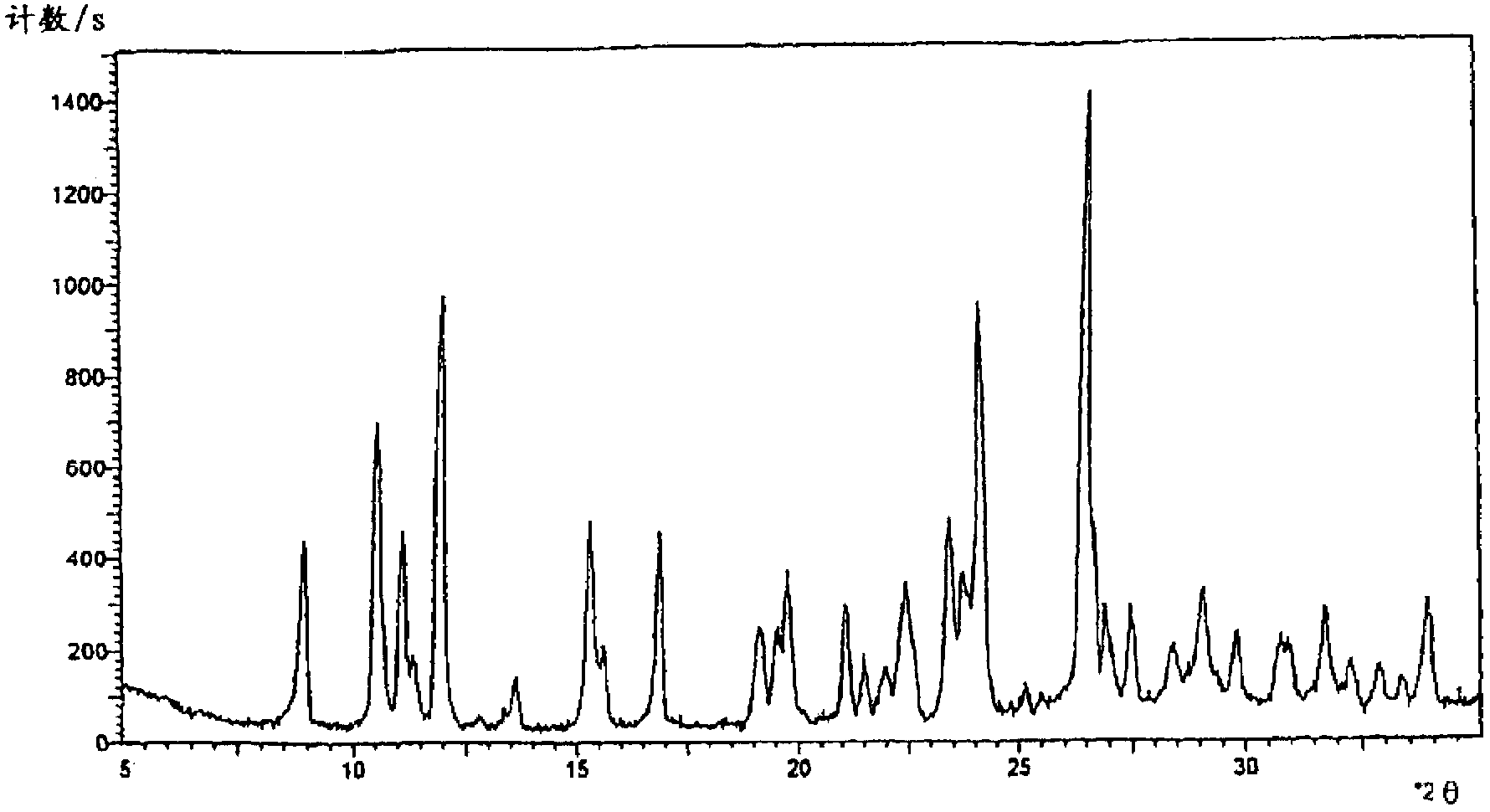
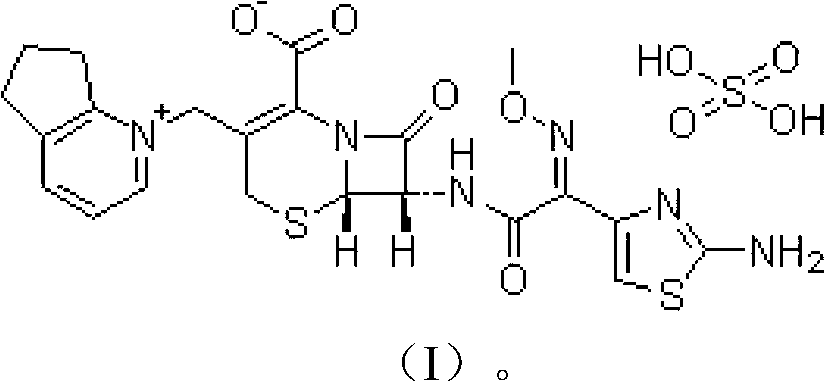
Cefpirome sulfate compound and composition thereof
A technology of cefpirome sulfate and its compound, which is applied in the field of medicine, can solve the problems of insignificant improvement of stability and curative effect, and achieve the effects of improving drug safety, improving stability, and simplifying prescriptions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Cefpirome sulfate of the present invention is prepared by the following method:
[0051] (1) Dissolve the crude cefpirome sulfate in purified water to form a 0.2 g / ml cefpirome sulfate aqueous solution;
[0052] (2) Add 0.4 times the amount of activated carbon to decolorize the crude product of cefpirome sulfate, filter, and heat the filtrate to 38°C;
[0053] (3) Add the ethanol / isopropanol / acetone mixed solution whose volume is 8 times of the weight of the cefpirome sulfate crude product to the filtrate twice; in the ethanol / isopropanol / acetone mixed solution, ethanol:isopropanol : The amount of acetone is 12:4.5:4.5; for the first time, at a stirring speed of 12rmp, add 1 / 14 ethanol / isopropanol / acetone mixed solution dropwise to the filtrate at a constant speed within 20 minutes, and dropwise at 1°C Cool the filtrate to 12°C at a speed of 1 / min; for the second time, at a stirring speed of 7rmp, add the remaining ethanol / isopropanol / acetone mixed solution to the filt...
Embodiment 2
[0057] (1) Dissolve the crude cefpirome sulfate in purified water to form a 0.02g / ml cefpirome sulfate aqueous solution;
[0058] (2) Add activated carbon of 0.1 times the amount of crude cefpirome sulfate for decolorization, filter, and heat the filtrate to 35°C;
[0059] (3) Add the ethanol / isopropanol / acetone mixed solution whose volume is 4 times the weight of the cefpirome sulfate crude product to the filtrate twice; in the ethanol / isopropanol / acetone mixed solution, ethanol:isopropanol : The amount of acetone is 8:3:3; for the first time, at a stirring speed of 8rmp, add 1 / 16 ethanol / isopropanol / acetone mixed solution dropwise to the filtrate at a constant speed within 5 minutes, and drop it at 0.5°C Cool the filtrate to 10°C at a speed of / min; for the second time, at a stirring speed of 4rmp, add the remaining ethanol / isopropanol / acetone mixed solution to the filtrate at a constant speed for 20 minutes;
[0060] (4) Stop stirring, under the ultrasonic field of 0.05KW,...
Embodiment 3
[0063] (1) Dissolve the crude cefpirome sulfate in purified water to form a 0.3g / ml cefpirome sulfate aqueous solution;
[0064] (2) Add 0.5 times the amount of activated carbon to decolorize the crude product of cefpirome sulfate, filter, and heat the filtrate to 40°C;
[0065] (3) Add an ethanol / isopropanol / acetone mixed solution whose volume is 12 times the weight of the cefpirome sulfate crude product to the filtrate twice; in the ethanol / isopropanol / acetone mixed solution, ethanol:isopropanol : The amount of acetone is 16:6:6; for the first time, at a stirring speed of 16rmp, add 1 / 12 ethanol / isopropanol / acetone mixed solution to the filtrate at a constant speed within 30 minutes, and drop it at the same time at 1.5℃ Cool the filtrate to 15°C at a speed of / min; for the second time, at a stirring speed of 10rmp, add the remaining ethanol / isopropanol / acetone mixed solution to the filtrate at a constant speed for 30 minutes;
[0066] (4) Stop stirring, under the ultrasonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com