Method for synthesizing cefpirome sulfate
A cefpirome sulfate and synthetic method technology, applied in chemical recovery, organic chemistry, etc., can solve the problems of many side reactions, high production cost, and long reaction time, so as to shorten the reaction time, increase the product yield, and reduce the production rate. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
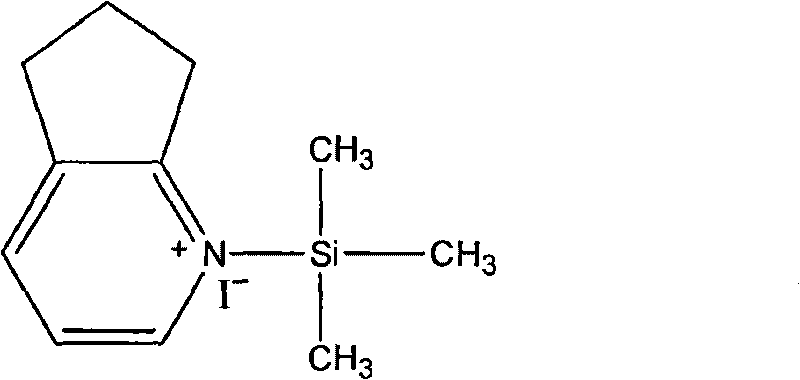
[0021] 1. Synthesis of quaternary ammonium salt intermediates
[0022] Add cyclopentenopyridine 26.61g (0.223mol) in the four-necked flask and be dissolved in 100ml cyclohexane, under N 2 Under protection, stirred and added TMSI 12.06g (0.06mol) dropwise, slowly raised the temperature to 70°C, refluxed for 4h, and cooled to room temperature.
[0023] 2.7-Synthesis of ACP
[0024] Add 100ml of dichloromethane and 13.6g (0.05mol) of 7-aminocephalosporanic acid (7-ACA) into the three-necked flask, stir and dropwise add 19.33g (0.12mol) of hexamethyldisilazane at 15°C, dropwise After the addition, react at 15°C for 50min, add the quaternary ammonium salt intermediate solution prepared in step (1), react at 5°C for 1.5h, then slowly raise the temperature to 15°C, and add 100ml DMF and 56ml concentrated Hydrochloric acid, stirred and cooled to 0°C, slowly added the above solution into the mixture, stirred and reacted for 30 minutes. 50ml of water was added thereto, and after stir...
Embodiment 2
[0028] 1. Synthesis of quaternary ammonium salt intermediates
[0029] Add cyclopentenopyridine 29.85g (0.25mol) in four-necked flask and be dissolved in 100ml cyclohexane, under N 2 Under protection, stirred and added TMSI 12.06g (0.06mol) dropwise, slowly raised the temperature to 70°C, refluxed for 4h, and cooled to room temperature.
[0030] 2.7-Synthesis of ACP
[0031] Add 100ml of acetonitrile and 13.6g (0.05mol) of 7-aminocephalosporanic acid (7-ACA) into the three-necked flask, stir and add 19.33g (0.12mol) of hexamethyldisilazane dropwise at 15°C, and the dropwise addition is completed React at 15°C for 50min, add the quaternary ammonium salt intermediate solution prepared in step (1), react at 5°C for 1.5h, then slowly heat up to 15°C, in a three-necked flask, add 100ml DMF and 56ml concentrated hydrochloric acid, stir After cooling to 0°C, the above solution was slowly added to the mixture, and stirred for 30 minutes. 50ml of water was added thereto, and after s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com