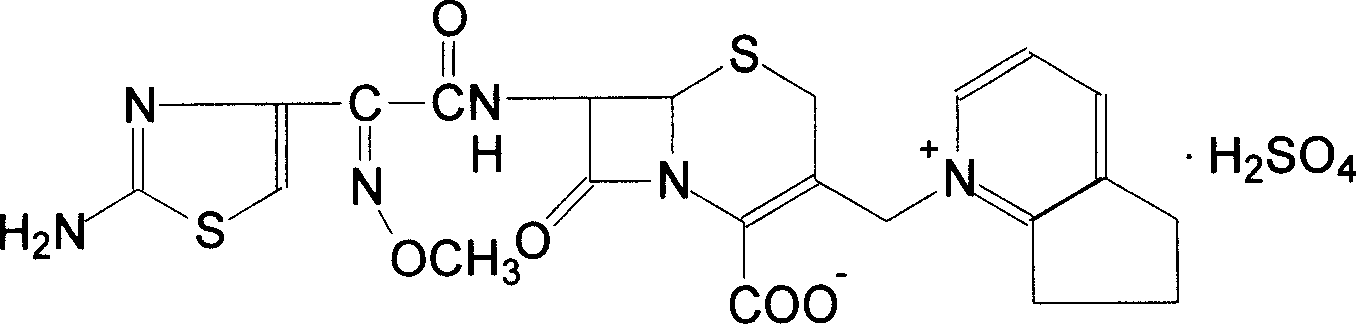
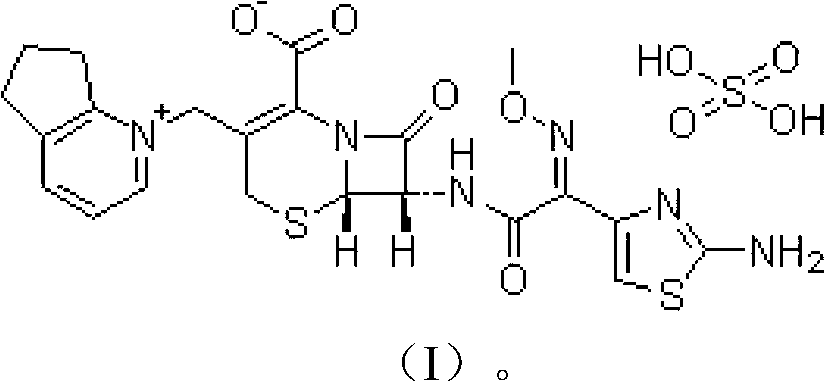
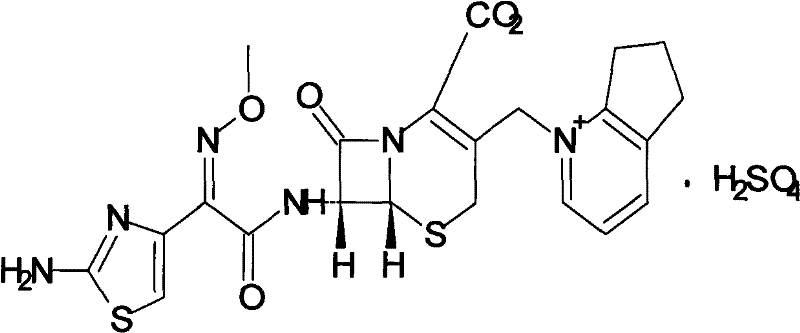
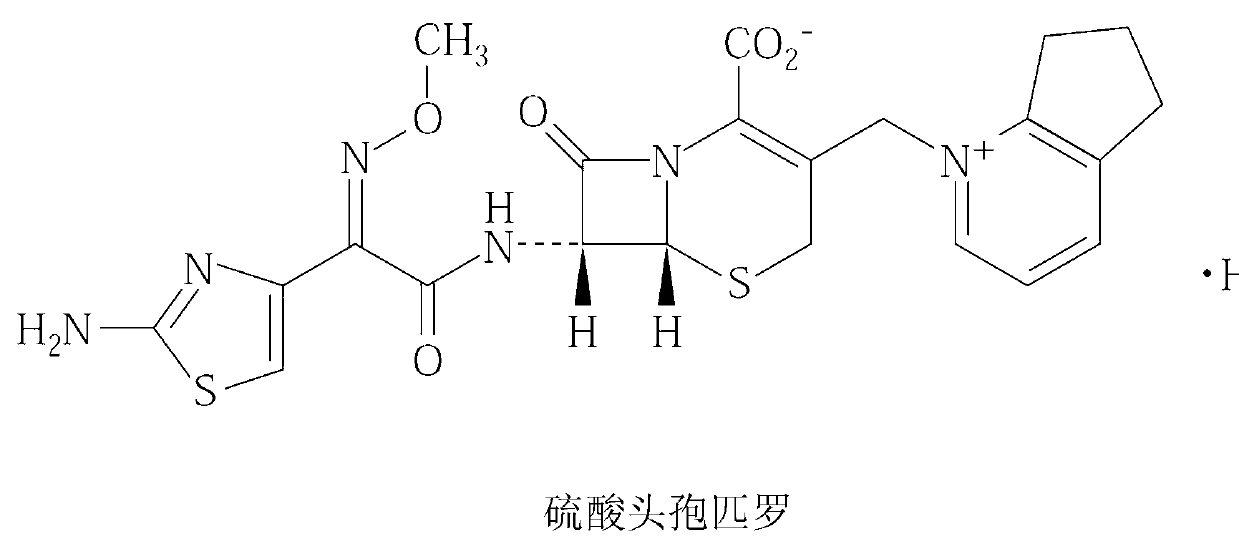
Cefpirome Sulfate is the sulfate form of cefpirome, a semisynthetic, broad-spectrum, fourth-generation cephalosporin with antibacterial activity. Cefpirome binds to and inactivates penicillin -binding proteins (PBPs) located on the inner membrane of the bacterial cell wall.
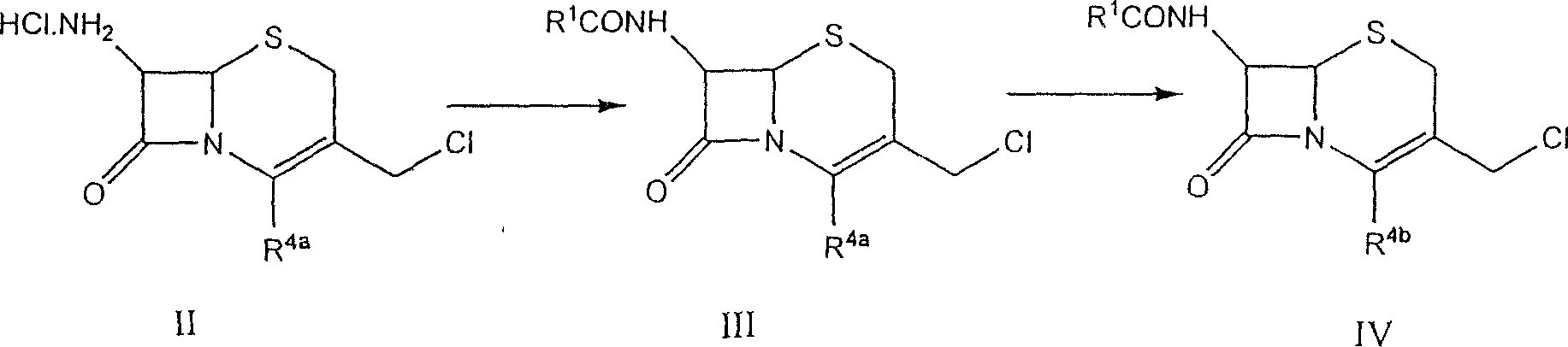
New combination of Cefpirome Sulfate and preparation method
InactiveCN1660116AAntibacterial agentsOrganic active ingredientsNeutral Amino AcidsCEFPIROME SULFATE
A cefpirome sulfate as a newm edicine and its preparing process which features use of alkaline or neutral amino acid as cosolvent are disclosed.
Owner:NANJING CHENXIANG MEDICAL RES
Cefpirome sulfate preparation technology
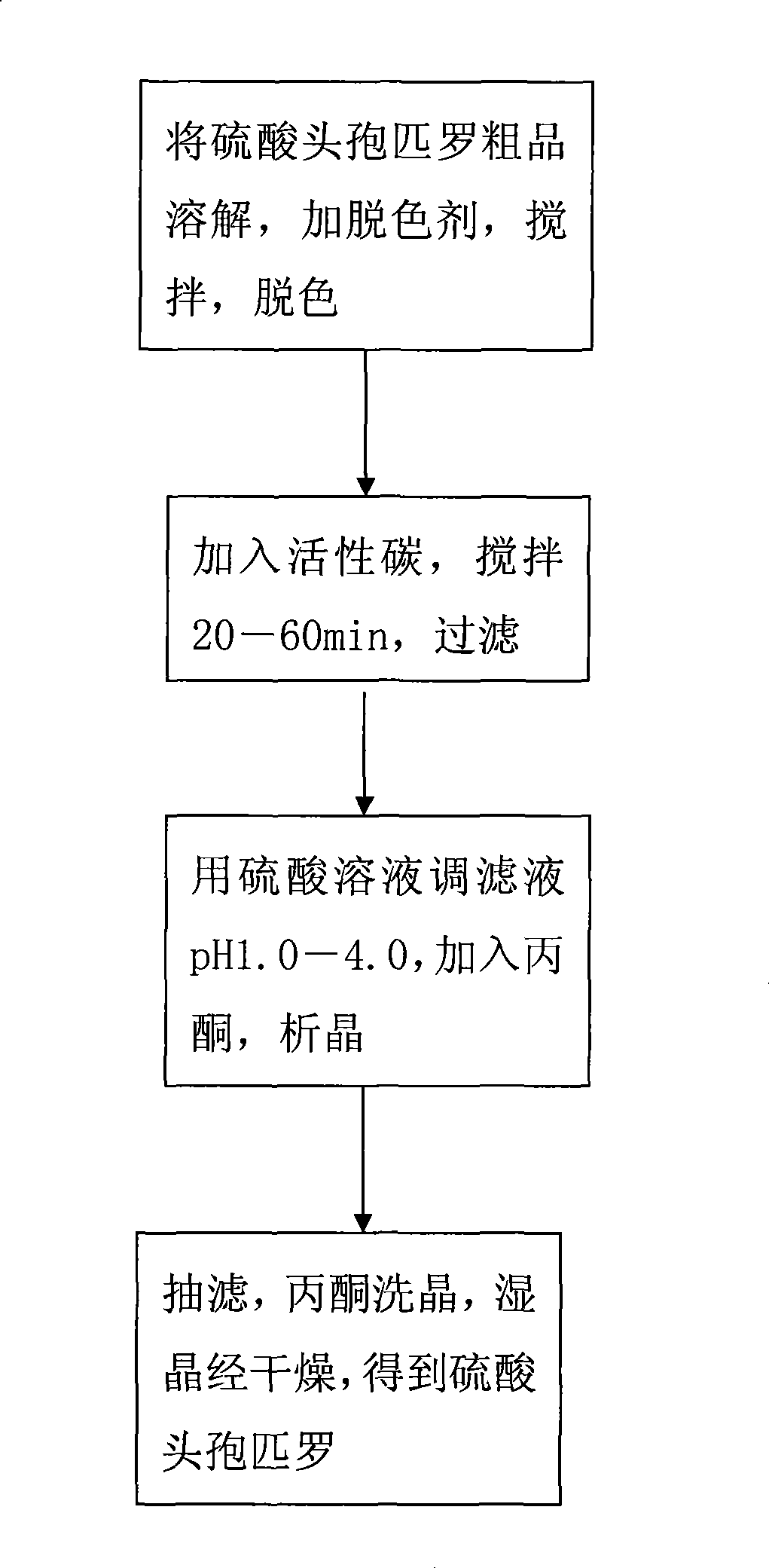
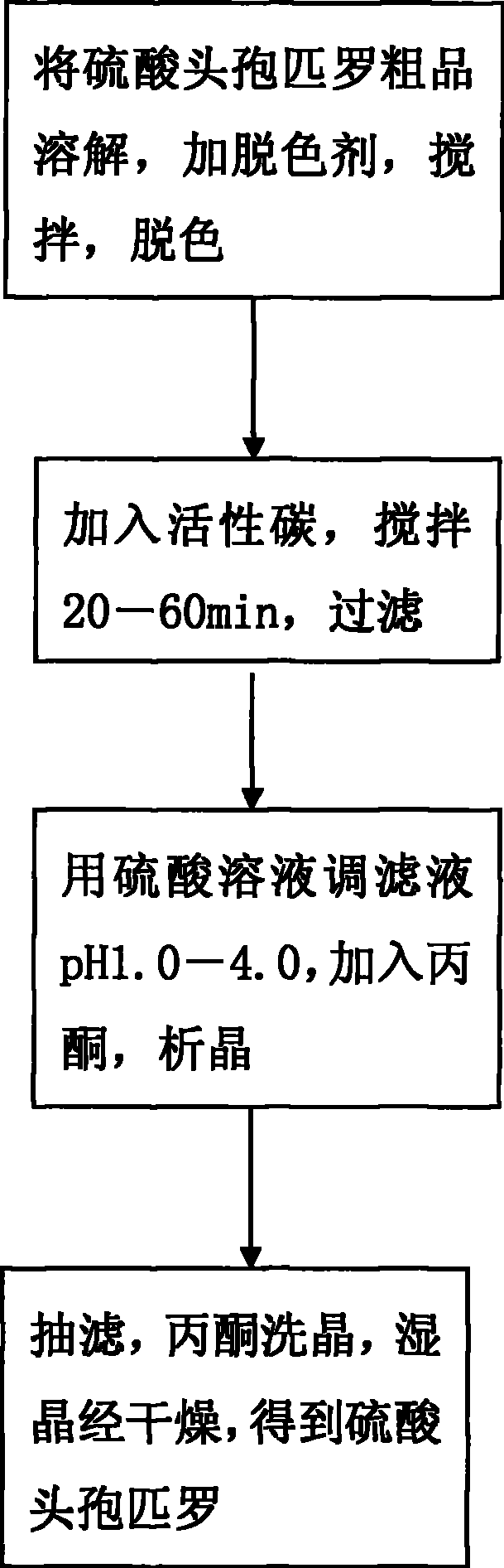
The invention discloses a preparation technique for cefpirome sulfate, which is carried out according to the following steps: first, a crude product of the cefpirome sulfate is put into water, or proper alcohol and alkaline solution are added to the cefpirome solution and stirred until pH value is between 3.0 and 6.0 so as to cause solid cefpirome sulfate to be dissolved; meanwhile, a discoloring agent is added, and stirring and discoloring are carried out, wherein, the mole ratio of the dosage of the discoloring agent to the dosage of the cefpirome is between 1 to 500 and 1 to 10; second, active carbon is added, stirred for 20min to 60 min and is filtered; third, sulphuric acid solution is used for adjusting the pH value of filter liquid to be 1.0 to 4.0; acetone is added and crystallization is carried out; fourth, vacuum filtration is carried out; acetone is used for cleaning crystals; the wet crystal is dried, thus obtaining the cefpirome sulfate. The preparation technique for preparing the cefpirome sulfate has high yield, high purity and good crystal form and can obtain the cefpirome sulfate without mixed color.
Owner:辽宁美亚制药有限公司
Synthesis process of cefpirome sulfate as antibiotic
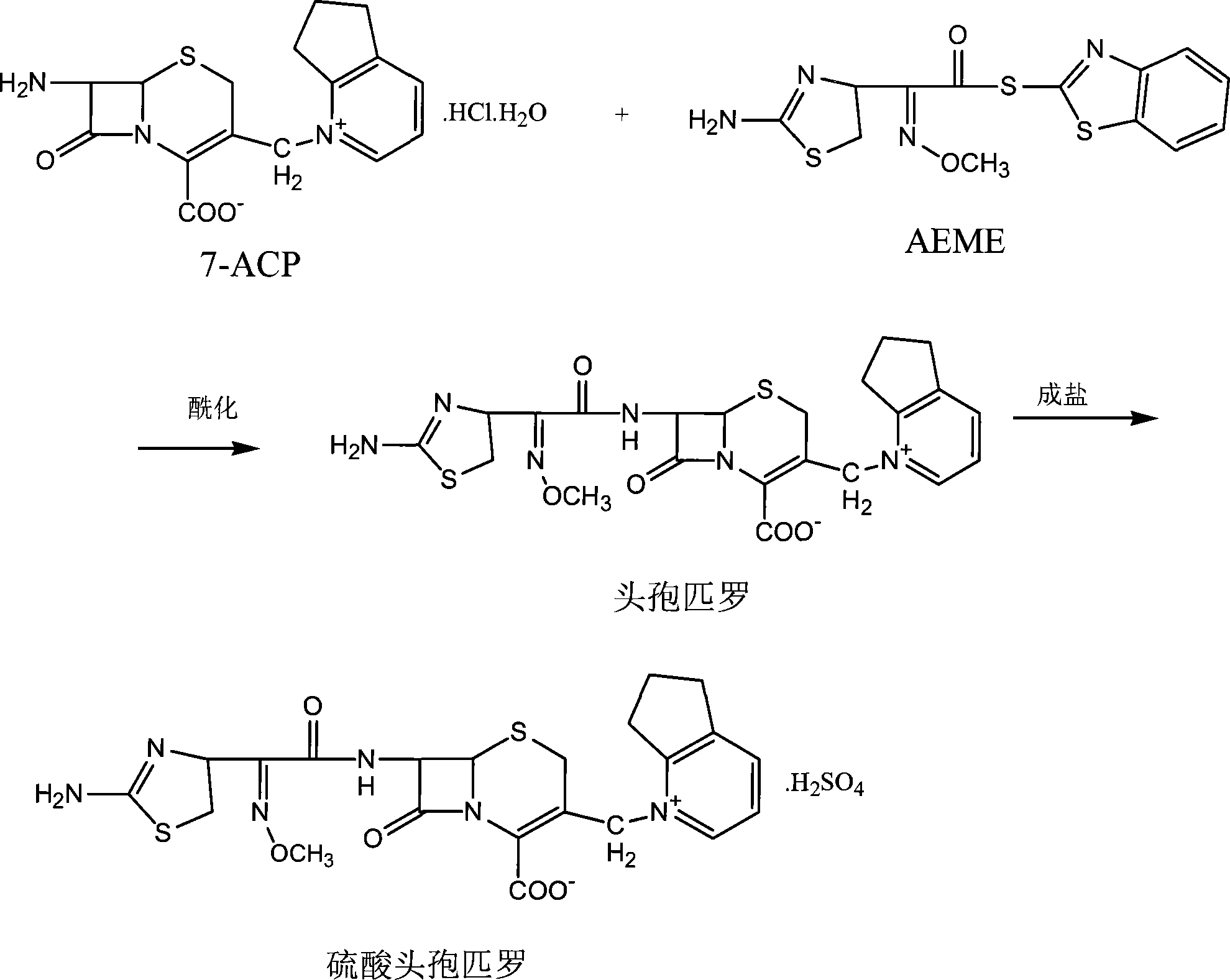
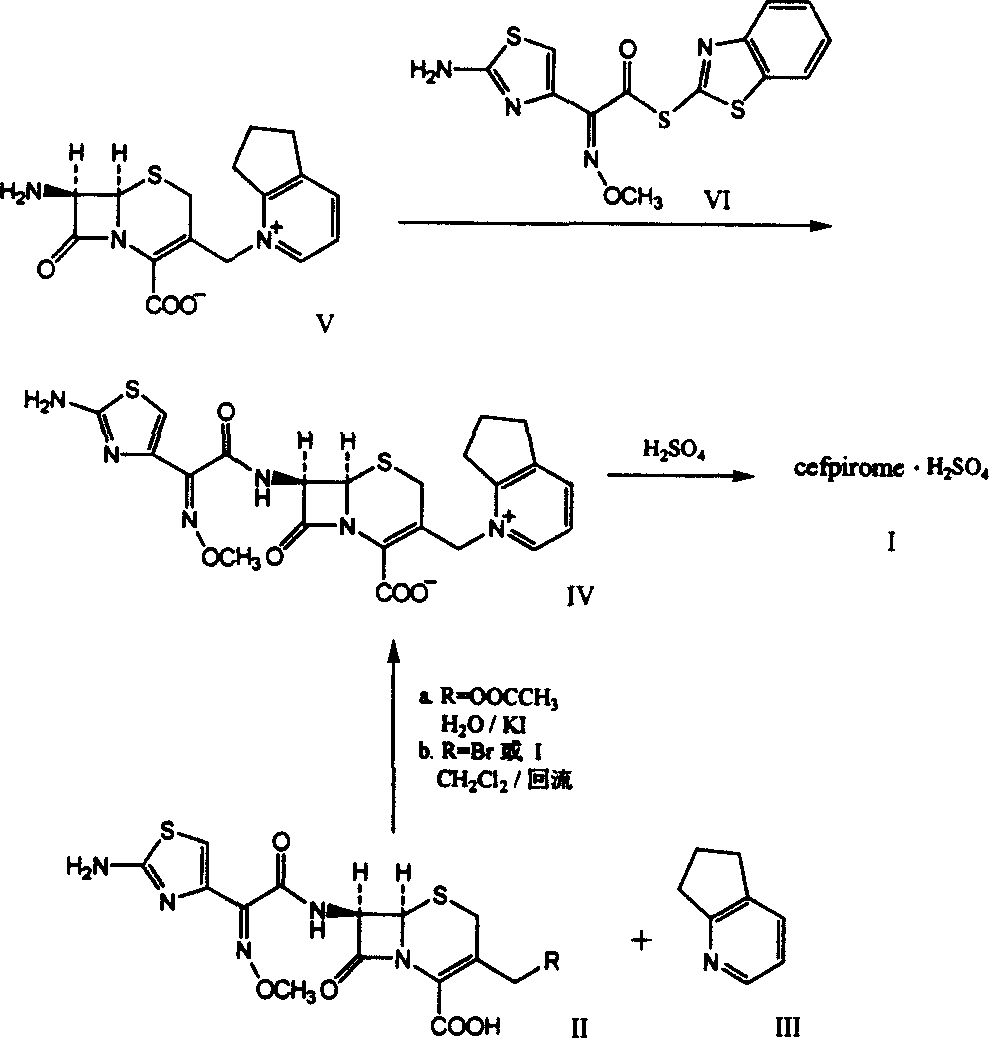
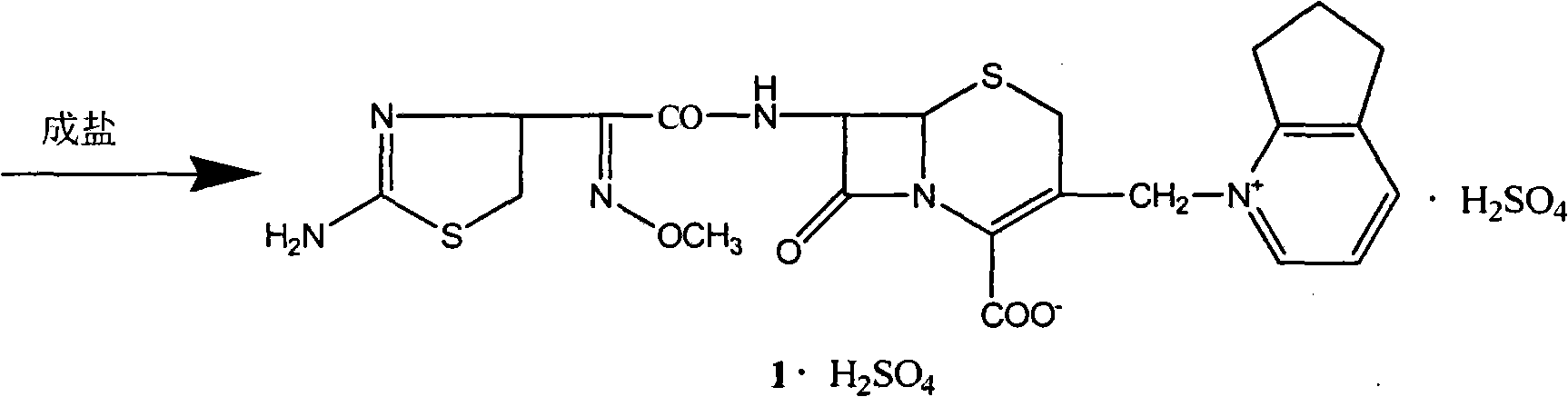
The present invention relates to synthesis process of cefpirome sulfate as one kind of antibiotic. The present invention synthesizes cefpirome sulfate by using 7-amino-3-[(2, 3-cyclopentenyl pyridyl)-1-methyl] cefo phytanic hydrochloride and 2-methoxyimino-2-(2-amino-4-thiazolyl) -(z)-thioacetic benzothiazole ester as the initial material, and through acylation reaction and salt forming reaction. Compared with available technology, the present invention has the advantages of short synthesis path, simple technological condition, low cost, high product yield, high product quality, and being suitable for industrial production.
Owner:苏州盛达药业有限公司
Technique for preparing high-purity cefpirome sulfate
InactiveCN101104621AIncrease productionSimple and fast operationOrganic chemistryOrganic solventCEFPIROME SULFATE
The present invention relates to a method for refining cefpirome sulfate. Sodium hydroxide and cefpirome sulfate are reacted to generate sodium sulfate and free cefpirome, and then organic solvent is added so that the generated sodium sulfate and the hydrate are precipitated. Cefpirome sulfate can be gained when the sulfuric acid salt formation is re-added to the decolored filtrate.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
Preparation methods of cefpirome intermediate and cefpirome
ActiveCN102391288AHigh purityHandling Recycling SimplifiedOrganic chemistryCyclopenteneCarboxylic acid
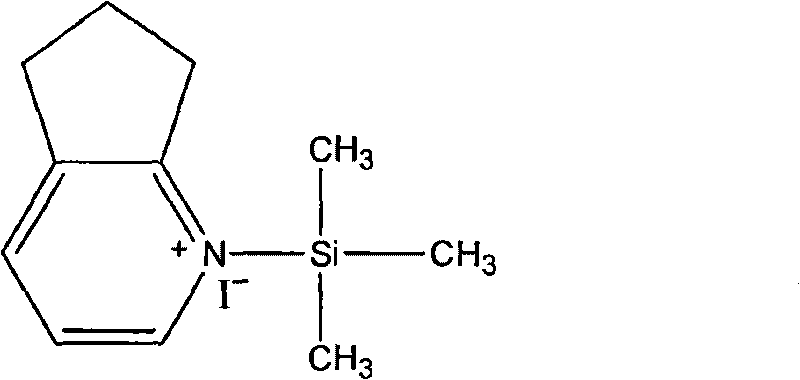
The invention relates to preparation methods of cefpirome intermediate and cefpirome; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the cefpirome intermediate (6R, 7R)-7-amino-3-[(2,3-cyclopentene-pyridine)methyl]ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing cefpirome sulfates by using the obtainedintermediate halogen acid salt. The cefpirome intermediate and cefpirome prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Synthetic method of cefpirome sulfate
ActiveCN101284840ALow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
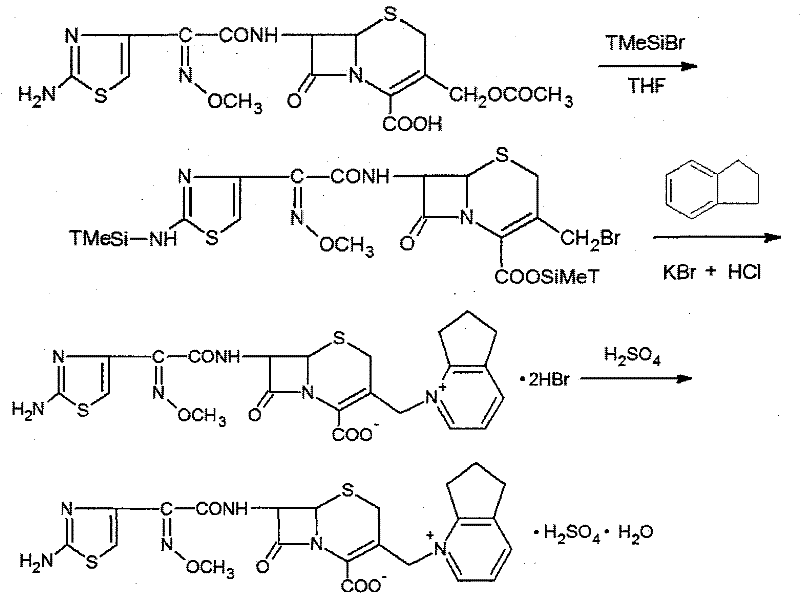
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
Reworking method of cefpirome sulfate mixed powder
The invention discloses a reworking method of cefpirome sulfate mixed powder. The reworking method comprises the following steps of: 1) dissolving the cefpirome sulfate mixed powder to be reworked in a certain amount of water, and setting the temperature at 0 DEG C-30 DEG C; 2) enabling the solution after dissolution to pass through a column loaded with an adsorbent, and collecting filtrate; 3) adding activated carbon into the filtrate, performing decolorization reaction, and controlling the reaction time at 30min; 4) filtering and collecting the filtrate; 5) dripping a sulfuric acid solution into the filtrate, and regulating the pH value to 1.2-2.4; 6) adding a crystallizing agent into the filtrate for crystallization, controlling the crystallization temperature at 0 DEG C-30 DEG C, and stirring for 2-4h; 7) filtering to obtain a crystal after filtration, and washing with one solution or a mixed solution of acetone and alcohol; and 8) controlling the temperature at 45 DEG C-55 DEG C, and drying to obtain the cefpirome sulfate. According to the reworking method disclosed by the invention, the unqualified cefpirome sulfate can be effectively reworked and refined to produce a qualified product, and the cost can be further reduced.
Owner:苏州盛达药业有限公司
Cefpirome sulfate preparation technology
The invention discloses a preparation technique for cefpirome sulfate, which is carried out according to the following steps: first, a crude product of the cefpirome sulfate is put into water, or proper alcohol and alkaline solution are added to the cefpirome solution and stirred until pH value is between 3.0 and 6.0 so as to cause solid cefpirome sulfate to be dissolved; meanwhile, a discoloringagent is added, and stirring and discoloring are carried out, wherein, the mole ratio of the dosage of the discoloring agent to the dosage of the cefpirome is between 1 to 500 and 1 to 10; second, active carbon is added, stirred for 20min to 60 min and is filtered; third, sulphuric acid solution is used for adjusting the pH value of filter liquid to be 1.0 to 4.0; acetone is added and crystallization is carried out; fourth, vacuum filtration is carried out; acetone is used for cleaning crystals; the wet crystal is dried, thus obtaining the cefpirome sulfate. The preparation technique for preparing the cefpirome sulfate has high yield, high purity and good crystal form and can obtain the cefpirome sulfate without mixed color.
Owner:辽宁美亚制药有限公司
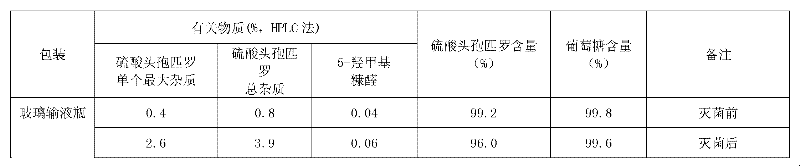
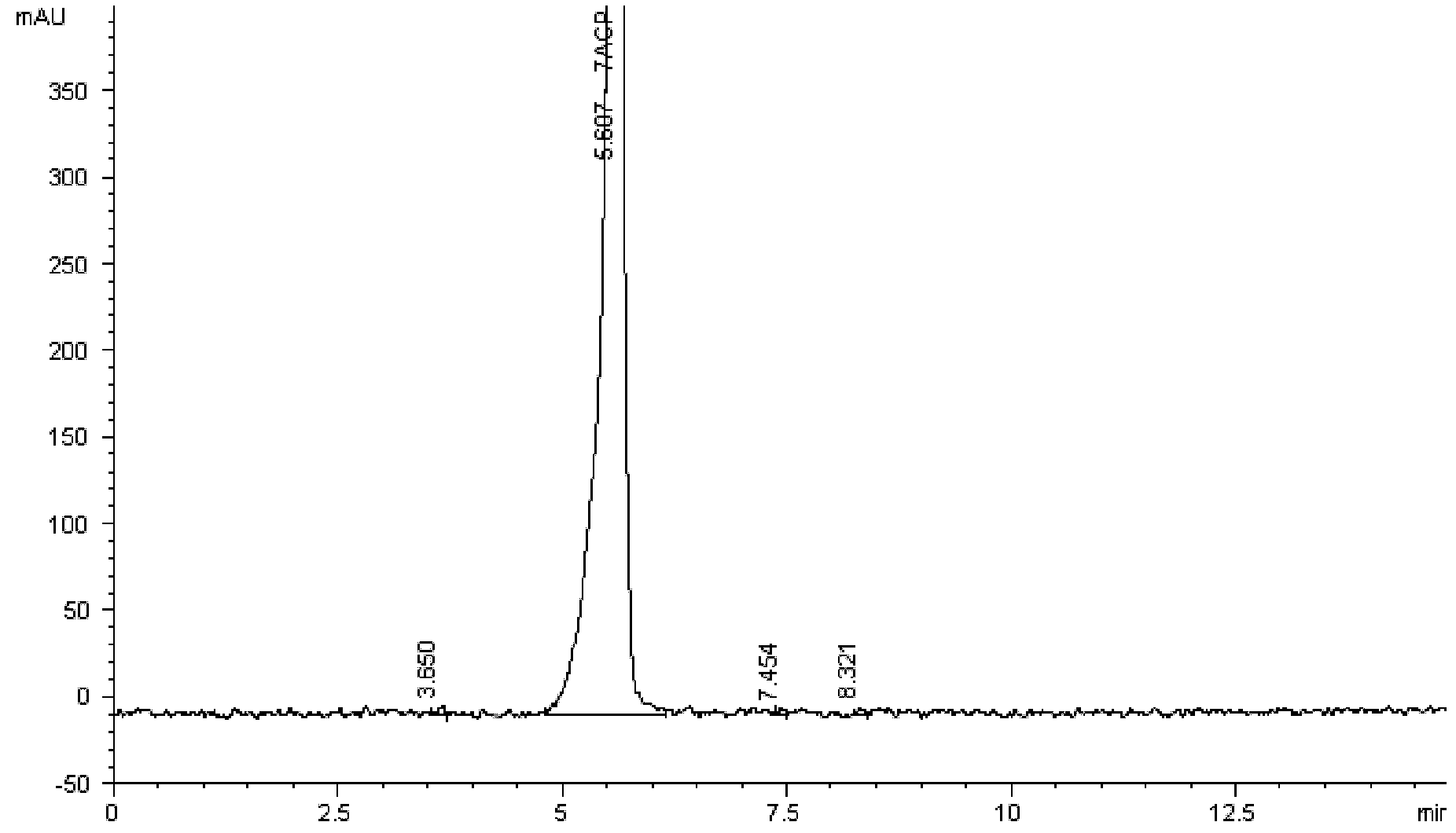
Method for detecting related substance II of cefpirome in cefpirome sulfate/sodium chloride injection
InactiveCN102928525AImprove accuracyStrong specificityComponent separationPhosphateSodium Chloride Injection
The invention provides a method for detecting a related substance II of cefpirome in cefpirome sulfate / sodium chloride injection. According to the method, experiments on chromatographic conditions and system suitability are performed by using high-performance liquid chromatography. For the color spectrum which is suitable for separating globular protein of which the molecular weight is 1,000-5,000, hydrophilic silica gel serves as a filler, and phosphate buffer solution-acetonitrile of which the volume ratio is 85:15 serves as a mobile phase, wherein the flow velocity is 0.5 ml / min, the phosphate buffer solution consists of 0.05 mol / L disodium hydrogen phosphate and 0.05 mol / L sodium dihydrogen phosphate which are mixed in the volume ratio of 50:50, and the measurement wavelength is 254 nm. The method comprises the following steps of: placing appropriate amount of cefpirome sulfate / sodium carbonate mixed powder which comprises 10 mg of cefpirome into a 10 ml measuring flask, wherein the mixed powder is taken out of an infusion bag; adding water to dissolve the mixed powder and diluting to certain scale, and shaking up; and injecting 20 mu l of the diluted solution into a liquid chromatograph to perform chromatography, and recording a chromatogram. The detection method is advanced, reliable, high in accuracy and high in specificity, the experimental apparatus is high in precision, high in reproducibility and high in stability, the related substance II of the cefpirome can be effectively detected, and the method can be used for quality control.
Owner:北京锐业制药有限公司
Method for preparing high-purity cefpirome sulfate by sodium salt precipitation method
InactiveCN101397304AIncrease productionSimple and fast operationAntibacterial agentsOrganic chemistryOrganic solventSulfate
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +1
Reworking method of cefpirome sulfate
The invention discloses a reworking method of cefpirome sulfate. The reworking method comprises the following steps of: 1) dissolving the cefpirome sulfate to be reworked in a certain amount of water; 2) dripping an organic base for dissolution, regulating the PH value to 4.0-8.0, and setting the reaction temperature at 0 DEG C-30 DEG C; 3) enabling the solution after dissolution to pass through a column loaded with an adsorbent, and collecting filtrate; 4) adding activated carbon into the filtrate, performing decolorization reaction for 30min, and filtering; 5) regulating the PH value of the filtrate after decolorization to 1.2-2.4; 6) adding a quantitative crystallizing agent into the filtrate for crystallization, controlling the crystallization temperature at 0 DEG C-30 DEG C, and stirring for 2-4h till the end of reaction; 7) filtering, and washing with one solution or a mixed solution of acetone and alcohol; and 8) drying at the temperature of 45 DEG C-55 DEG C to obtain the cefpirome sulfate. According to the reworking method disclosed by the invention, the unqualified cefpirome sulfate can be effectively reworked and refined to produce a qualified product, and the cost can be further reduced.
Owner:苏州盛达药业有限公司
Compound of onium salt in Cephalosprins, preparation method, and method for synthesizing vitriolic cefpyrazole from the compound
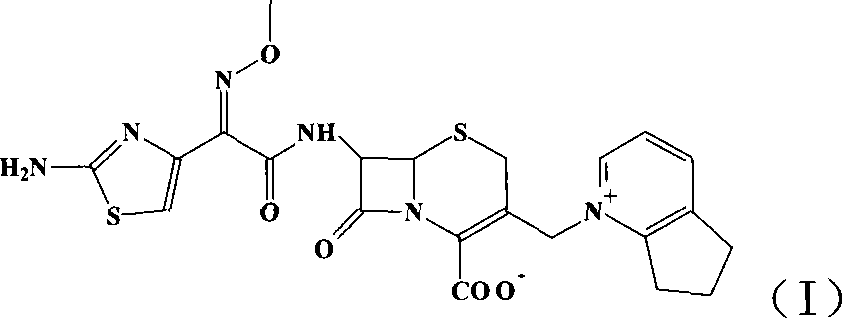
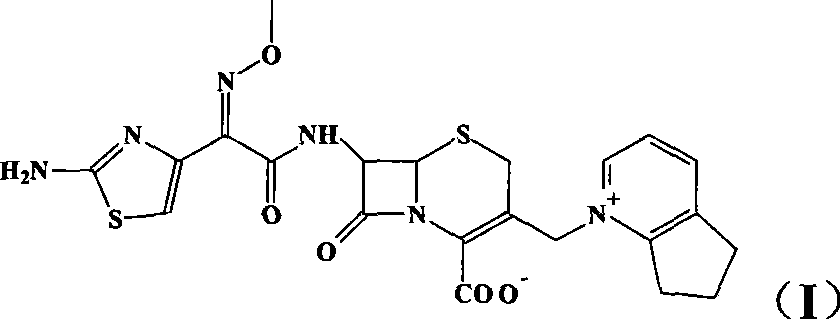
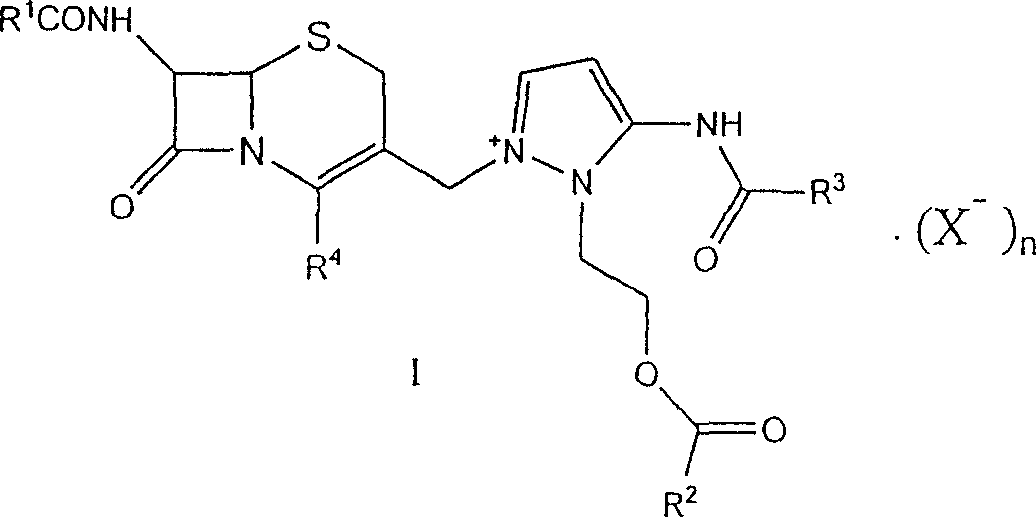
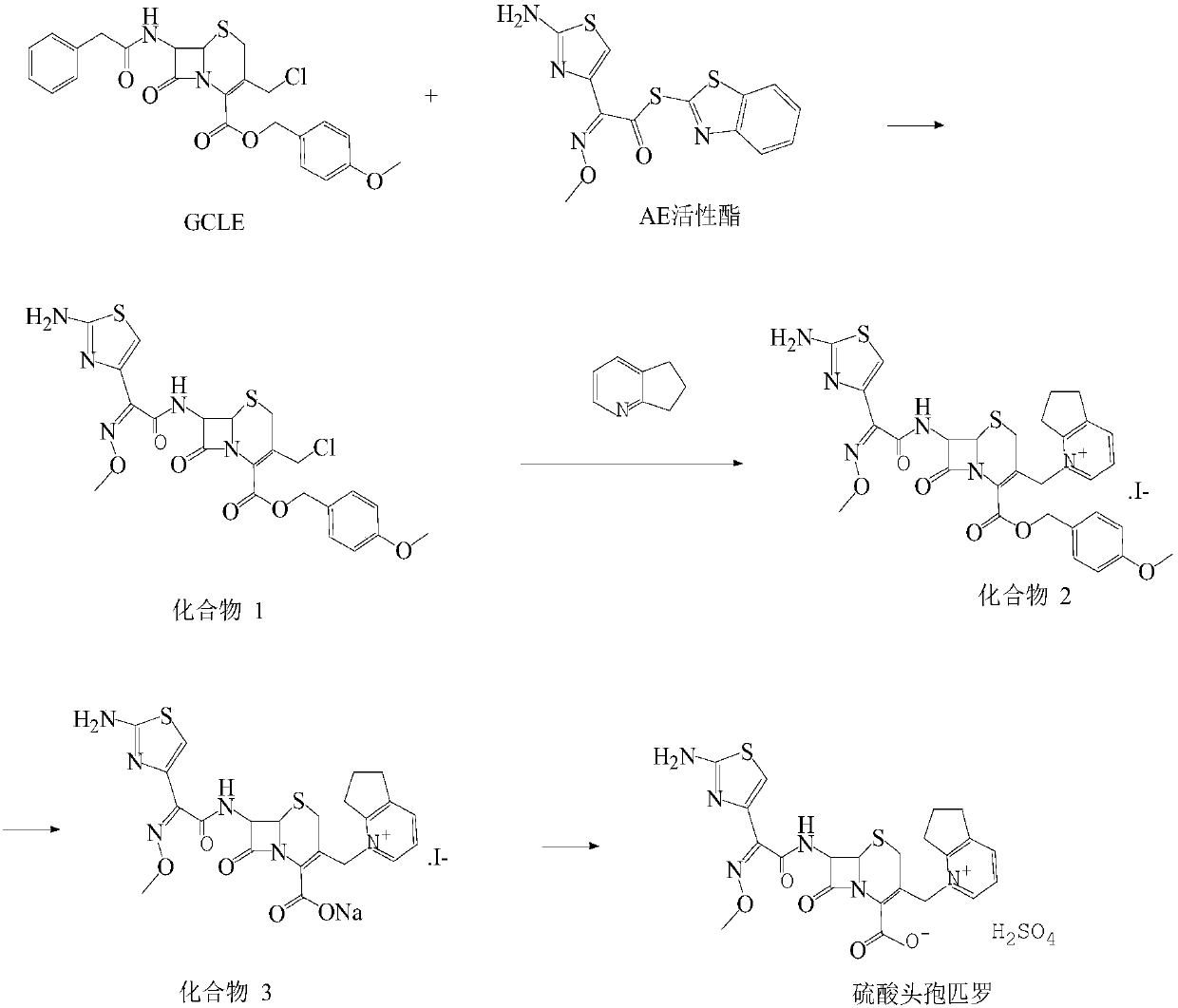
This invention discloses a method for preparing 7beta-alkylamido-3-[3-alkylamido-2-(2-alkyloyloxyethyl)-1-pyrazole]methyl-3-cephem-4-carboxylate (I) as an important intermediate for cefpirome sulfate synthesis. Besides, this invention also discloses a method for synthesizing cefpirome sulfate, which comprises: utilizing (I) as the raw material, hydrolyzing, and reacting with AE active ester. The method has such advantages as mild reaction conditions; simple process, and avoids expensive or stimulating reagents.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
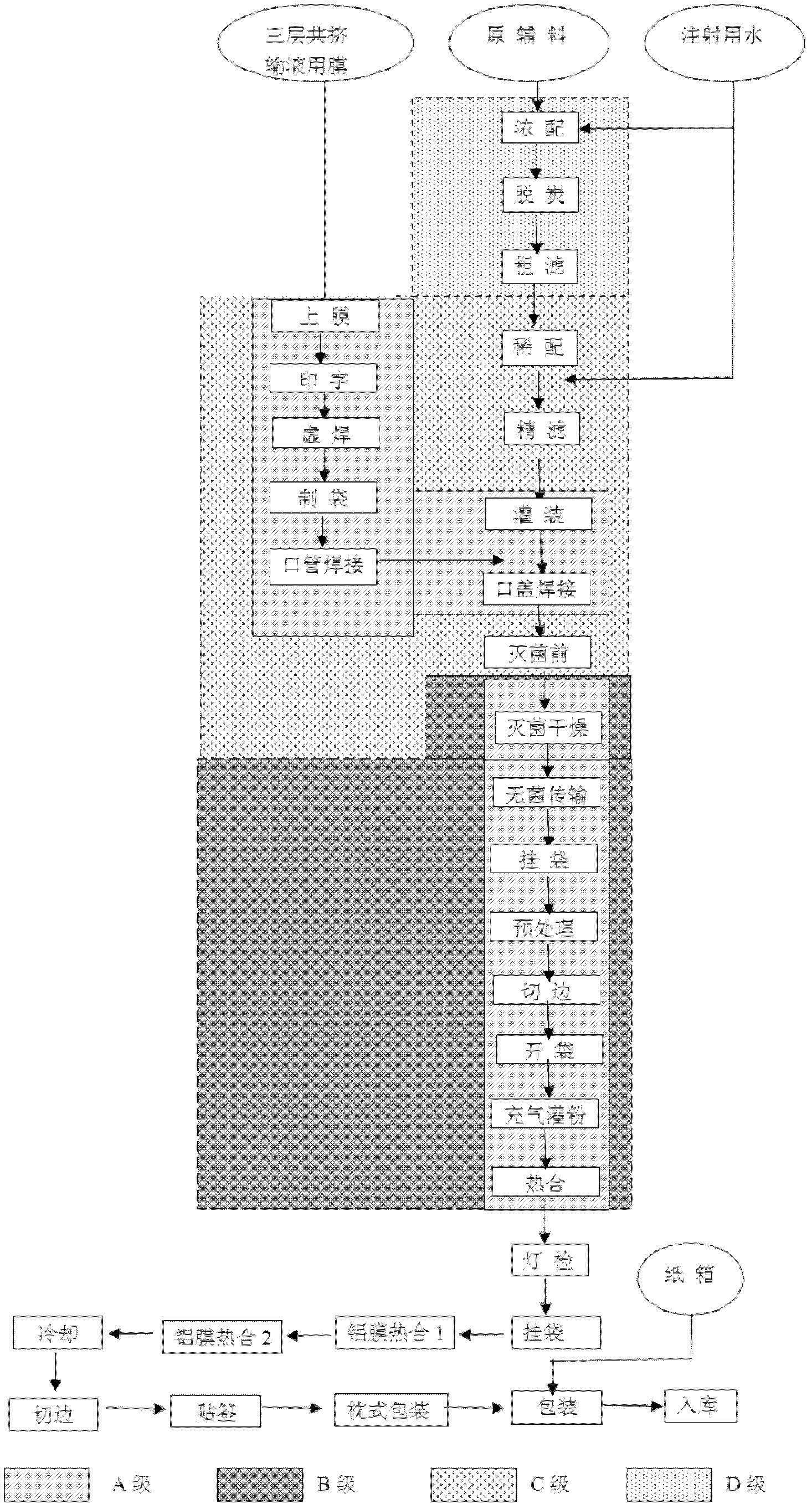
Production method of cefpirome sulfate/ sodium chloride injection non-PVC (polyvinyl chloride) instant matched injection
InactiveCN102431664AGood isolation securityEasy to operateAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionPolyvinyl chloride
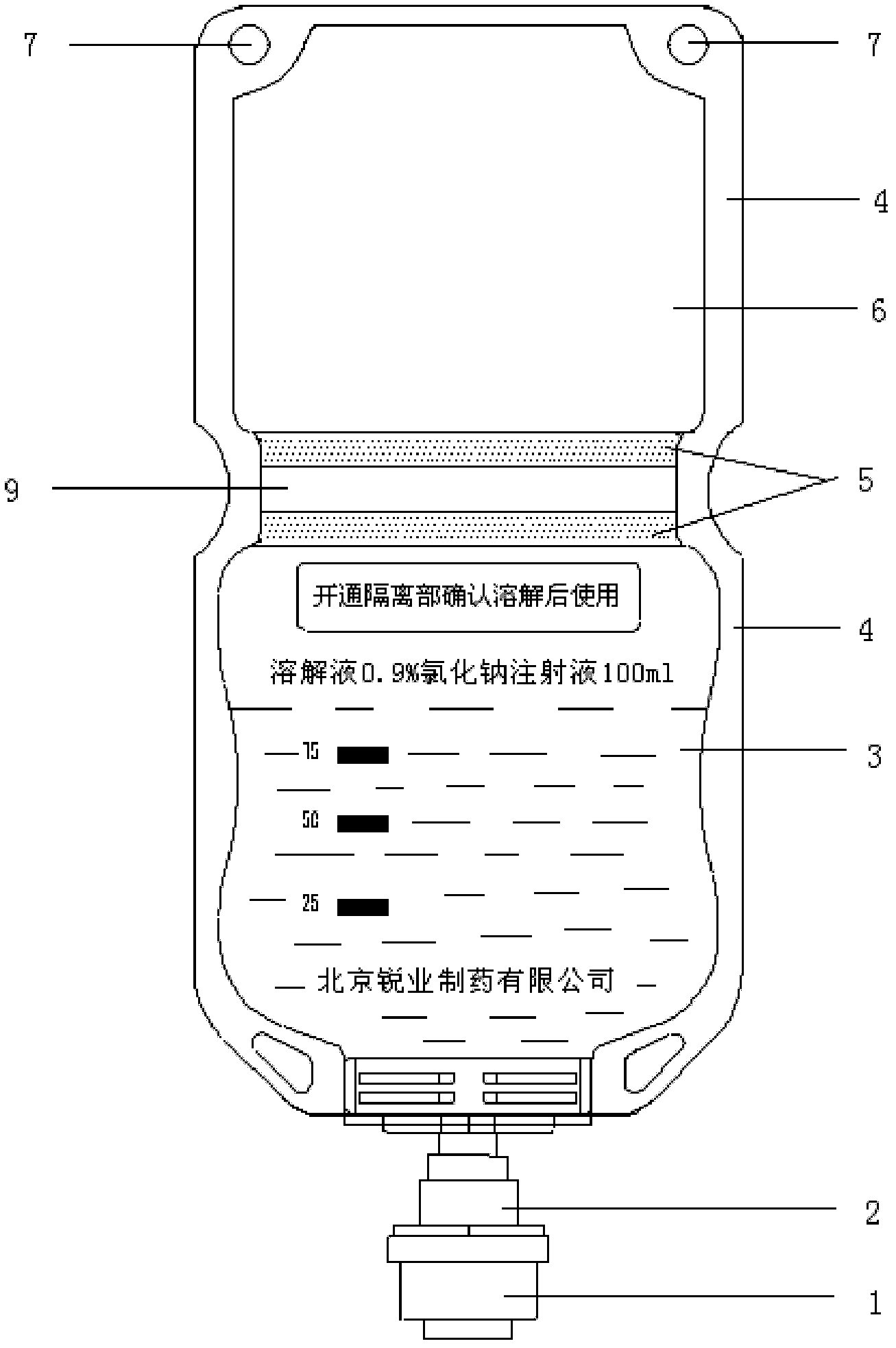
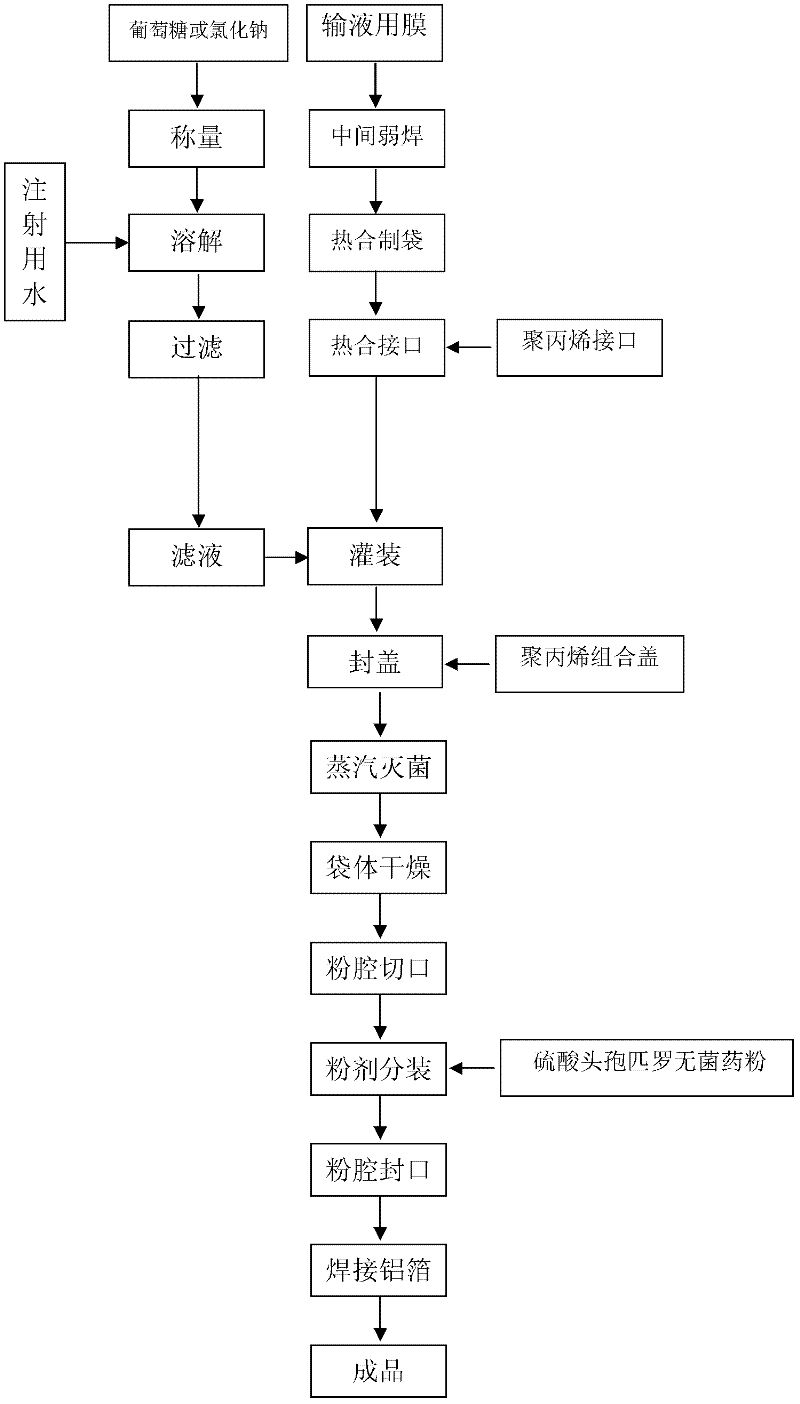
The invention provides a production method of cefpirome sulfate / sodium chloride injection non-PVC (polyvinyl chloride) instant matched injection, which comprises the following steps of: preparing liquid medicine, bagmaking and filling, sterilizing and drying, sterile split charging, and the like; and is characterized in that the bagmaking and filling comprises the following steps of: utilizing a film for multilayer coextrusion infusion to make a bag body with a solid chamber and a liquid chamber through middle pseudo soldering and periphery soldering, wherein the pseudo soldering part divides the bag body into the solid chamber and the liquid chamber; after further processing and testing the bag body, filling sodium chloride injection into the liquid chamber; and then sealing a combined cover and a port by fusing. The production method of the cefpirome sulfate / sodium chloride injection non-PVC (polyvinyl chloride) instant matched injection has the following advantages that: the separating safety of the solid chamber and the liquid chamber is good; the operation in opening is convenient; each processing step has no adverse impact on drugs and the bag body; and the impact on the drugs and the bag body, and various test items for the produced product conform to the standard.
Owner:北京锐业制药有限公司
Method for synthesizing cefpirome sulfate
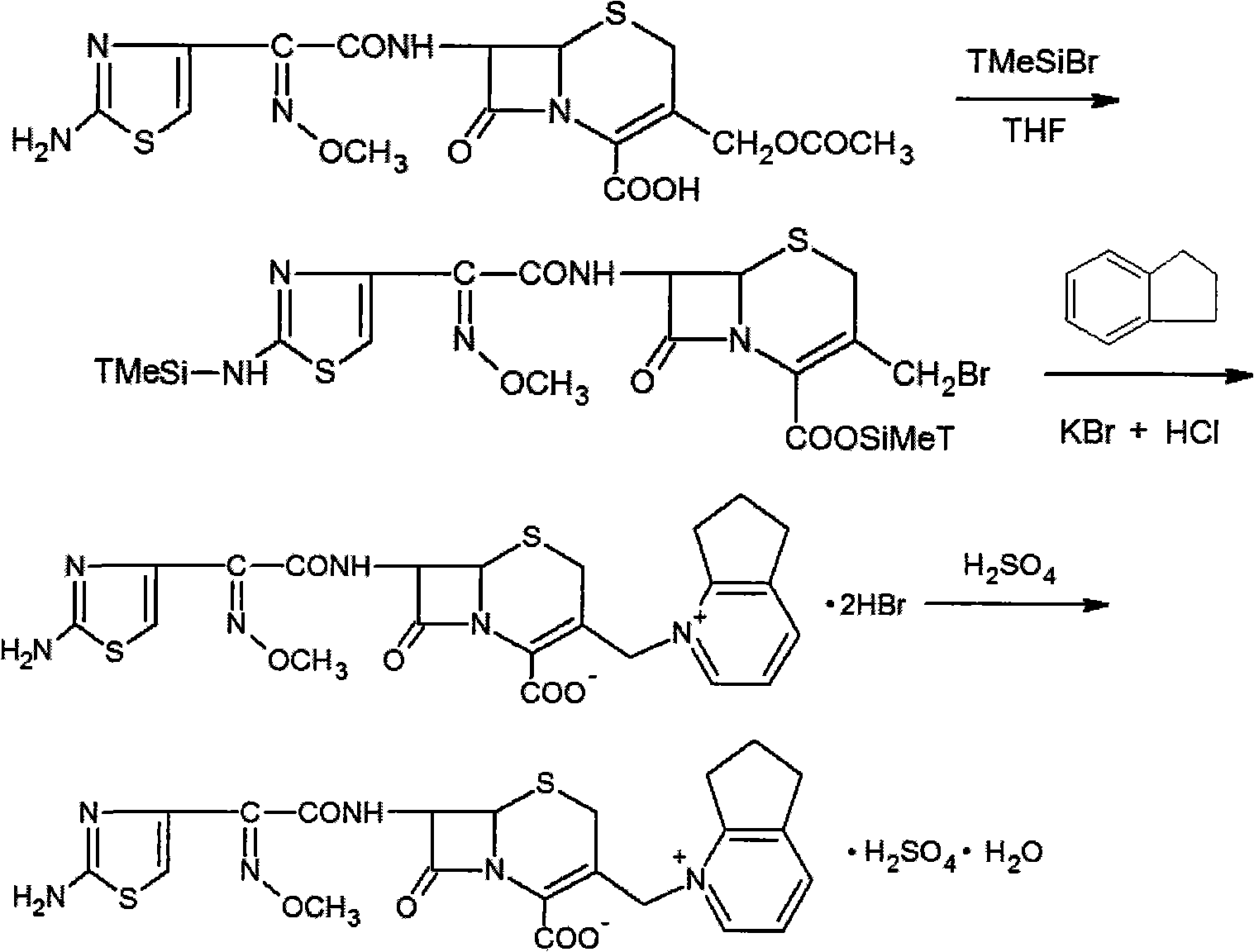
InactiveCN101747349AOvercome the disadvantage of azeotropic separationOvercome the disadvantages of strong alkalinity and more side reactionsOrganic chemistryChemical recyclingAlkalinityQuaternary ammonium cation
The invention relates to a method for synthesizing cefpirome sulfate. The method comprises the following steps: firstly, preparing 2,3-cyclopentenopyridine and trimethylsilyl iodide into quaternary ammonium salt intermediate compound; Secondly, performing carboxyl and amido protection for 7-ACA through hexamethyl disilylamine, and finally adding quaternary ammonium salt intermediate compound into the well protected 7-ACA solution for rapid reaction to obtain cefpirome mother nuclide 7-ACP; Thirdly, performing N- acidylation reaction to 7-ACP and AE active ester in the mixed phase of DMF and water, and obtaining cefpirome sulfate after salt forming reaction. By using step one to obtain quaternary ammonium salt intermediate compound, not only N,N- diethylaniline is saved, but also the side chain 2,3-cyclopentenopyridine is easy to rectify and recycle, thereby greatly reducing the production cost; simultaneously, the disadvantages of strong alkalinity and a large amount of side reaction of 2,3-cyclopentenopyridine are overcome. By using the method, the yield rate and the purity of the product can be improved.
Owner:YIYUAN XINQUAN CHEM
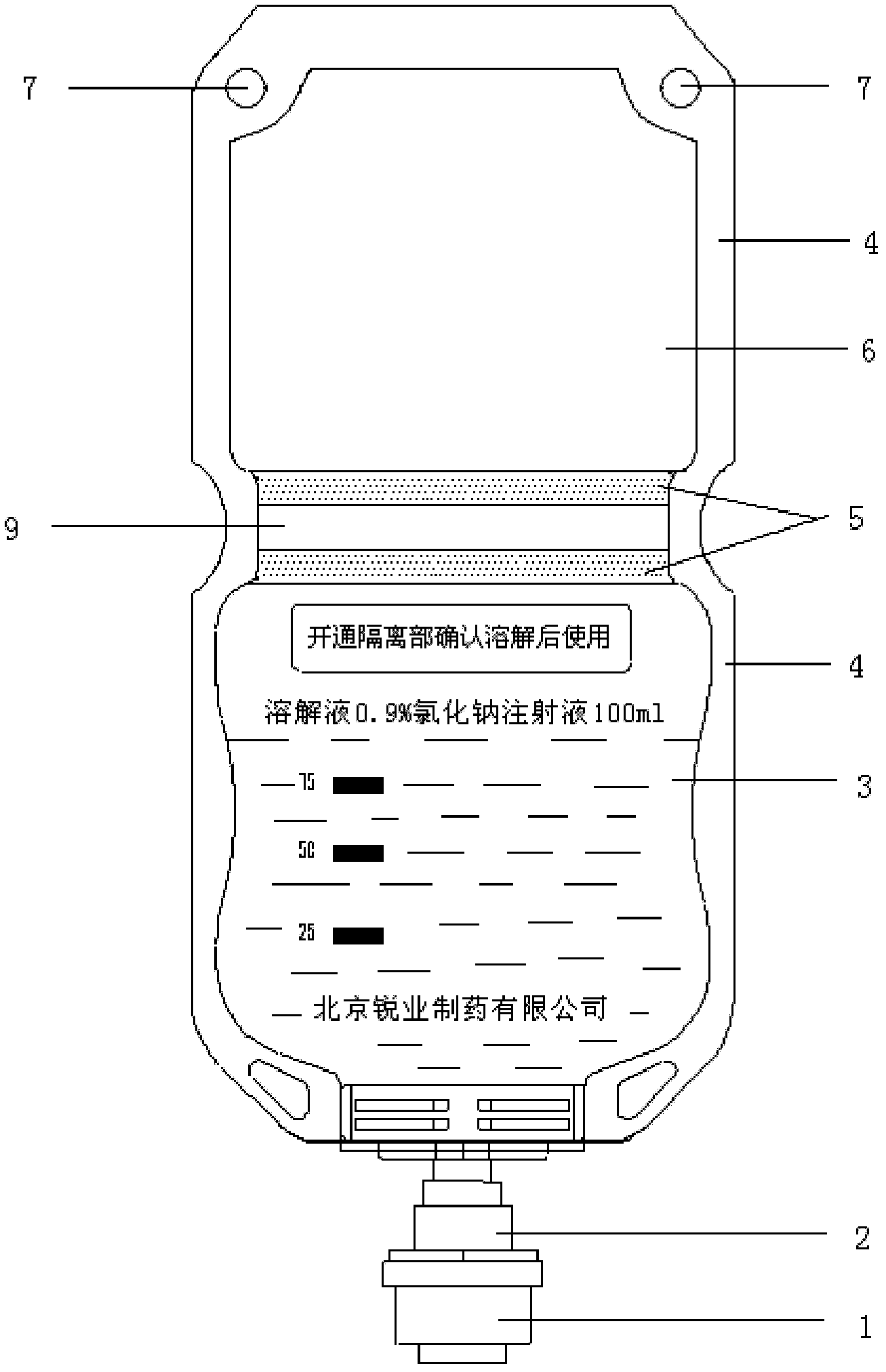
Double-cavity bag packed cefpirome sulfate injection and preparation method thereof
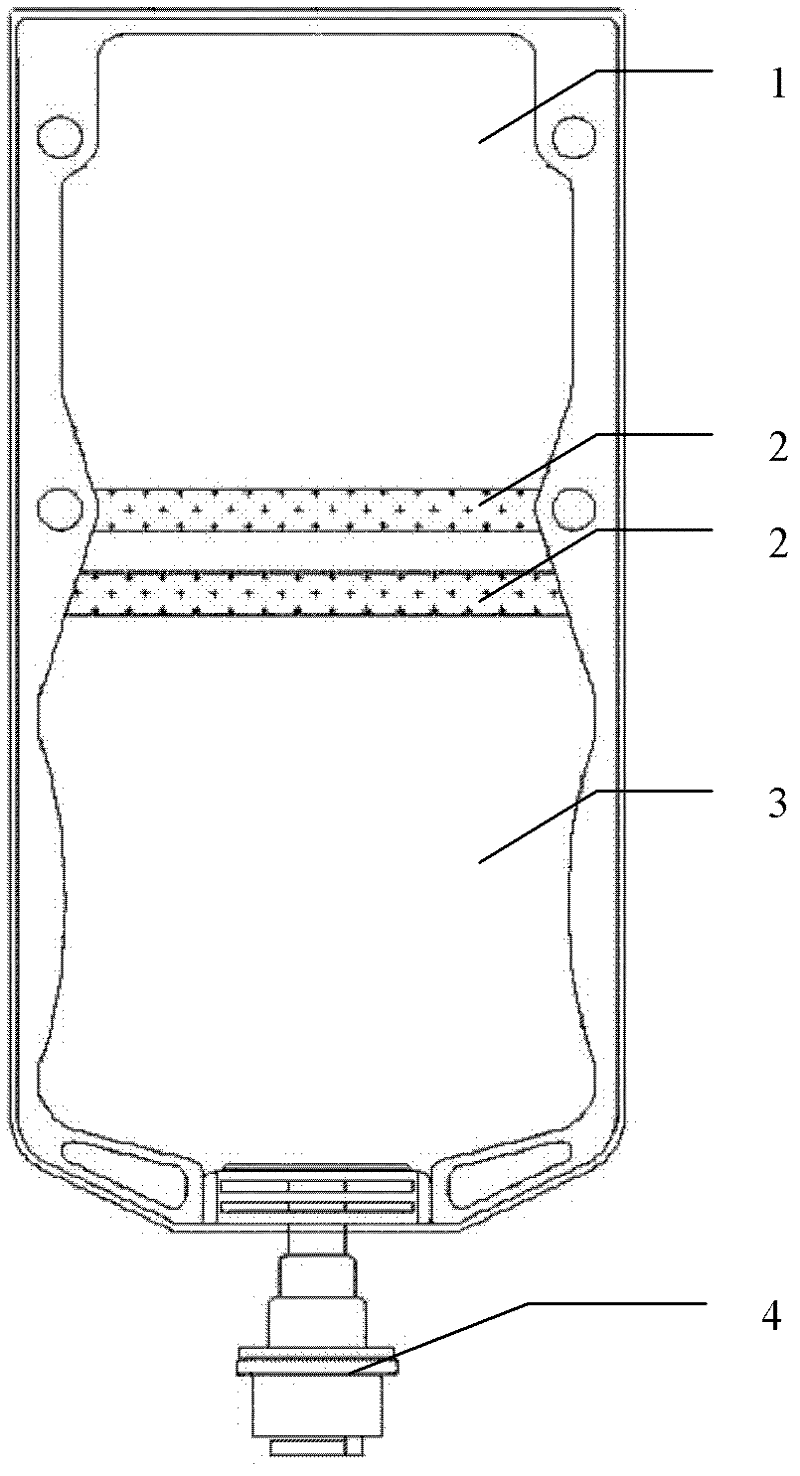
InactiveCN102429860APrevent precipitationAvoid reactionAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionGlucose polymers
The invention discloses double-cavity bag packed cefpirome sulfate injection. The injection medicinal composition consists of the following Chinese herbal medicines: 0.5 to 2 weight parts of cefpirome sulfate (based on cefpirome) in a powder cavity, and 100 volume parts of glucose injection with 4 to 11 weight parts of glucose or 100 volume parts of sodium chloride injection with 0.8 to 1 weight part of sodium chloride in a liquid cavity. The cefpirome sulfate injection is packed in a double-cavity bag, and cefpirome sulfate sterile medicine powder and a solvent (the sodium chloride injection or the glucose injection) are filled in the two cavities separated by cold joint; and a cold joint isolating bar can be opened only by extruding the medicine bag during use, so that the cefpirome sulfate sterile medicine powder and the solvent can be quickly dissolved and mixed under the closed sterile condition, and full medicine treatment mixed liquid is formed and is directly applied to a patient.
Owner:HUNAN KELUN PHARMA
Reworking method of cefpirome sulfate mixed powder
The invention discloses a reworking method of cefpirome sulfate mixed powder. The reworking method comprises the following steps of: 1) dissolving the cefpirome sulfate mixed powder to be reworked in a certain amount of water, and setting the temperature at 0 DEG C-30 DEG C; 2) enabling the solution after dissolution to pass through a column loaded with an adsorbent, and collecting filtrate; 3) adding activated carbon into the filtrate, performing decolorization reaction, and controlling the reaction time at 30min; 4) filtering and collecting the filtrate; 5) dripping a sulfuric acid solution into the filtrate, and regulating the PH value to 1.2-2.4; 6) adding a crystallizing agent into the filtrate for crystallization, controlling the crystallization temperature at 0 DEG C-30 DEG C, and stirring for 2-4h; 7) filtering to obtain a crystal after filtration, and washing with one solution or a mixed solution of acetone and alcohol; and 8) controlling the temperature at 45 DEG C-55 DEG C, and drying to obtain the cefpirome sulfate. According to the reworking method disclosed by the invention, the unqualified cefpirome sulfate can be effectively reworked and refined to produce a qualified product, and the cost can be further reduced.
Owner:苏州盛达药业有限公司
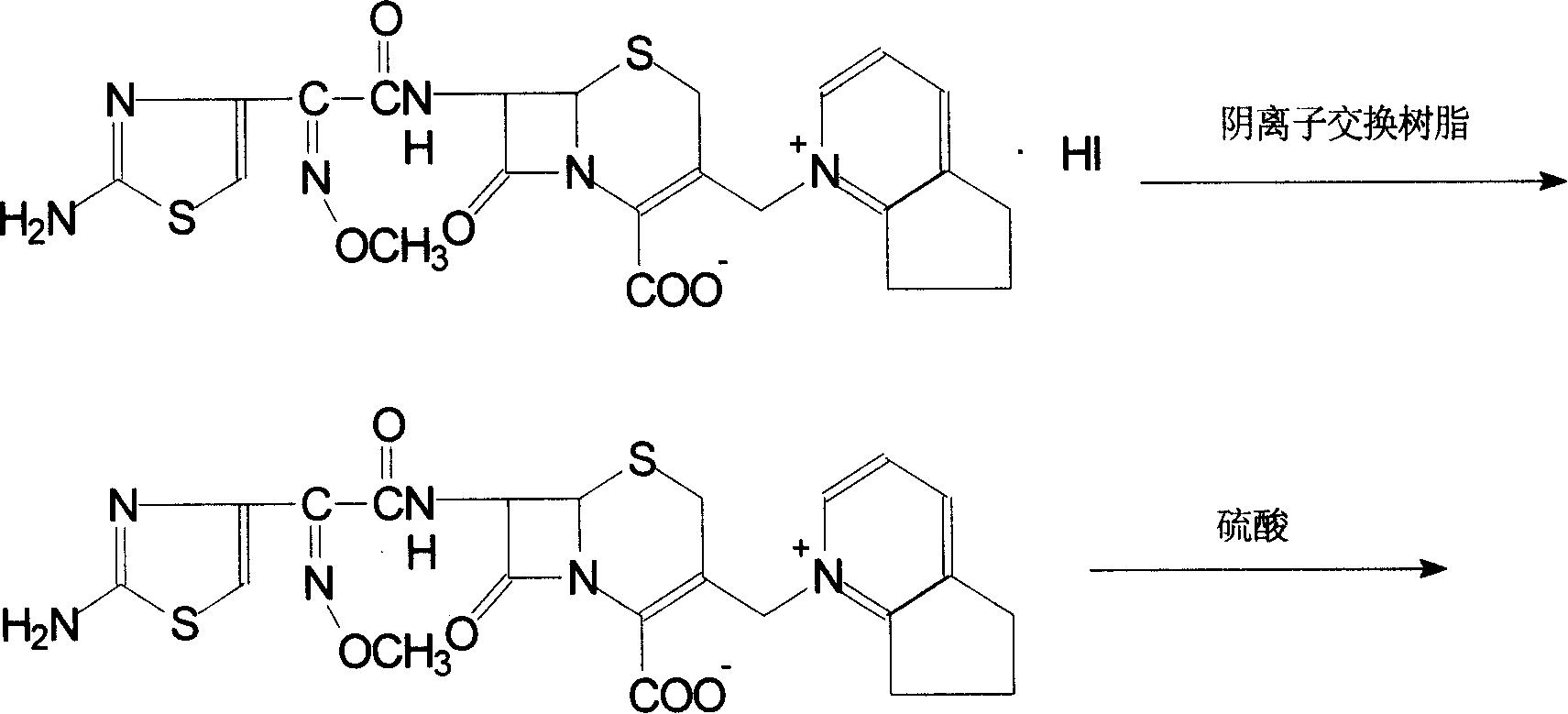
Method of preparing cefpirome sulfate
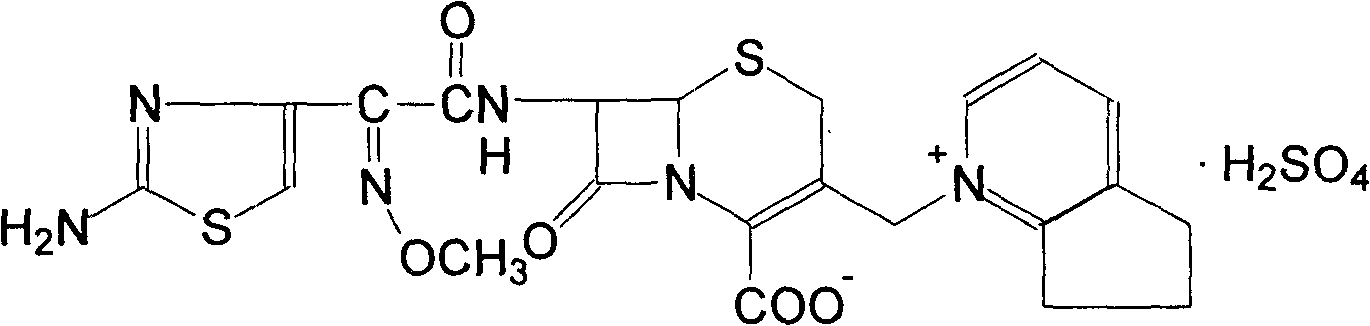
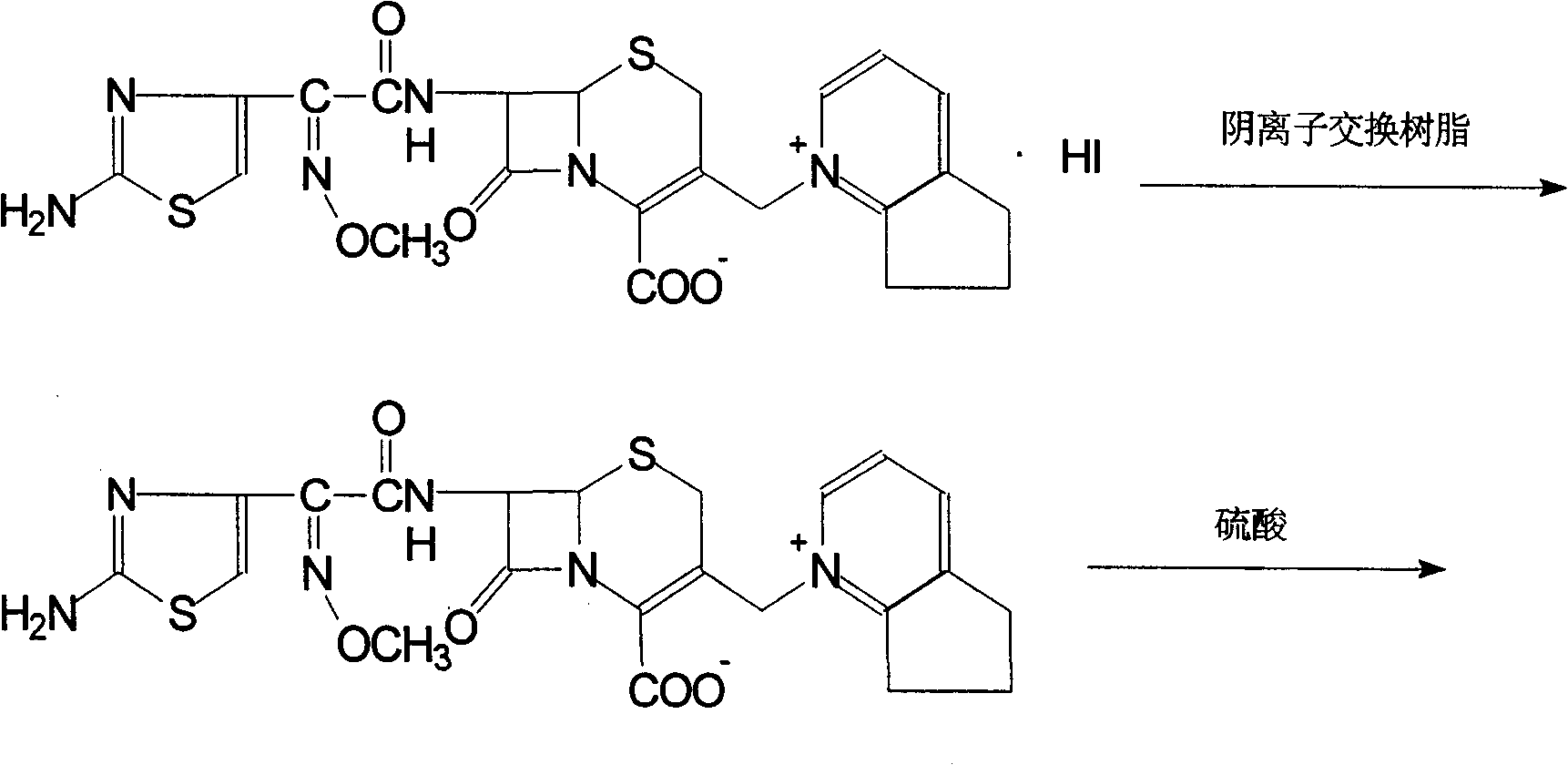
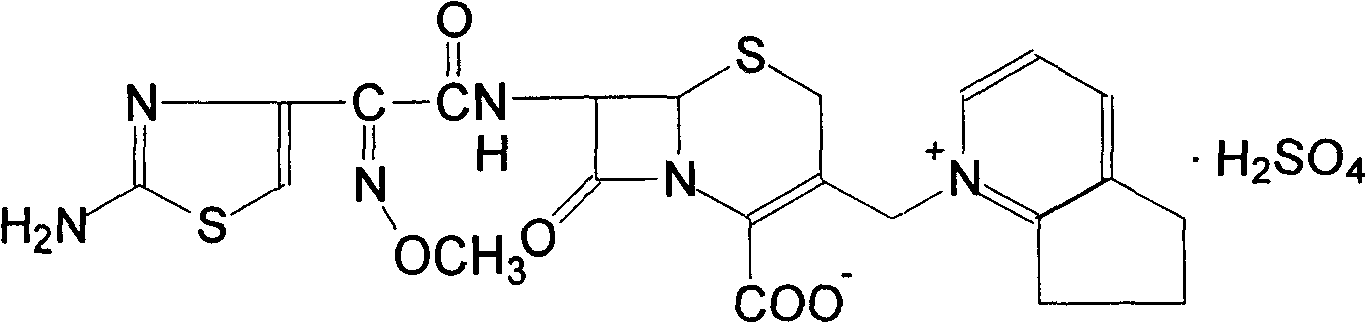
The invention discloses a preparation method of cefpirome sulfate. The method comprises the following steps: treating cefpirome hydroiodide salt with basic anion exchange resin in water as a solvent to obtain cefpirome, and then forming a salt with a sufficient amount of sulfuric acid , adding ethanol to precipitate crystals, and obtain cefpirome sulfate. The invention is easy to operate, the obtained product has better quality and higher yield, and avoids the use of highly toxic solvents such as toluene, thereby reducing the pollution of three wastes.
Owner:河北凯盛医药科技有限公司
Cefpirome sulfate compound and composition thereof
InactiveCN102796119ASimple prescriptionImprove self stabilityAntibacterial agentsOrganic active ingredientsArginineCEFPIROME SULFATE
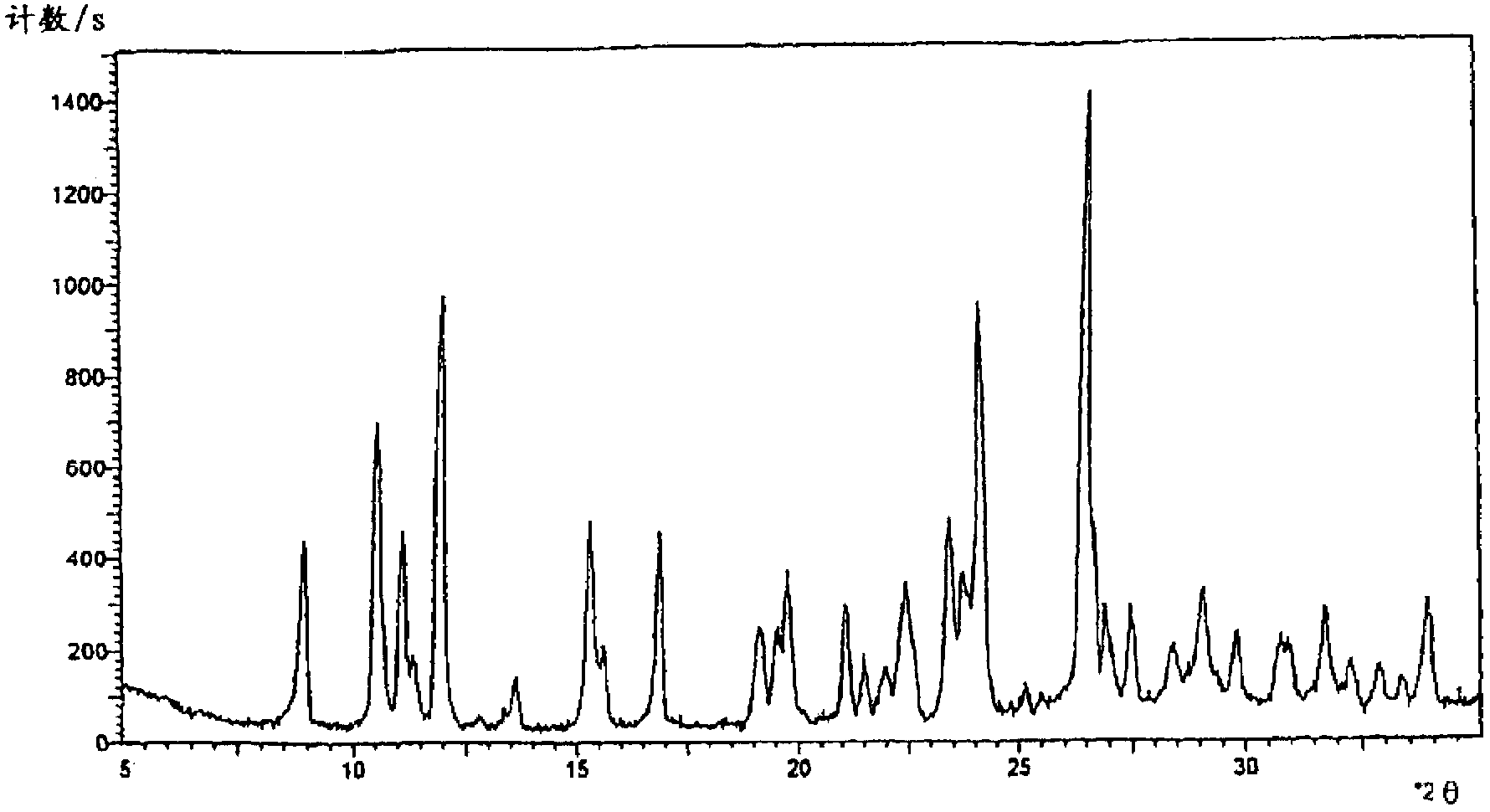
The invention relates to a cefpirome sulfate compound and a composition thereof. The cefpirome sulfate compound is measured with a power X-ray diffraction measuring method. The X-ray powder diffraction represented by the diffraction angle of 2theta+ / -0.2 degrees shows characteristic peaks at the angles of 8.92 degrees, 10.55 degrees, 11.12 degrees, 11.98 degrees, 15.23 degrees, 16.80 degrees, 19.59 degrees, 20.88 degrees, 22.40 degrees, 23.32 degrees, 23.54 degrees, 24.11 degrees, 26.52 degrees, 26.88 degrees, 27.47 degrees, 29.18 degrees, 31.76 degrees and 33.76 degrees. The composition contains arginine; the molar ratio of cefpirome sulfate to arginine is 1:1-1:3, and is preferably 1:1.5-1:2; and the arginine is preferably L-arginine. The stability of the cefpirome sulfate compound provided by the invention is enhanced remarkably, the cefpirome sulfate compound does not change easily after standing for a long time, and the administration safety of a patient is enhanced greatly.
Owner:JIANGXI KANGHUA MEDICAL TECH
Refining process of 7-ACP.HCL
ActiveCN101759709AHigh yieldHigh purityAntibacterial agentsOrganic chemistryMethylene DichlorideIodide
The invention discloses a refining process of 7-ACP.HCL, comprising the following steps of: 1, adding a hydrochloric solution to an aqueous 7-ACP.HCL crude product solution, stirring, dissolving, and regulating a pH value between 0.5 and 2.0; 2, adding a right amount of hydrogen peroxide, stirring, then adding activated carbon, stirring for 15-30 minutes, filtering, washing the carbon by using deionized water, and merging washing liquor; 3, adding methylene dichloride, stirring for 15-30 minutes, standing and demixing, and separating an aqueous phase; and 4, adding a right amount of acetone to the aqueous phase, dripping an alkali solution, regulating the pH value between 2.5 and 4.0, stirring, crystallizing, and growing crystals for 1-2 hours after the crystallization. The invention has high yield and purity of the 7-ACP.HCL, is beneficial to synthesizing high-purity cefpirome sulfate, enhances the purity of the 7-ACP.HCL by removing iodide impurities contained in the 7-ACP.HCL by adding the hydrogen peroxide before the recrystallization process of the 7-ACP.HCL, has simple operation process and reaction condition, and is suitable for industrialized production.
Owner:SHANGHAI NEW ASIA PHARMA
Synthetic method of cefpirome sulfate
ActiveCN101284840BLow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
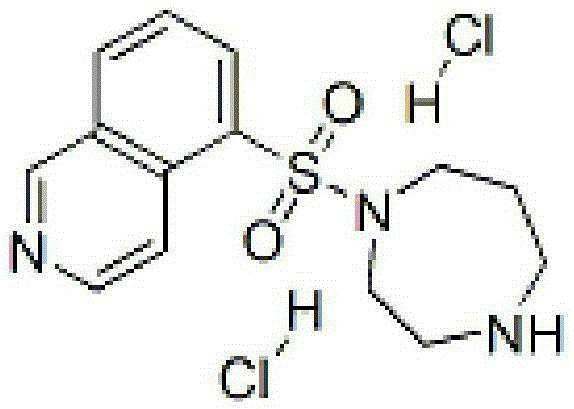
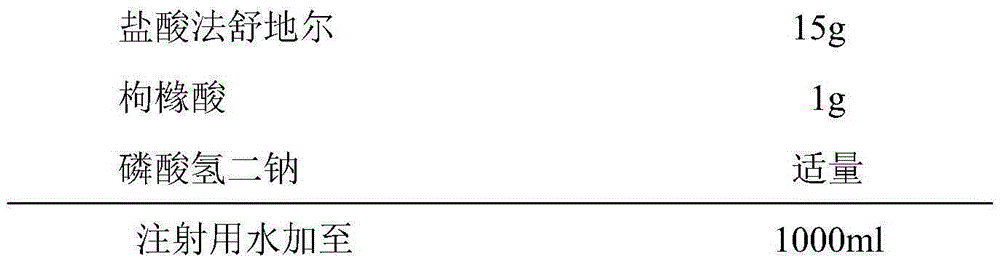
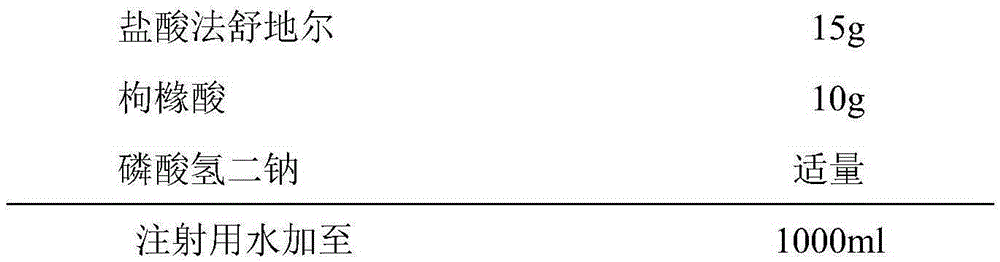
Fasudil hydrochloride injection and preparation method thereof
InactiveCN105287372AOrganic active ingredientsPharmaceutical delivery mechanismForeign matterCEFPIROME SULFATE
The invention provides a fasuadil hydrochloride injection and a preparation method thereof. The fasuadil hydrochloride injection is prepared from fasuadil hydrochloride with the concentration of 15mg / mL, citric acid with the concentration of 1 to 10mg / mL, and disodium hydrogen phosphate; the disodium hydrogen phosphate is adopted to adjust a solution pH value to be 4.0 to 6.5. Preferably, the concentration of the citric acid is 3 to 7mg / mL, and the pH value is 5.5 to 6.5. More preferably, the concentration of the citric acid is 5mg / mL, and the pH value is 5.7 to 6.3. By adopting the fasuadil hydrochloride injection prepared by the method, visible foreign matters meet quality standard provisions in a freezing and thawing test, and the compatible stability with cefpirome sulfate is improved.
Owner:BEIJING LANDAN PHARMA TECH
Method for synthesizing cefpirome sulfate
InactiveCN101161655AReduce degradation damageReduce dosageOrganic chemistryBis(trimethylsilyl)amineIon exchange
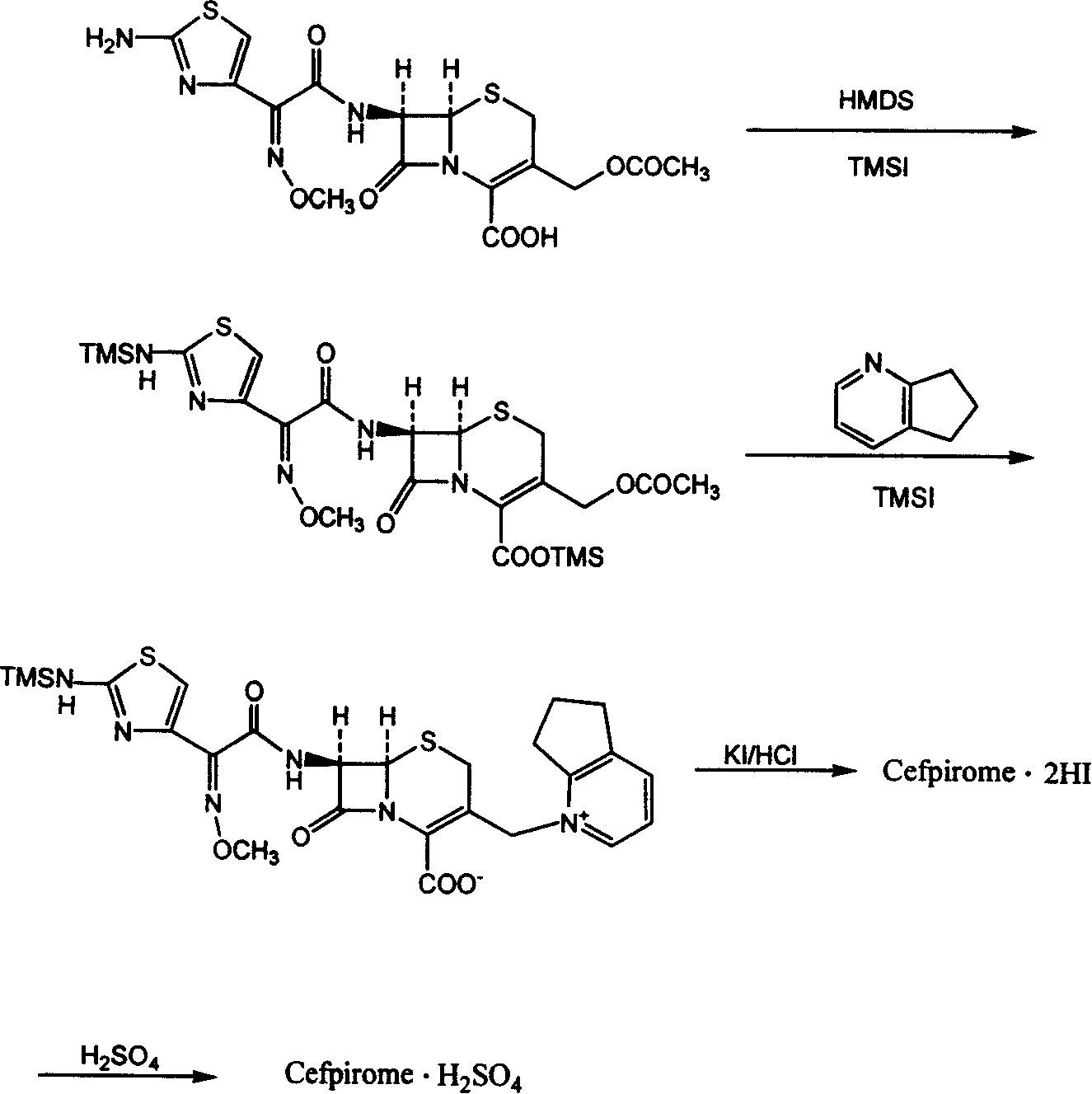
The present invention relates to a preparation method of cefpirome sulfate, and is characterized in that hexamethyldisilazane (HMDS) with low price combined with a water-ethanol mixture solvent is added as the ion exchange medium, so that the needed dosage of 2,3-cyclopentenopyridine and odotrimethylsilane is reduced, as well as the fabrication cost. The synthetic technics provided by the present invention has the advantages of feasibility, low cost, and stable quality of the product.
Owner:上海慈瑞医药科技股份有限公司
New combination of Cefpirome Sulfate and preparation method
InactiveCN100356921CAntibacterial agentsOrganic active ingredientsNeutral Amino AcidsPharmaceutical Substances
A cefpirome sulfate as a newm edicine and its preparing process which features use of alkaline or neutral amino acid as cosolvent are disclosed.
Owner:NANJING CHENXIANG MEDICAL RES
Composition of cefpirome sulfate and sodium citrate
ActiveCN101695494BGood water solubilitySolve the shortcomings of inconvenient clinical useAntibacterial agentsPowder deliverySolubilitySide effect
The invention discloses a composition with antibacterial effect. The composition is composed of cefpirome sulfate and sodium citrate. The aseptic powder of cefpirome sulfate and sodium citrate is combined, and sodium citrate is used as cefpirome sulfate. The co-solvent of Pilo, can effectively increase the water solubility of Cefpirome Sulfate, solve the shortcoming of the inconvenient clinical use of Cefpirome Sulfate, Cefpirome Sulfate can play its antibacterial effect well, the composition obtained after the two ratio It has good stability, and the prepared powder injection can be stored for a long time. The antibacterial active ingredient cefpirome sulfate has a stable structure, and it is easy to formulate quality standards, and does not produce impurities with toxic and side effects, and the curative effect is stable and safe.
Owner:国药集团致君(苏州)制药有限公司
Preparation methods of cefpirome intermediate and cefpirome
ActiveCN102391288BHigh purityHandling Recycling SimplifiedOrganic chemistryCyclopenteneCarboxylic acid
The invention relates to preparation methods of cefpirome intermediate and cefpirome; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the cefpirome intermediate (6R, 7R)-7-amino-3-[(2,3-cyclopentene-pyridine)methyl]ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing cefpirome sulfates by using the obtained intermediate halogen acid salt. The cefpirome intermediate and cefpirome prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Synthesis method of cefpirome sulfate
InactiveCN109651400AReduce usageReduce manufacturing costOrganic chemistrySynthesis methodsDissolution
Belonging to the technical field of cefpirome drug synthesis, the invention in particular relates to a synthesis method of cefpirome sulfate. The method includes: taking GCLE and AE active ester as the raw materials for reaction under the action of alkali and a catalyst, performing extraction, using dilute hydrochloric acid to adjust pH and performing crystallization to obtain a compound A; addingan activating agent into an organic solvent, performing stirring till dissolving clarification, adding the compound A, adding 2, 3-cyclopentenopyridine dropwise to precipitate solid, thus obtaining acompound B; adding the compound B into a mixed solvent of water and alcohol, conducting heating stirring till dissolution, using sulfuric acid to adjust the pH value and performing crystallization toobtain cefpirome sulfate. The method provided by the invention adopts GCLE as the raw materials, avoids the use of expensive catalyst, and the used solvent and activating agent are cheap and easily available, thus greatly reducing the production cost. The synthesis method provided by the invention has the advantages of simple synthetic process route, few reaction steps, convenient operation and few side reaction, and the prepared cefpirome sulfate has high purity and yield.
Owner:淄博鑫泉医药技术服务有限公司
Cefpirome sulfate pharmaceutical composition powder injection
InactiveCN103127128AReduce dosageReduce or avoid side effectsAntibacterial agentsPowder deliverySide effectTreatment effect
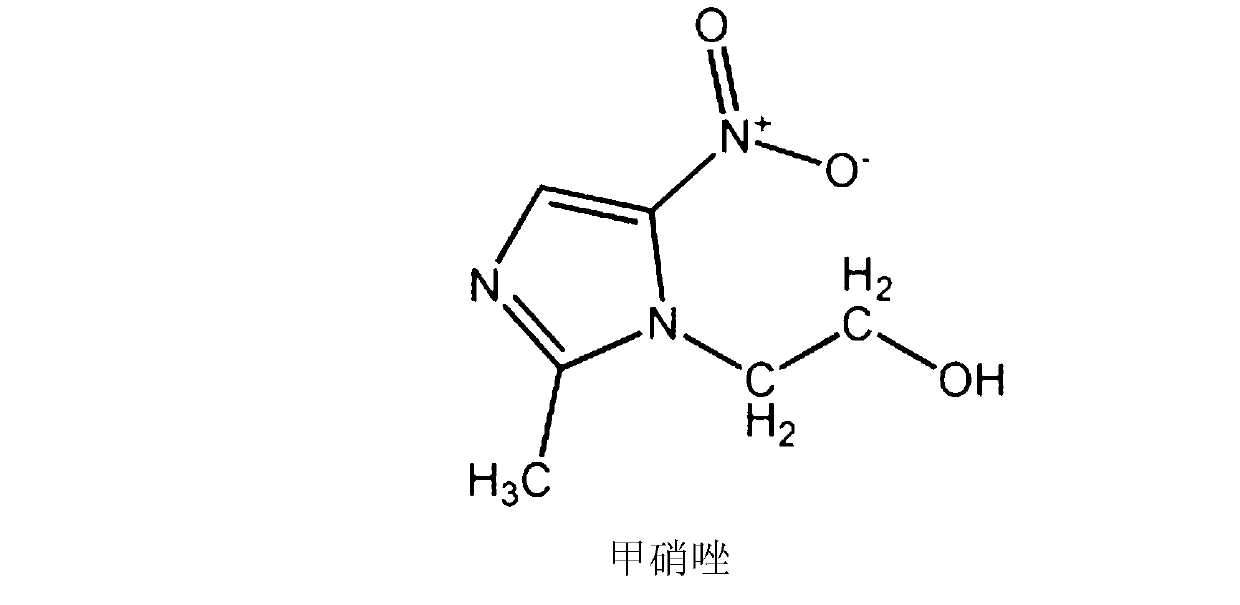
The invention provides a cefpirome sulfate pharmaceutical composition powder injection, relating to the field of a pharmaceutical preparation and a preparation method thereof. The invention solves the problems of high dosage of single cefpirome sulfate and metronidazole preparation, poor treatment effect after combination, and great side effect at present. The main materials of the pharmaceutical composition are cefpirome sulfate and metronidazole lipid microspheres, the auxiliary material is sodium carbonate, the weight ratio of the cefpirome sulfate (on the basis of cefpirome) to the metronidazole lipid microspheres (on the basis of metronidazole) is 100:6-100:20, and the weight ratio of the cefpirome sulfate (on the basis of cefpirome) to the sodium carbonate is 1000:242; and after the pharmaceutical composition is prepared into the powder injection, the pH value of the water solution of the pharmaceutical composition is 5.5-7.0. The pharmaceutical composition powder injection provided by the invention has high curative effect, and can reduce the consumption of the metronidazole by more than 80%, thereby greatly relieving or avoiding the toxic and side effects of the metronidazole, especially digestive tract reactions, including nausea, vomit, inappetence, abdominal colic and neurotoxicity, and lowering the incidence rate of adverse reactions.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method for synthesizing antibiotic cefpirome sulfate
InactiveCN101337970BSimple process conditionsEasy to operateAntibacterial agentsOrganic chemistryHexamethyldisilaneSynthesis methods
The invention relates to a synthesis method of cefpirome sulfate that is a bacteriophage. 7-amin cethalosporanic acid (7-ACA) is used as starting material and reacts with hexamethyldisilane amine (HMDS) and iodotrimethylsilane (TMSI) first to obtain 7-ACA for protecting amino and carboxyl; then 7-ACA, amino and carboxyl of which are protected, reacts with iodotrimethylsilane and 2, 3-cyclopenopyridine to synthesize an intermediate 7-ACP through a one-pot method; then 7-ACP reacts with active ester to prepare a product of cefpirome sulfate through acidylation reaction and salifying reaction. Compared with the existing technical route, the synthesis method has the advantages that the process conditions are simple, the operation is convenient, the product yield is high, the product quality is stable, the method is suitable for the large-scale industrialized production, etc.
Owner:国药集团致君(苏州)制药有限公司
Cefpirome sulfate composition freeze-dried powder injection for injection
InactiveCN103550177ABiocompatibleBiodegradableAntibacterial agentsPowder deliveryChitosan nanoparticlesNon toxicity
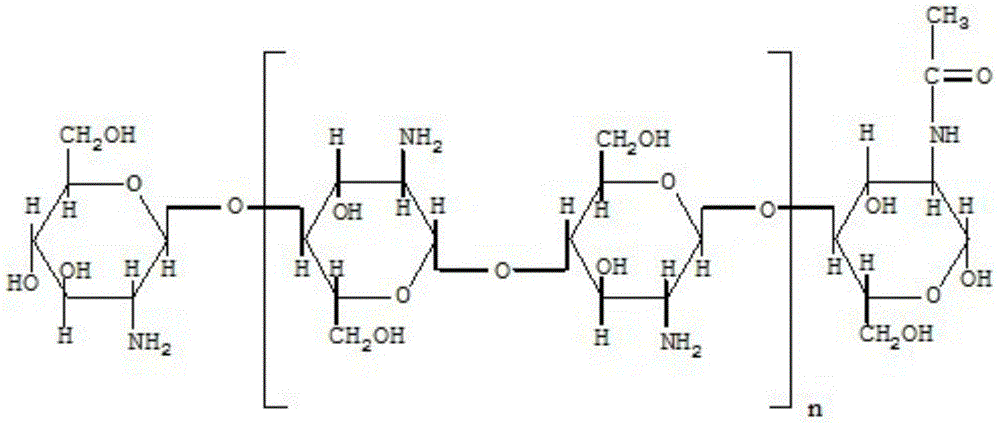
The invention provides a cefpirome sulfate composition freeze-dried powder injection for injection, and relates to the technical field of medicines and medicine preparation. The cefpirome sulfate composition freeze-dried powder injection for injection comprises the following raw material components in parts by weight: 7.26-9.17 parts of cefpirome sulfate, 4.36-5.50 parts of chitosan nanoparticles and 84.38-89.10 parts of water for injection. The cefpirome sulfate composition freeze-dried powder injection for injection has the advantages that 1) the chitosan nanoparticles occupy the active center of beta-lactamase, so that the beta-lactamase in drug-resistance bacteria is deactivated, the antibacterial spectrum of cefpirome is widened and the antibacterial activity of cefpirome is remarkably enhanced; 2) the chitosan nano carrier material has biocompatibility, biodegradability and non-toxicity; 3) the composition can maintain body circulation for a longer time; 4) the chitosan nanoparticles can replace mannitol as a freeze-dried skeleton agent of the freeze-dried powder injection, so that the activity of mannitol on a human body is eliminated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method of preparing cefpirome sulfate
The invention discloses a preparation method of sulfuric acid Cefpirome, and has the steps as follows: Cefpirome hydriodate is changed by the resin through using alkaline negative ion and is processed to get the Cefpirome when using water as solvent, thereby forming salt with full sulfuric acid, ethanol is added to separate out crystal, and then sulfuric acid Cefpirome is obtained. The invention has simple operation, and the obtained product has better quality with higher obtaining rate, moreover, and the solvents with bigger toxicity like toluene are avoided from being used, thereby reducingthe three waste pollution.
Owner:河北凯盛医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com